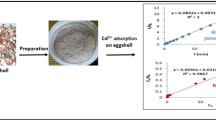
Abstract
The aim of this study was to investigate the feasibility of using a raw eggshell matrix as an affordable and ecology adsorbent for the removal of As(V) from aqueous solutions. The surface morphologies of the raw eggshell matrix were analyzed by Fourier transform infrared spectroscopy and scanning electron microscopy. Operating parameters such as the concentration of raw eggshell matrix, contact time, pH, and temperature were investigated in the removal of As(V) from aqueous solutions. In addition, desorption studies were carried out. In this study, an As(V) removal efficiency of approximately 91.43% was achieved under the optimum conditions (raw eggshell matrix = 1.0 g/L, pH = 5.97, time = 10 min). The ΔHº, ΔSº, and ΔGº provided during the process indicated that the raw eggshell matrix was applicable, spontaneous, endothermic for the removal of As(V). The Langmuir was used to calculate the maximum adsorption capacity (9.143 mg/g) of the raw eggshell matrix, and it was observed that the pseudo-second-order was suitable for the removal of As(V). It was also determined that 100 µg/L As (V) and the parameters that affect the adsorption process act as an antimicrobial agent and inhibit Escherichia coli bacteria on the raw eggshell matrix surface. According to the findings of this study, raw eggshell matrix was determined as a successful adsorbent for the removal of As(V) without any modification. Thus, it was demonstrated that the study has the potential to influence industrial symbiosis and industrial ecology approaches. This perspective will directly or indirectly contribute to cleaner production.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is a basic requirement needs for survival. However, the pollution of water resources has become a serious problem. Among the main pollutants, the spread of heavy metals, in particular, has increased considerably in recent years. Arsenic is among the top 20 most dangerous heavy metals (Pathan and Bose, 2018), and its contamination is an issue concerning the whole world as the constant utilization of arsenic-contaminated water can lead to arsenic-concerned illnesses (Mejia-Santillan et al., 2018). Today, mining activities such as ore processing and smelters; wood combustion and processing industry; coal and oil use, production, and burning; geothermal exploitation; agrochemical industry all produce arsenic-containing wastewater (Hayat et al. 2017).
Various methodologies have been being developed in order to eliminate arsenic from aqueous solutions. Some of these include chemical oxidation, coagulation, electrochemistry, nanofiltration, reverse osmosis, electro-dialysis, ion exchange, solvent extraction, membrane separation and adsorption using natural and artificial materials (Altowayti et al. 2020). However, all of these methodologies require high initial and maintenance costs and a qualified labor force and include huge concentrations of harmful mud and both heavy and weak contaminant sedimentation, which need more refinement. Today, adsorption is accepted as the most suitable elimination method due to features such as easy operation, simple design, renewability, small amount of sludge production, and easy integration into existing processes. Researchers have tested various adsorbents including oxides and hydroxides, metal phosphates, agricultural and industrial wastes, polymer resins and activated carbon (Kloster et al. 2020). Eggshells, which are considered as agricultural and industrial waste, are also an effective adsorbent.
Agricultural waste is a significant issue arising in the food industry due to the cost of its disposal. However, finding new uses for these residual materials is an opportunity for the bio-economic community. Eggshells are produced in large quantities by egg processing industries, and large amounts of these solid residues are still disposed of as waste in landfills without being pre-treated and, thus, are a source of organic pollution (Laca et al. 2017). By using eggshells as an adsorbent, the food industry could dispose of their waste and industries that produce arsenic-containing wastewater could save on the cost of adsorbents. In turn, this could lead to the emergence of industrial symbiosis and industrial ecology approaches. In biology, the word symbiosis refers to the interactions between organisms and the interdependencies of species in ecosystems. In terms of an industrial approach, symbiosis refers to the sectors that interchange their waste and by-products in order to decrease their raw material requirements (Lybæk et al. 2021). The ultimate aim of industrial symbiosis is to collaboratively use waste or by-products from other companies to minimize energy or resource use. Thus, a zero-waste level can be achieved and the matter cycle can be locked (Neves et al. 2020; Mirabella et al. 2014).
In the present study, the elimination of As (V) from aqueous solutions was investigated using a raw eggshell matrix (eggshell + membrane, RESM), a low-cost adsorbent. Within this context, the aim was to both directly and indirectly contribute to cleaner production by developing industrial symbiosis and an industrial ecology perspective for researchers and producers. There are several studies in the literature that have focused on adsorption using modified eggshells to remove arsenic ions from wastewater. Compared to these studies, the most significant difference of the present study is that no activation, coating or, modification was applied to the adsorbent. By doing so, it was ensured that the pathogens that may be present in the eggshells were inhibited by the effect of the arsenic and adsorption factors. In addition, the possibility of generating biological pollution was prevented by using RESM as an adsorbent and microbial safety of the aqueous environment was ensured.
Within the scope of this study, the surface morphologies of the RESM were analyzed by Fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy (SEM–EDX). Escherichia coli analyses, which can be found on the RESM, were carried out in two steps with 3M Petrifilm high-sensitivity coliform count plates. Factors that affect the removal efficiency of arsenic such as RESM concentration, contact time, solution pH, and temperature were investigated. Different adsorption isotherms and kinetics were measured.
