Abstract
Solar energy storage systems hold a key for those seeking for a potential solution of energy issues. The experimental work established in this investigation is based on a composite organic phase change material (PCM) comprised of a technical grade paraffin wax/nanofiller synthesized via ultrasonic dispersion. Various mass fractions of ZnO nanorods synthesized via thermal decomposition technique or silica-coated zinc oxide (SZR) prepared via hydrolysis route were used as a nanofiller embedded in PCM. PCM was applied in a vertical type pipe-in-pipe (PIP) thermal heat storage system connected with a flat plate solar collector where water is used as the heat transfer fluid (HTF). The mass flow rate of the HTF was selected (1.3 g/s) according to the experimental results. The solar intensity data showed the solar collector energy gained was around 170 W, and it was related to the daytime. Results showed the heat transfer rate was affected by the change in the nanofiller type and the mass fraction. Finally, an increase in the heat was gained from 7 to 140 kJ/min with increasing the nanofillers up to a certain limit. Almost 200% system enhancement is achieved for ZSR rather than pristine PW-PCM which makes the system attractive for water heating.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Egypt is a country located on the eastern north of the borderline of Africa continent, famously noted for its sunny weather and dry climate over the year especially on the non-coastal regions. Although industry represents a significant proportion of the Egyptian economy, there is a challenge associated with energy crisis (Farahat et al. 2016; Ashour et al. 2014).
As regards a sustainable practical solution and environmental pollution control, solar energy storage in the form of latent heat could reserve as a sustainable energy solution for the electricity shortage, fossil fuels depletion and pollution crises (Sharma et al. 2009; Hajare and Gawali 2015; Chaichan et al. 2015). Sustainable energy plays a vital role not only as a renewable energy source, but also for its rapid distribution (Goldemberga and Coelh 2004). The fundamental purpose of the solar energy storage is to aid energy management by storing the heat in the form of latent heat at periods that sun is abundant and releasing them when it is required as heat and/or hot water in an eco-friendly approach with zero emissions (Kaviarasu and Prakash 2016).
While Egypt is well endowed with sunshine, the renewable solar energy application is still limited in comparison with the global energy need (Tony and Tayeb 2016). To overcome the energy depletion crisis, the Egyptian government vision by 2020 is to substitute about 20% of the total generated energy by electricity with a renewable source (El-Khayat and Ameen 2010).
Although a lot of benefits are associated with the application of solar energy as a sustainable source (Tony et al. 2016), there are downsides such as the high installation cost, inefficiency and inconsistency due to unsteady environmental condition and daytime dependence. Thus, solar energy storage needs to be evolved to improve efficiency of using solar energy (Canbazoglu et al. 2005). In general, solar energy storage is an effective technique to harness solar energy radiation for further uses to cross the unavailability periods of solar radiation to meet energy demand all the times (Sharma et al. 2009). Therefore, in order to overcome the gap between energy needs and supply, latent heat energy storage systems are used to compromise solar energy storage that should retain high storing capacity, be cost efficient with high efficiency and hazard free to the environment (Kaygusuz and Sari 2005; Kaviarasu and Prakash 2016). Solar water heating (SWH) is considered a potential application since it is considered a high-energy storage technique. However, the selection of appropriate storing medium depends on the storing material melting point within the temperature range. Such materials are efficient on storing and releasing a vital amount of heat with slight temperature variation (Kaygusuz and Sari 2005). Recently, numerous types of organic and inorganic PCM materials were applied such as salt hydrates (Kaygusuz and Sari 2005), paraffin wax (Ho and Gao 2009), fatty acids (Kaviarasu and Prakash 2016) and eutectic materials (Skovajsa et al. 2017). However, the insufficient thermal conductivity and diffusivity make them useful energy storing materials for heating purposes (Kaviarasu and Prakash 2016). Nevertheless, according to the authors’ knowledge, there are a few trails in the literature on the application of the so-called PCM as a storing medium on SWH. A pervious investigation conducted by Otanicar and Golden (Otanicar and Golden 2009) compared the conventional and nanofluid SWH technology. In addition, Zeng et al. (2010) examined the thermal performance of PCM for active solar water heating system. Dwivedi et al. (2016) reported that PCM can be successfully integrated with the solar thermal water heating system.