Materials and methods
Materials
High purity As2O5 standard (100 µ/L As(V) concentration) was obtained from Sigma-Aldrich Chemie GmbH, Germany. All of the chemicals used in the study were of analytic purity (99%). For the adjustment of pH, 0.1 M HNO3 was used as the standard acid, while 0.1 M NaOH was used as the base solution. The glass and plastic materials used in the batch adsorption analysis were washed with 2% HNO3 and Milli-Q water (resistance, 18.2 MΏ cm; Millipore Corp.).
Preparation of the RESM
In this study, various processes, namely drying, grinding, and sieving, were applied to use the RESM as an adsorbent. The RESM was firstly washed with pure water (Milli-Q water, resistance, 18.2 MΏ cm; Millipore Corp.) and dried at 25 °C for 24 h. After the drying process, the RESM with a size of 1–1.5 cm2 was ground using a steel blade shredder. Then, the RESM was sieved, and the particles ranging between 1.5–3 mm mesh size were used in the adsorption studies. The RESM prepared at the beginning of each experimental study was dried again at 25 °C, brought to a fixed weight and used.
Escherichia coli tests in the RESM
The 3 M Petrifilm Coliform Count Plate is a ready-culture-medium that contains Violet Red Bile (VRB) nutrients, a cold-water-soluble gelling agent, and a tetrazolium indicator that facilitates colony enumeration in samples. Escherichia coli measurements were taken using this system. The homogenized samples were directly inoculated on Petrifilm, and the results obtained at the end of the required incubation were presented in terms of colony-forming unit (CFU).
Batch experimentations
Adsorption tests were carried out in glass equipment in accordance with the batch method. 250-mL Erlenmeyer flasks were used in the experiments. The schematic representation of the adsorption of As(V) onto the RESM is shown in Fig. 1. The laboratory-scale ZHICHENG analytical model thermal shaker was evaluated in the studies. A LABQUEST2 brand device was used to determine pH. For the adjustment of pH, 0.1 M HNO3 (Sigma-Aldrich Chemie GmbH, Germany) was used as the standard acid, while 0.1 M NaOH (Sigma-Aldrich Chemie GmbH, Germany) was used as the base solution. The effect of temperature on the adsorption of As(V) was examined between a range of 10 °C and 35 °C. The experiments were conducted by using different doses of the adsorbent ranging from 0.5 to 10 g/L in order to identify the optimum dose of RESM. All batch experiments were repeated three times. The As(V) concentration was analyzed by using an Inductively Couple Plasma-Optical Emission Spectrometer (ICP-OES, 2100DV, Perkin Elmer, USA). The As(V) yield (%) from the aqueous solution was calculated using the Eq. (1):
where Co is the input concentration (mg/L), Ce is the output concentration (mg/L). (qe) is the adsorption capacity (mg/g), m is the dry weight of the RESM (g), and V is the volume (L) of the aqueous solution (Eq. (2)):
Desorption experiments
Desorption tests were applied to examine the reusability of the RESM in the experiments. Firstly, in order to clear any non-adsorbed arsenic, the RESM was filtered and then gently washed by using distilled water. Secondly, the As (V)-loaded RESM was instantly blended with As (V) desorbing solvents and shaken for 5 h. Then, to determine the arsenic desorbed from the RESM, the blend was filtered and the supernatant was analyzed. The 0.1 M NaOH solution was found to be successful in the desorption of As (V) compared to KOH, NaOH, and HCl (Sigma-Aldrich Chemie GmbH, Germany). Accordingly, desorption was carried out using 25 mL of 0.1 M NaOH solution. The percentage of desorption was determined with Eq. (3):
where Cdes (mg/L) and Csorb (mg/L) are As(V) released in the solution and adsorbed onto RESM, respectively.
Morphological RESM characterizations
A SEM–EDX examination (Hitachi-SU 1510) was applied using a microscope equipped with an energy-dispersive analytic method operating at a pickup tension of 5–15 kV. FTIR spectroscopy (Excalibur 3000MX) was used for the characterization of the RESM surface. The surface area was determined with the Brunauer, Emmett and Teller (BET) method. A PerkinElmer 2400 brand elemental analyzer was used to determine the elemental composition of the synthesized RESM.
Results and discussion
Escherichia coli experiments
The eggs were stored at a temperature of ≤ 7 °C to reduce or control the microbial growth on the surface of the shell. In addition, eggshell membranes contain matrix proteins such as ovotransferrin, lysozyme, ovalbumin, clusterin, osteopontin, ovocleidin-17, ovocleidin-116, ovocalyxin-32, and ovocaloxin-36 all of which have antimicrobial properties (Jalili-Firoozinezhad et al. 2020; Lesnierowski and Stangierski, 2018). As arsenic species are toxic to living organisms, great care should be taken in adsorption and other treatment methods (Kim et al. 2020; Fontecha-Umaña et al. 2020). Adsorbents, especially those that form the basis of adsorption, may have various living species on their surfaces. Therefore, due to the possibility of the presence of Escherichia coli on the egg shells used in the present study, it was investigated whether adsorption factors and arsenic had an inhibitory effect on them. The RESM was exposed to arsenic at the optimum adsorption parameters (RESM concentration = 0.1 g/L, pH = 5.97, contact time = 10 min) and evaluated. As shown in Fig. 2, it was determined that both arsenic and other experimental parameters may lead to the inhibition for Escherichia coli. In particular, parameters in which arsenic reaches its maximum removal efficiency were preferred. This is because, with these selected parameters, the arsenic was removed in the receiving water environment and the microbial load of the eggshells was reduced.
An unprocessed control set was created to detect the existing microflora of the eggshells. For this purpose, 1 g of eggshells was weighed and kept in 10 ml of water at 37 °C and a pH of 7 for 24 h, which are the ideal breeding conditions of Escherichia coli. In addition, one more control set was created at 20 °C. At the end of the determined time period, the RESM was incubated at 20 °C and 37 °C for 24 h after being inoculated on Petrifilm, in accordance with, the manufacturer's instructions. At the end of the incubation process, the results were determined in CFU/1 g by counting the blue colonies that had grown in the Petrifilm. Different trial sets were also created for different doses, pH, and temperature combinations used in the study. In order to determine the antimicrobial effect of these conditions on the Escherichia coli load on the eggshells, inoculation was performed with the same method and the results were evaluated as CFU/1 mL. The conditions and results of the control and other experimental sets are given in Table 1. When these results were evaluated, it was observed that 100 µg/L As(V) and parameters affecting the adsorption, namely pH, temperature, and time, acted as an antimicrobial agent and inhibited Escherichia coli bacteria on the surface of the RESM.
Morphological and elemental characterization of the RESM
Table 2 shows several properties and the elemental analysis of the RESM as detected by ICP-OES (Zaman et al. 2018). The SEM images of the RESM are presented in Fig. 3. As can be seen from figure, there were uneven and spiral surfaces with a high amount of messy pores, which produce great free surface field. In addition, the images showed the adsorption ability of As(V). EDX was performed to see the elemental exchange in the RESM before and after As(V) treatment.
As can be observed in Fig. 3, before the As(V) removal from the structure, the percentages of C, S, O, and Ca were 75.13%, 1.52%, 25.68%, and 1.67%, respectively. However, after the removal, the elemental composition of As(V) changed to: 77.26% for C, 1.56% for S, 20.60% for O, and 0.58% for Ca (Fig. 3). Similar values were reported by Kusmierek et al. (2017). As eggshell characterization has been determined in many studies, the physicochemical properties of eggshell are well known (Shi et al. 2020; Mejia-Santillan et al. 2018; Kusmierek et al. 2017). According to the EDX analysis results, O and Ca bonded with As(V) during adsorption process. During the removal of As(V), O and Ca decreased in percentage. However, the percentages of S and C values increased, albeit at a low rate. This increase was thought to be due to the last vital activities of E. coli in the medium.
The FTIR peaks are presented in Fig. 4. The spectrum of 3000–3500 cm−1 indicated the existence of an OH set, while the pointed peak at 1465 cm−1 demonstrated the existence of a C = O set. The spectrums between 800 and 1320 cm−1 that demonstrate the C–O tension vibrations are given in Fig. 4. In Fig. 4, the band between 3000 and 3500 cm−1 showed less peak compared to before the adsorption, process; however, it still indicated an OH stretch; the small peak between 1500 and 2235 cm−1 indicated a C=C stretch; the spectrums around 2000–2500 cm−1 defined the presence of specific peaks; and the wide area at approximately 3500 cm−1 could be determining the presence of O–H and N–H tension (Hasrin et al. 2020). In addition, the presence of a -OH group indicated that the RESM had a moisture adsorption capability. The bands at 1631 and 1440 cm−1 represented C=O and C=O stretching, respectively. However, the existence of O–H and C=O stretching bands in the FTIR of the RESM demonstrated the existence of –COOH, which is consistent with the previous studies (Seeharaj et al. 2019; Shakoor et al. 2019). Based on the FTIR analysis, it can be assumed that the surface functional groups were suitable for adsorption.
pH stability studies
The As (V) removal efficiencies at different pH values obtained in the first stage of determining the optimum adsorption conditions are presented in Fig. 5. When the pH reached 5.97, it was observed that the As(V) removal percentage for RESM increased significantly from 70.21% to 87.02%. Increasing the pH value of the solution from 5.97 to 12.0 resulted in a decrease in yield from 87.02% to 63.71%. Among the different pH values studied, the highest removal yield for the adsorption of As(V)was found as 87.02% when pH was 5.97.
As a result of the increase in pH from 2.0 to 12.0, the removal efficiency of As(V) decreased swiftly from 87.02% to 63.71%. The reason for this was that as As(V) types, H2AsO4− and HAsO42− are dominant in the pH range of 3–10 (H3AsO4 has three pKa values: pK1 = 2.20, pK2 = 6.97, and pK3 = 11.53) (Wu et al. 2018). As the solubility of calcium carbonate is low (less than 1%) in the pH range of 2–12, the experiment for the adsorption of As(V) was carried out in this pH range (Flores-Cano et al. 2013). The pHzpc (zero-point charge) of any absorbent is a very significant feature that identifies the pH value at which the surface has net electrical neutrality. The pHzpc of RESM was determined as 7.43 and was comparable with the values obtained in previous studies (Kusmierek et al. 2017). According to the studies in the literature, decreases in adsorption efficiency can be observed depending on the electrostatic repulsion force at optimum conditions. At the condition in which the pH value is below the pHzpc of the material (6.5), the hydroxyl groups of the surface will be protonated to form OH2+, which makes the ligand interchange with the arsenate anion easier (Niazi et al. 2018; Bibi et al. 2017). The gradual de-protonation of the surface hydroxyl groups due to the increase in the pH of the solution causes the adsorbent to be charged negatively. As this condition imposes repulsion with the anionic As(V), it is unfavorable for As(V) adsorption. It has been stated in the literature that as the pH of the solution increases, the adsorption level of strong acid anions by hydroxides and metal-oxides decreases (Zhang et al. 2013).