Among the various PCMs types, paraffins are considered the most recommended organic PCM because they are available in low cost, safe, chemically stable, nonreactive, have considerable latent heat of fusion and low vapor pressure in melting, and besides, are thermally efficient although there are a large number of thermal cycles. Conversely, a remarkable low heat transfer rates during the melting/solidification process are as a result of its low thermal conductivity. Therefore, researchers put more efforts into enhancing the thermal conductivity of paraffin PCM such as introducing nanoparticles. For instance, Al2O3 nanoparticles (Ho and Gao 2009; Hajare et al. 2014), carbon nanotubes (wang et al. 2009), CuO nanoparticles (Karunamurthy et al. 2012), graphene nanoplatelets (Fang et al. 2013) and TiO2 and ZnO nanoparticles (Kaviarasu and Prakash 2016) were applied as embedded materials in paraffin-PCM. Although various nano-PCMs have been tested to essentially improve the thermal conductivity, there are limitations in examining their performances on the integrated solar collector applications systems. Such limitations are related to the nanocomposites, i.e., how metal oxide nanoparticles influence the thermal behavior of PCM nanocomposites and what is the possible loading concentration of nanoparticles providing an optimum degree of dispersion throughout PCM, which achieves the unique characteristics of the PCM nanocomposites (Kaviarasu and Prakash 2016). Moreover, the compatibility between nanofiller and the host PCM provides improvement in thermal properties and durability. Many researchers work on surface treatment of nanoparticles by coupling agents to enhance the utilization of nanocomposites (Muller et al. 2017).
This emphasizes the essentials for a new approach in energy storing facilities. Herein, this paper presents the feasibility of two organic paraffin/nanofiller PCM systems derived from synthesized ZnO nanorods and SiO2-coated ZnO nanorods for latent heat energy storage. The coating SiO2 layer could achieve the stability of nanorods in PCM as well as limiting the photodegradation of PCM due to the photoactivity of ZnO nanoparticles (Wen et al. 2011). The heat gained during the charging/discharging processes was used to determine the mechanism of these materials.
Materials and methods
Materials
Commercial paraffin wax (PW) with a melting point around 53 °C and a latent heat of fusion 190 kJ/kg was selected as a base organic PCM material in all experiments. Zinc acetate dihydrate (Winlab, purity 99.999%) used as a precursor for ZnO nanorods. However, tetraethyl orthosilicate, TEOS, assay (GC, area %) ≥ 99.0 area %, purchased from Merck KGaA, and ethanol (Sigma Aldrich ≥ 99.8%) have been used for capping ZnO nanorods by SiO2.
Synthesis of ZnO nanorods
Thermal decomposition route has been used to synthesize ZnO nanorods. In such route, zinc acetate dihydrate was placed in a covered 50-mL alumina crucible. Thereafter, the crucible was placed in an oven and heated to 350 °C and maintained for 3 h. The heating condition was selected to be sufficient for complete thermal decomposition process of the precursor (Lin and Li 2009). The obtained powder was in dark yellow color. In fact, ZnO is originally off-white powder. However, the heating conditions in the used route of preparation and relative long time of heating in the presence of air could lead to a probable formation of zinc interstitials and oxygen vacancies in the obtained ZnO nanocrystals (Xu et al. 2013; Mallika et al. 2015). So, zinc interstitials should produce lattice defects that switch the color to dark yellow. The final product without surface coating is named ZR referring to non-treated ZnO nanorods.
Silica-coated zinc oxide was prepared according to the typical hydrolysis method for TEOS in the presence of ZnO nanorods as reported by Ramasamy et al. (2014). In this respect, 4 g of as-synthesized ZnO nanorods was dispersed with TEOS (50 wt% to the relevant amount of ZnO). The mixture was sonicated for 10 mim and then stirred overnight to achieve the complete hydrolysis of TEOS. The obtained treated ZnO nanorods were collected by centrifugation and washing with ethanol for four times. The treated sample was dried at 60 °C for 10 h. The obtained surface-coated nanorods with silica were labeled ZSR.
Preparation of PCM nanocomposites
Two types of PCM nanocomposite samples were prepared by mixing treated or non-treated ZnO nanorods with PW. The amount of PW, 15 g, was fixed for all the investigated PCM nanocomposite samples. The concentrations of nanofiller, ZR or ZSR, for each type of nanocomposite were ranged from 0 to 1 wt% from PW. In order to obtain good dispersion of nanorods, the desired amount of PW was melted on hot plate at 60 °C. Thereafter, the desired amount of nanofiller was added, and then, the mixture was exposed to ultrasonic radiation at 60 °C using ultrasonic bath (DAIHAN Wisd model WUC-A03H, 40 kHz).