Effect of contact time
Contact time is a significant parameter in determining the equilibrium time required for the absorption of metal ions on an adsorbent, as there is a positive relationship between the number of metal ions removed from the aqueous solutions and contact time (Gaur et al. 2018). The influence of contact time was examined at 25 °C by changing the length of the contact time from 5 to 120 min. In addition, the RESM amount and pH level were fixed as 1 g/100 ml and 5.97, respectively. Figure 6 shows the removal efficiency of arsenic on the RESM according to contact time. According to figure, it can be observed that the arsenic removal efficiency firstly increased quickly and then achieved balance. This may be due to the larger surface area and specific properties of the RESM for the adsorption of As (V). Similar behavior has also been mentioned by various studies in the literature (Nistico et al. 2018). Under the presented situation, the maximum As(V) removal efficiency was determined as 91.43%. As can be seen from Fig. 6, the reaction for As (V) was fairly quick and the elimination yield reached equilibrium within 30–120 min. Gaur et al. (2018) carried out a study by using apple pomace activated with NaOH and determined that the ultimate amount of As(V) was adsorbed within a 60-min incubation period, and then, it reduced with the increase in the incubation period.
Effect of RESM dosage
The influence of RESM dose was investigated at 25 °C, 5.97 pH, and a contact time of 10 min by modifying the adsorbent amount from 0.5 to 10 g/100 ml. The removal percentage of As(V) decreased from 71.57%, 83.39%, 70.00%, and 69.56% when the RESM dose was 0.5, 1.0, 2.0, and 5.0 g/L, respectively. The increased dose of RESM and the decrease in efficiency may be due to the saturation of the RESM, in which case it can no longer adsorb As(V). In this study, the maximum removal of ~ 83.39% was achieved at an optimum RESM dose of 1.0 g. After 1.5 g RESM dose, the arsenic removal efficiency remained constant at doses between 2 and 10 g (Fig. 7). Similar results have been reported by studies in the literature for the adsorption of arsenic by utilizing different adsorbents (Zeng et al. 2020; Sawood and Gupta 2020).
Effect of temperature changes
During the adsorption stage, temperature affects the velocity of the reaction between the adsorbent and adsorbate in the aqueous solution. The influence of temperature on the adsorption of arsenic is shown in Fig. 8. It can be understood that there was a positive relationship between temperature and adsorption of As(V). As can be observed in Fig. 8, with the temperature increasing from 10 °C to 45 °C, the As(V) removal efficiency increased from 76.51% to 87.21%. However, a higher increase in temperature had very little effect on the adsorption of arsenic. Regarding the literature, it can be said that this issue was also observed in other adsorbents (Pokhrel and Viraraghavan 2008). The reason for this relationship between the arsenic removal efficiency and the temperature is not clear yet. The increased adsorption capacity or rising kinetic activity may be shown as a reason; however, further research is required for the determination of the exact reason (Wu et al. 2018).
Thermodynamic effects of temperature changes
Thermodynamic parameters are important factors in determining the level of spontaneous formation of adsorption and energy requirements. Gibbs energy (ΔG°, kJ/mol), entropy change (ΔS°, J/mol/K), and enthalpy change (ΔH°, kJ/mol)) are determined using Eqs. 4 and 5. Kd is the thermodynamic distribution coefficient and was calculated using Eq. 6 (Heiba et al. 2018). R [8.314 (J/mol/K)]
Thermodynamic calculations were performed according to Fig. 9. The ΔG° was −2.643, −2.045, −1.779, −1.180, −1.346, −1.085 kJ/mol at 283, 288, 293, 298, 303, 308 K, respectively. All ΔGº values were negative, which clearly reveals the spontaneous nature of the adsorption. In addition, negative values of ΔG° indicate that the bond energy between the RESM and As(V) was strong. According to the literature, ΔGº is between zero and − 20 kJ/mol for physical adsorption, and −80 and −400 kJ/mol for chemical adsorption. Mudzielwana et al. (2020) determined that in As(V) treatment with RESM, ΔG° remained below −20 and the limit value was not exceeded despite the temperature increase. The positive values of ΔH° and ΔS° showed that As(V) adsorption was endothermic and the randomness was found to increase between the As(V) solution and the inactive regions of RESM. As the temperature increased, the degree of the self-realization of the process increased. Additionally, the positive ΔHº value of 23.589 kJ/mol for the adsorption of As(V) indicated that the adsorption to the RESM was endothermic in physical sorption. The positive ΔS° value of 7.295 J/mol/K was obtained from the interaction of RESM and As(V) in the solid–liquid interface. Similar findings were obtained in relevant studies in the literature (Rahdar et al. 2019; Tran et al. 2017). The ΔG° values obtained for all temperature values used in the study were found to be between −1.085 and −2.643 kJ/mol. According to the literature, ΔG° is between 0 and −20 kJ/mol for physical adsorption. The values obtained in the present study were found to be, within this range. As a result, it can be said that the adsorption of As (V) by RESM is of a physical adsorption.
Evaluation of different adsorption isotherms and kinetic models
Four different isotherms were used to describe the relationship between the RESM and As(V) during the adsorption process. According to the experimental results, the Freundlich (7), Langmuir (8), Dubinin-Radushkevich (9), and Temkin (10) isotherms and the separation factor (RL) (11) were determined (Çelebi et al. 2020):
Ce: balance amount of As(V) (mg/L); qe: the number of As(V)-RESM at equilibrium (mg/g); KL: Langmuir constant (L/mg); KF and 1/n: Freundlich constants; Ɛ and R (8.314 J/mol K) constants; AT (L/mg), bT (J/mol): Temkin constants. In order to evaluate the goodness of fit of the isotherms, the normalized standard deviation (Δq) was measured by using the following formula (Kusmierek et al. 2017):
In Eq. 12, N refers to the number of data points. It was thought that the isotherm model with the lower Δq value and higher correlation coefficient (R2) would be better fitted to the experimental data. Table 3 shows the values of the equilibrium constants, and the correlation coefficient for each isotherm model. As can be seen from the table, the Langmuir isotherm model had a higher R2 value. A maximum absorption capacity of 9.143 mg/g for RESM was obtained with the Langmuir isotherm equation.
When the values of separation factors (RL) obtained by using Eq. 11 are observed, it can be said that for the value of 0, the process of adsorption was irreversible, for the values between 0 and 1, it was unfavorable, and for the values above 1, it was linear. As can be seen in Table 3, the separation factor values found in this study were between 0 and 1, indicating that the adsorption of As(V) onto RESM was favorable at equilibrium. By comparing the values of the R2 and the normalized standard deviation (Δq), it can be said that the Langmuir and Freundlich isotherms were efficiently in line with the adsorption process of the RESM particles. Compared to the Langmuir, the Freundlich was less suitable (R2 = 0.983, Δq = 2.554%). The experimental (qexp) and calculated (qcal) adsorption capacities for the isotherms are shown in Fig. 10, while Fig. 11 shows the adsorption isotherms of As(V) onto RESM. The results obtained with RESM were consistent with the results obtained in previous studies (Hua 2018). Alkurdi et al. (2020) used bone char for arsenic species and found that the Langmuir isotherm was suitable. Sawood and Gupta (2020) evaluated the Azadirachta indica/Fe for the removal of As(V) from aqueous solutions and determined that the Langmuir and pseudo-second-order were successful for this process. In the present study, as can be seen in Table 4, the maximum arsenic ion adsorption capacities of the RESM determined by using the Langmuir isotherm were within the same range of both modified and natural absorbents used in previous studies. It can also be said that the adsorption capacity of eggshells is lower than other adsorbents. The adjustment of the equilibrium data to the Langmuir isotherm was decisive for a single-layered absorption of the arsenic ion on the homogeneous surface areas of the RESM mixtures.
Çelebi et al. (2020) identified the absorption process by applying the pseudo-first-order and pseudo-second-order kinetic and intra-particle diffusion models in order to examine the adsorption rate of the arsenic ion via the RESM adsorbent. In the present study, pseudo-first-order (Eq. 13), pseudo-second-order (Eq. 14), Elovich (Eq. 15), and intraparticle diffusion (Eq. 16) kinetics were tested for the adsorption of As (V).
In these equations, k1 is the rate constant of the pseudo-first-order; k2 refers is the rate constant of the pseudo-second-order; qt and qe are the arsenate adsorption capacity for any time (t) and at equilibrium, respectively; kp is the rate constant of the intra-particle diffusion; and c is the intercept of the intra-particle diffusion model. Table 5 shows the values of the As(V) adsorption kinetics of the pseudo-first order and pseudo-second order.
According to Table 5 and Fig. 11, it can be explicitly said that the kinetic data can be explained better by the pseudo-second-order model as this model had a higher R2 for RESM compared to the first-order model. For this reason, inferences indicating that the adsorption of arsenic ion via RESM follows pseudo-second-order kinetics can be made. The term adsorption refers to the physical bonding of ions and molecules to the surface of a solid material. Kinetic models can clarify the behavior of the adsorption process under flexible operational parameters (Wu et al. 2018). The pseudo-second-order model with higher R2 values better explains the fact that adsorption may be the speed limiting stage, which may include the exchange of electrons or valence forces sharing between RESM and As(V). Other studies in the literature conducted on heavy metal adsorption using different techniques have emphasized that the pseudo-second-order kinetic model is appropriate to remove various heavy metals (Lee et al. 2018). The present study obtained similar results to those acquired in the mentioned studies.
Desorption of the spent RESM
Desorption efforts can reveal the adsorption mechanism. In addition, they can be used to evaluate the feasibility of the regeneration of the used adsorbent and the recovery of the toxic materials, which are adsorbed, before discharging them into the environment. The reusability of adsorbents with good adsorption efficiency is economically important. For real-scale wastewater applications, reusing active areas of worn adsorbents will reduce both the operating costs and the risk of secondary pollutants. In the batch experiments, conducted in the present study, distilled water and different solvents with various concentrations were utilized in order to obtain the ideal desorption efficiency. In terms of bases, the highest desorption efficiency of 52.61% belonged to NaOH that had 2 M concentrations. The hydroxyl ions discharged from the bases might have been replaced with the negatively charged arsenic ions which were on the RESM surface. In the NaOH-based desorption study, strong bonds can be observed between the As and the RESM surface as the adsorption mechanism occurred by chemical absorption. Moreover, by using 0.1 M HCl, ∼96% of the arsenic ion was removed from the solution. However, the adsorbent lost 73% of its relative weight. Depending on these findings, it can be suggested that NaOH can be used to generate RESM, and HCl can be utilized to achieve the full recovery of As(V) from the studied RESM. The arsenic released as a result of the regeneration of the eggshells after the desorption stage can be precipitated using different chemical precipitation (Al2O3, lime, FeCl3 etc.) and electrocoagulation methods. The chemical sludge formed after settling can be sent to hazardous waste disposal facilities to remove the arsenic from the water.