Characterization techniques
The X-ray diffraction (XRD) measurements for ZR were achieved via XRPhillips X’pert (MPD3040) X-ray diffractometer supported by a monochromatic source CuKa (k = 1.5406 A). The XRD measurement was done under step-scan mode, and the registered intensities of the diffracted X-rays were detected every 0.02° over 2 \(\theta\) range of 20°–80°. Additionally, the morphology of the synthesized treated and non-treated ZnO nanorods, ZSR and ZR, respectively, was checked by high-resolution transmission electron microscopy (HR-TEM, JEM-2100).
Methodology
Description of the experimental facility
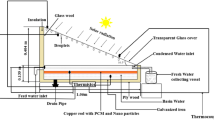
The pilot-scale experimental facility testing the PCM was designed and constructed by integrating four parts. The schematic representation of the pilot plant facility is shown in Fig. 4. The first part of the setup is the heating system through a flat plate collector that heats up water, the heat transfer fluid (HTF), which acts as the energy source for the charging PCM installation. Then, the second part is the cooling system, which is based on water HTF in the heat exchanger through the discharging process that in the real facility simulates the energy consumption by the consumers based on the heat exchanger concept. The third part consists of the shell and tube heat exchanger that stores the PCM in the tube of the heat exchanger. Thermal energy is stored in PCM in heat exchanger during the charging process, however releasing it need during the discharging process. The fourth part is a well thermally insulated SWH storage tank that stores the heat released in the solar thermal storing process in the form of hot water. All of the four parts are linked together through rubber tubes piping system transferring the HTF at a certain flow rate. Moreover, the heat loss to the surroundings is minimized using a thermal insulation on the heat exchanger, rubber tubes and the hot water storage tank.
Concept of flat plate solar collector configuration and geographical location
Experiments performed in this study were under the meteorological conditions at Advanced Materials/Solar Energy and Environmental Sustainability (AMSEES) Lab., Faculty of Engineering, Menoufia University, Shebin El-Kom City, Menoufia Governorate, in the north of Egypt. The region is characterized by abundant sunshine and moderate dry climate especially in the summer periods, and the sun shines all over the year. The latitude and longitude of the location are 30°58′N and 31°01′E, respectively, 11 m above sea level (Tony and Tayeb 2011; Farahat et al. 2016). The temperature ranged in the summer season from 28 to 43 °C, and the solar intensity reached 1145 W/m2.
Solar collector represents the active constituent of the solar heating technique since it gathers the solar radiant energy and then transfers it into heat through the HTF. A typical flat plate collector type is used in this study, which is manufactured from an insulated metal box with a highly transparent glass cover. A steel sheet absorber connected with serpentine form copper tubes acting as the HTF carrier are subjected inside the metal box. The absorbing surface and the copper tubes are painted in a dull black color in order to increase the absorptivity with a collection area of solar radiation of 0.5 m2, and the mass flow rate is 1.3 g/s. In order to dismiss the heat loss, all sides and the bottom of the collector are thermally insulated. The collector was placed facing the south with a slope angle of 30° with respect to the horizontal line which is suitable for the geographical location. The useful energy gain from this collector controls the temperature rise of the HTF. This collector increases the temperature of the HTF up to 70 °C.
Collector thermal efficiency and energy analysis
The solar collector efficiency (\(\xi , \%\)) during any period of time is evaluated by the ratio of the amount of useful heat collected through the collector (\(Q_{\text{uc}} , {\text{W}}\)), [Eq. (2)], to the amount of solar radiation (\(I_{\varepsilon } , {\text{W}}/{\text{m}}^{2}\)) incident on the area of the absorber (\(A_{c} , {\text{m}}^{2}\)) as given in Eq. (1) (Kurtbash and Durmush 2004; Hematian et al. 2012):
where \(m^{.}\) is the rate of water mass flow (kg/s), Cp is the specific heat capacity of water (kJ/kg.K), \(T_{\text{wic}}\) is the inlet collector water temperature and \(T_{\text{woc}}\) is the outlet water temperature from the collector.
Description of measurement procedure
The measured variables recorded at time intervals include inlet and outlet HTF temperatures through the collector, ambient temperature, air temperature, PCM temperature, water in the SWH storage tank temperature and the solar radiation.
The intensity of solar radiation incident was measured using an Eppley Black and White solarimeter, model 8–48. This meter was placed adjacent to the collector cover at the same plane.