Cost analysis of the RESM
In the literature, the potential use and removal efficiency of adsorbents are often mentioned, while cost analysis is not. In this study, RESM was chosen as an adsorbent due to its renewable characteristics, availability, and low cost. RESM is characterized as a waste that is produced in various industries and must be disposed of, thus resulting in an additional cost to companies. If another industry that produces wastewater of a high arsenic content does not choose RESM as an adsorbent, it will face a new cost. However, these costs can be avoided if both firms adopt the industrial symbiosis approach. A preliminary economic evaluation of the cost of RESM was made in this section. However, the calculated price may vary from region to region according to the change in various costs. A simple cost estimate was made for the manufacture and the use of RESM as follows: Approximately, € 255 was calculated for one ton of RESM production, taking into account transportation, production (crushing, separation, storage, etc.), and electrical energy costs. The price of commercially active carbon, which is widely used in adsorption, is around 5866 €/ton (GAPS Water Treatment 2020). Therefore, the cost of RESM is significantly more affordable and economical than commercially available activated carbons.
Conclusion
The method of adsorption has a significant potential for water treatment as it is both cost-effective and energy efficient. In order to increase this potential, alternative adsorbents are required. In this context, various wastes that are considered as rubbish can be used. This study evaluated the performance of RESM as an inexpensive adsorbent to remove As(V). As previously discussed, the optimum parameters for the removal efficiency of RESM are a temperature of 25 °C and a pH value of 5.97. Under these optimum conditions, approximately 91.43% of As(V) was removed by using the RESM at a dose of 1 g/L and As(V) at an initial concentration of 100 µg/L. It was observed that rapid adsorption occurred within the first 10 min. The thermodynamic parameters ensured during the adsorption determined that the RESM was feasible, natural, and endothermic for the removal of As(V). The FTIR results revealed that –OH, alkyl, –COO–, and –NH2 groups were active in As (V) removal. The Langmuir isotherm was determined as the most suitable for the adsorption of As(V) onto the RESM. According to the findings of this study, RESM was determined as a successful adsorbent for the removal of As(V) without any modification. Thus, it was demonstrated that the study has the potential to influence industrial symbiosis and industrial ecology approaches. This perspective will directly or indirectly contribute to cleaner production. It was also revealed that 100 µg/L As(V) and parameters affecting the adsorption, namely pH, temperature, and time, acted as an antimicrobial agent and inhibited Escherichia coli bacteria on the RESM surface. Further studies must be carried out in order to validate the performance of RESM in the adsorption process. All results showed that RESM was an environmentally friendly, affordable, and easily available adsorbent in removing As(V) from aqueous solutions.
References
Alkurdi SSA, Al-Juboori RA, Bundschuh J, Bowtell L, Marchuk A (2020) Inorganic arsenic species removal from water using bone char: a detailed study on adsorption kinetic and isotherm models using error functions analysis. J Hazard Mater inpress. https://doi.org/10.1016/j.jhazmat.2020.124112
Altowayti WAH, Haris SA, Almoalemi H, Shahir S, Zakaria Z, Ibrahim S (2020) The removal of arsenic species from aqueous solution by indigenous microbes: batch bioadsorption and artificial neural network model. Environ Technol Innov 19:100830. https://doi.org/10.1016/j.eti.2020.100830
Asif Z, Chen Z (2017) Removal of arsenic from drinking water using rice husk. Appl Water Sci 7:1449–1458. https://doi.org/10.1007/s13201-015-0323-x
Bibi S, Farooqi A, Yasmin A, Kamran MA, Niazi NK (2017) Arsenic and fluoride removal by potato peel and rice husk (PPRH) ash in aqueous environments. Int J Phytoremediation 19:1029–1036. https://doi.org/10.1080/15226514.2017.1319329
Çelebi H, Gök G, Gök O (2020) Adsorption capability of brewed tea waste in waters containing toxic lead(II), cadmium (II), nickel (II), and zinc(II) heavy metal ions. Sci Rep 10:17570. https://doi.org/10.1038/s41598-020-74553-4
Flores-Cano JV, Leyva-Ramos R, Mendoza-Barron J, Guerrero-Coronado RM, Aragon-Pina A, Labrada-Delgado GJ (2013) Sorption mechanism of Cd(II) from water solution onto chicken eggshell. Appl Surf Sci 276:682–690. https://doi.org/10.1016/j.apsusc.2013.03.153
Fontecha-Umana F, Rios-Castillo AG, Ripolles-Avila C, Rodriguez-Jerez JJ (2020) Antimicrobial activity and prevention of bacterial biofilm formation of silver and zinc oxide nanoparticle-containing polyester surfaces at various concentrations for use. Foods 9:442. https://doi.org/10.3390/foods9040442
GAPS water treatment, Chemviron ENVIROCARB 207C Activated Carbon. https://www.gapswater.co.uk/cgi-bin/ca000001.pl/ (accessed 03 November 2020)
Gaur N, Kukreja A, Yadav M, Tiwari A (2018) Adsorptive removal of lead and arsenic from aqueous solution using soya bean as a novel biosorbent: equilibrium isotherm and thermal stability studies. Appl Water Sci 8:98. https://doi.org/10.1007/s13201-018-0743-5
Hasrin NI, Othman SA, Harun SNI, Sufian AAM (2020) Applications of egg shell. Asian J Appl Sci 1:9–13
Hayat K, Menhas S, Bundschuh J, Chaudhary HJ (2017) Microbial biotechnology as an emerging industrial wastewater treatment process for arsenic mitigation: a critical review. J Clean Prod 151:427–438. https://doi.org/10.1016/j.jclepro.2017.03.084
Heiba HF, Taha AA, Mostafa AR, Mohamed LA, Fahmy MA (2018) Synthesis and characterization of CMC/MMT nanocomposite for Cu2+ sequestration in wastewater treatment. Korean J Chem Eng 35:1844–1853. https://doi.org/10.1007/s11814-018-0096-7
Hua J (2018) Adsorption of low-concentration arsenic from water by co-modified bentonite with manganese oxides and poly(dimethyldiallylammonium chloride). J Environ Chem Eng 6:156–168. https://doi.org/10.1016/j.jece.2017.11.062
Jalili-Firoozinezhad S, Filippi M, Mohabatpour F, Letourneur D, Scherberich A (2020) Chicken egg white: hatching of a new old biomaterial. Mater Today 40:193–214. https://doi.org/10.1016/j.mattod.2020.05.022
Kim H, Jeon Y, Lee W, Jang G, Yoon Y (2020) Shifting the specificity of E. coli biosensor fromi arsenic to phenylarsine oxide through genetic engineering. Sensors 20:3039. https://doi.org/10.3390/s20113093
Kloster GA, Valiente M, Marcovich NE, Mosiewicki MA (2020) Adsorption of arsenic onto films based on chitosan and chitosan/nano-iron oxide. Int J Biol Macromol 165:1286–1295. https://doi.org/10.1016/j.ijbiomac.2020.09.244
Kusmierek K, Idzkiewicz P, Swiatkowski A, Dabek L (2017) Adsorptive removal of pentachlorophenol from aqueous solutions using powdered eggshell. Arch Environ Prot 43:10–16. https://doi.org/10.1515/aep-2017-0029
Laca A, Laca A, Díaz M (2017) Eggshell waste as catalyst: a review. J Environ Manage 197:351–359. https://doi.org/10.1016/j.jenvman.2017.03.088
Lee ME, Jeon P, Kim J-G, Baek K (2018) Adsorption characteristics of arsenic and phosphate onto iron impregnated biochar derived from anaerobic granular sludge. Korean J Chem Eng 35:1409–1413. https://doi.org/10.1007/s11814-018-0057-1
Lesnierowski G, Stangierski J (2018) What’s new in chicken egg research and technology for human health promotion? - a review. Trends Food Sci Technol 71:46–51. https://doi.org/10.1016/j.tifs.2017.10.022
Lybæk R, Christensen TB, Thomsen TP (2021) Enhancing policies for deployment of industrial symbiosis – What are the obstacles, drivers and future way forward? J Clean Prod 280:124351. https://doi.org/10.1016/j.jclepro.2020.124351
Martinez VS, Martinez AI, Hemandez-Beteta EE, Mijangos-Ricardez OF, Vazquez-Hipolito V, Patino-Carachure C, Lopez-Luna J (2018) As(III) and As(V) adsorption on manganese ferrite nanoparticles. J Mol Struct 1154:524–534. https://doi.org/10.1016/j.molstruc.2017.10.076
Mejia-Santillan ME, Pariona N, Bravo-C J, Herrera-Trejo M, Montejo-Alvaro F, Zarate A, Perry DL, Mtz-Enriquez AI (2018) Physical and arsenic adsorption properties of maghemite and magnetite sub-microparticles. J Magn Magn Mater 451:594–601. https://doi.org/10.1016/j.jmmm.2017.11.111
Mezbaul Bahar Md, Mahbub KR, Naidu R, Megharaj M (2018) As(V) removal from aqueous solution using a low-cost adsorbent coir pith ash: equilibrium and kinetic study. Environ Technol Innov 9:198–209. https://doi.org/10.1016/j.eti.2017.12.005
Mirabella N, Castellani V, Sala S (2014) Current options for the valorization of food manufacturing waste: a review. J Clean Prod 65:28–41. https://doi.org/10.1016/j.jclepro.2013.10.051
Mubarak S, Zia-Ur-Rehman M, Nawaz Chaudhry M (2015) Modified eggshells as cost effective adsorbent for the treatment of arsenic(III) contaminated industrial effluents. Asian J Chem 27:1995–2000. https://doi.org/10.22270/jddt.v8i6-s.2063
Mudzielwana R, Gitari MW, Ndungu P (2020) Enhanced As(III) and As(V) adsorption from aqueous solution by a clay based hybrid sorbent. Front Chem 7:913. https://doi.org/10.3389/fchem.2019.00913