Thermocouples were positioned evenly; the inlet and outlet water (HTF) temperatures, PCM temperatures and SWH storage tank were measured by two well-insulated thermocouples. All experiments were conducted from 9 am to 5 pm.
Results and discussion
XRD characterization of the nanostructure samples
Figure 1 shows the XRD pattern of the NZRs powder. The registered XRD pattern reveals the formation of ZnO with a wurtzite structure without other ZnO phases or impurity phases.
From the line broadening of the X-ray diffracted lines, the crystallite size (D) of the NZRs was estimated using Williamson–Hall plot (Mote et al. 2012). The equation of the Williamson–Hall approach is given by \(\beta_{\text{hkl}} \cos \theta = \frac{k\lambda }{D} + 4\varepsilon \sin \theta\), where \(\beta_{\text{hkl}}\) represents the full width at half maximum (FWHM) of the peak, k is a constant called the shape factor with the value of 0.94, \(\theta\) is the Bragg angle and \(\varepsilon\) is the strain in the sample as a result of crystal imperfection and/or distortion. Figure 2 shows the relation between \(4\sin \theta\) and \(\beta_{\text{hkl}} \cos \theta\) for the most predominant diffracted peaks. According to the Williamson–Hall and the linear square fitting of such plot, the calculated crystallite size (D) was found to be 53.4 nm, which confirms the nanosize of the investigated sample. Also, the obtained value of a slope, which is equal to \(\varepsilon\), was found to be 0.00125. Such positive value of \(\varepsilon\) is referring to a tensile strain in the sample.
Morphological characterization of the nanostructure samples
Figure 3a shows HR-TEM micrograph of ZR which reveals the successful formation of ZnO nanorods with diameters ranging from 20 to 70 nm. The inset of Fig. 3a shows a single nanorod with high magnification and it has sharply clear surface, whereas Fig. 3b and its inset, for ZSR sample, show the formation of thin layer of SiO2 as a result of treating ZnO nanorods with SiO2. Such layer could enhance the dispersion of nanorods in paraffin wax as will be discussed later. On the other hand, the obtained nanorods were loosely aggregated for both samples. In fact, the aggregation zones of nanoparticles can be almost overcome by exposure to ultrasonic radiation (Ramasamy et al. 2014) (Fig. 4).
Solar thermal performance analysis and temperature profile for the flat plate collector
On clear sky condition days, the collector performance investigation was done. Experiments had been conducted during the summer season from May to July 2018. Figure 5 shows different temperature variations throughout the daytime. The ambient temperature (Tab), air temperature (Ta) and the inlet (Twic) and outlet (Twoc) temperatures of the HTF subjected to the solar collector at a mass flow rate of 1.3g/s were monitored. The measured solar radiation is also shown in Fig. 5. As expected, the solar radiation increased in the morning from 505 W/m2 to a peak value of 1025 W/m2 at noon and thereafter began to decrease in the afternoon gradually reaching 500 W/m2 before the sunset. As shown in the figure, all the temperatures increase to reach their maximum values around the solar noon at 12:00 pm and then, it starts to decrease again later in the afternoon. The temperature ranged from 28 to 34 °C.
Figure 6a depicts the useful heat gained from the collector variation with daytime, which is obtained from Eq. 2. It is seen from the figure that the amount of heat gained from the collector reached its maximum in the solar noon. As expected, the results reveal that the collector inlet and outlet temperatures increase with the solar radiation (\(I_{\varepsilon }\)). Thus, Quc is increased with increasing temperature as the inlet and outlet temperatures of the HTF increased at a mass flow rate of 0.0013 kg/s. The maximum inlet water temperature reached its maximum value of 34 °C at the solar noon (13:00 pm), while it decreases again during the afternoon. Furthermore, the corresponding peak of the outlet temperature HTF is reached at 62 °C; thereafter, it decreases again as the solar radiation drops after the solar noon.
According to Fig. 6b, the maximum HTF inlet and outlet temperatures difference of the collector occurred at the time of solar noon (12:30 to 14:30 pm). Thus, the higher collector efficiency calculated from Eq. 1 is gained at the solar noon. Thereafter, even the solar intensity is reduced after the solar noon, the collector efficiency is still high compared to the beginning of the day as the collector’s absorber stored the solar energy.