Neves A, Godina R, Azevedo SG, Matias JCO (2020) A comprehensive review of industrial symbiosis. J Clean Prod 247:119113. https://doi.org/10.1016/j.jclepro.2019.119113
Niazi NK, Bibi I, Shahid M, Ok YS, Shaheen SM, Rinklebe J, Wang H, Murtaza B, Islam E, Farrakh Nawaz M, Lüttge A (2018) Arsenic removal by Japanese oak wood biochar in aqueous solutions and well water: investigating arsenic fate using integrated spectroscopic and microscopic techniques. Sci Total Environ 621:1642–1651. https://doi.org/10.1016/j.scitotenv.2017.10.063
Nistico R, Celi LR, Prevot AB, Carlos L, Magnacca G, Zanzo E, Martin M (2018) Sustainable magnet-responsive nanomaterials for the removal of arsenic from contaminated water. J Hazard Mater 342:260–269. https://doi.org/10.1016/j.jhazmat.2017.08.034
Pathan S, Bose S (2018) Arsenic removal using “green” renewable feedstock-based hydrogels: current and future perspectives. ACS Omega 3:5910–5917. https://doi.org/10.1021/acsomega.8b00236
Pokhrel D, Viraraghavan T (2008) Arsenic removal from an aqueous solution by modified A. niger biomass: batch kinetic and isotherm studies. J Hazard Mater 150:818–825. https://doi.org/10.1016/j.jhazmat.2007.05.041
Qi J, Zhang G, Li H (2015) Efficient removal of arsenic from water using a granular adsorbent: Fe-Mn binary oxide impregnated chitosan bead. Bioresour Technol 193:243–249. https://doi.org/10.1016/j.biortech.2015.06.102
Rahdar S, Taghavi M, Khaksefidi R, Ahmadi S (2019) Adsorption of arsenic (V) from aqueous solution using modified saxaul ash: isotherm and thermodynamic study. Appl Water Sci 9:87. https://doi.org/10.1007/s13201-019-0974-0
Sahu UK, Mahapatra SS, Patel RK (2018) Application of Box-Behnken design in response surface methodology for adsorptive removal of arsenic from aqueous solution using CeO2/Fe2O3/graphene nanocomposite. Mater Chem Phys 207:233–242. https://doi.org/10.1016/j.matchemphys.2017.11.042
Sawood GM, Gupta SK (2020) Kinetic equilibrium and thermodynamic analyses of As (V) removal from aqueous solution using iron-impregnated Azadirachta indica carbon. Appl Water Sci 10:131. https://doi.org/10.1007/s13201-020-01217-z
Seeharaj P, Sripako K, Promta P, Detsri E, Vİttayakorn N, (2019) Facile and eco-friendly fabrication of hierarchical superhydrophobic coating from eggshell biowaste. Int J Appl Ceram Technol 16:1895–1903. https://doi.org/10.1111/ijac.13235
Shakoor MB, Niazi NK, Bibi I, Shahid M, Saqib ZA, Nawaz MF, Shaheen S, Wang H, Tsang DCW, Bundschuh J, Ok YS, Rinklebe J (2019) Exploring the arsenic removal potential of various biosorbents from water. Environ Int 123:567–579. https://doi.org/10.1016/j.envint.2018.12.049
Shi Y, Liu G, Li M, Wang L (2020) Egg shell waste as an activation agent for the manufacture of porous carbon. Chin J Chem Eng 28:896–900. https://doi.org/10.1016/j.cjche.2019.09.014
Tran HN, You S-J, Hosseini-Bandegharaei A, Chao H-P (2017) Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: a critical review. Water Res 120:88–116. https://doi.org/10.1016/j.watres.2017.04.014
Wu L-K, Wu H, Liu Z-Z, Cao H-Z, Hou G-Y, Tang Y-P, Zheng G-Q (2018) Highly porous copper ferrite foam: a promising adsorbent for efficient removal of As(III) and As(V) from water. J Hazard Mater 347:15–24. https://doi.org/10.1016/j.jhazmat.2017.12.048
Zaman T, Mst-S M, Al Mahmood M-A, Rahman M-S (2018) Evolution and characterization of eggshell as a potential candidate of raw material. Ceramica 64:236–241. https://doi.org/10.1590/0366-69132018643702349
Zeng H, Yu Y, Wang F, Zhang J, Li D (2020) Arsenic(V) removal by granular adsorbents made from water treatment residuals materials and chitosan. Colloids Surf A 585:124036. https://doi.org/10.1016/j.colsurfa.2019.124036
Zhang G, Ren Z, Zhang X, Chen J (2013) Nanostructured iron(III)-copper(II) binary oxide: a novel adsorbent for enhanced arsenic removal from aqueous solutions. Water Res 47:4022–4031. https://doi.org/10.1016/j.watres.2012.11.059
Acknowledgements
Experiments were carried out in the accredited laboratories of Aksaray University Environmental Engineering Department.
Author information
Authors and Affiliations
Contributions
HÇ was involved in conceptualization, validation, writing—review and editing. ÖÇ was involved in methodology, editing, writing—original draft preparation. İŞ was involved in methodology, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: Maryam Shabani.
Rights and permissions
About this article
Cite this article
Çelebi, Ö., Şimşek, İ. & Çelebi, H. Escherichia coli inhibition and arsenic removal from aqueous solutions using raw eggshell matrix. Int. J. Environ. Sci. Technol. 18, 3205–3220 (2021). https://doi.org/10.1007/s13762-021-03216-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-021-03216-2