Pilot plant performance of PCM analysis
Charging/discharging cycles
PCM charging (Tω) and discharging (Tα) temperatures variation cycles are shown in Fig. 7a, b for ZR-PCM, respectively, with respect to time period of charging/discharging at different times. Different ZR-PCMs conditions are compared with the commercial PCM (PW). ZnO nanoparticles at different concentrations (0.05, 0.01, 0.15, 0.2, 0.3 and 1 wt%) are added to the base PW to locate the optimum ZR addition to the PCM. It is noted from Fig. 7a the ZR addition to the PW show a range of melting temperatures. An increase in the temperature was observed by the ZR addition to PW PCM. The most significant system was noted for 0.3% ZR PCM as the temperature of charging reached 62 °C compared to 53 °C for PW PCM without nanoadditives. However, above this amount of ZR, the PCM melting temperature starts to reduce. In particular, according to the literature (Kurtbash and Durmush 2004), the addition of nanoparticles helps to convince a change in the shape of the heat flow of the PCM, thus modifies the value of the melting temperature of the PCM with nanoadditives. Moreover, it is expected that the addition of nanoparticles enhances the latent heat of the PCM. Additionally, the addition of ZnO nanorods controls the photodegradation of PCM (Ramasamy et al. 2014).
The results of discharging cycle of the ZR augmented with that of the pure paraffin PCM are illustrated in Fig. 7b at different ZR compared to PW PCM. As a result of increasing the melting temperature with the addition of ZR, the solidification temperature further increases. Comparing the solidification temperature to that of the pure paraffin, the nanoparticles included in paraffin PCM have a higher solidification temperature, depending on the percentage of nanoparticles mass fraction. The data discerned in Fig. 4b markedly illustrated a relative enhancement of the process when the ZR is together with PW by the increase in the solidification time. Thus, the overall heat stored from the process is increased. The significant enhancement is observed for the 0.3% ZR PCM. However, above 0.3% nanoparticles addition, Tα reduces and thus the process becomes unfavorable. This phenomenon is usually observed experimentally, and this could be illustrated by the fact that excess nanoparticles reduce the stability of the PCM owing to the agglomeration and sedimentation. Therefore, selecting the optimum value of additive nanoparticles into PCM is essential in improving the charging/discharging process performance. This is reported previously in the literature (Teng and Yu 2012; Pise et al. 2013; Chieruzzi et al. 2015). Here, it is worth to mention that the excess of nanofillers in PCM could lead to an increase in dynamic viscosity of PCM which will cause a reduction in heat transfer rate of PCM (Harikrishnan et al. 2014).
Furthermore, comparing Fig. 7c and d with a and b, the results reveal that ZSR-PCM showed a pronounced noticeable effect than ZR-PCM. The treated nanocapsulated PCMs showed a higher charging (Tω) and discharging (Tα) temperatures than the untreated ZR-PCM as seen in Table 1. The enhanced performance showed an increase of 4 °C for the charging temperature than the untreated ZR. Thus, the nanocapsulated PCMs have a higher thermal stability than the non-capsulated one due to shell-coated ZnO particles. Furthermore, as the amount of nanocapsules mixture increased in the PCM, the heat storage capacity increased. This may be explained by the presence of the coated layer of SiO2 on ZnO enabling a good dispersion throughout the host paraffin wax. This provides more sites for inorganic nanoparticle materials, which enhances the probability of absorbing heat leading to an increase in the latent heat of fusion of paraffin wax.
This result indicates that the heat storage capacity is related to the amount of the nanocapsules added to the PCM and the optimal addition was found to be 0.3 wt%. This phenomenon is previously stated in the literature (Harikrishnan et al. 2014).
Heat profile yield
Figure 8 illustrates the temperature gained, \(T_{\beta ,}\), and the amount of heat rate released, \(Q_{{{\varvec{\upbeta}},}}\), by the PCM during discharging processes in order to extrapolate from the results the optimal conditions for application in SWH. Consequently, in selecting the optimal additives, it should be considered the heat performance and the range of phase change temperature gained and phase change heat. The experimental results reveal that adding ZnO nanoparticles to paraffin can increase the temperature range and the amount of heat gained, thereby increasing the temperature range of PCM that can be applied to heat storage. Moreover, the ZSR with PW increases the temperature by 10.5 °C that increases the storing up heat compared to the pure paraffin PCM to the optimal 0.3% ZSR PCM (Fig. 5a), maintaining the optimum heat storage capacity. As seen from Fig. 5b, the rate of heat gained from the PCM is increased in the initial period from 1.3 to 6.0 kJ/min for PW and 0.3% ZSR augmented PW PCM. This could be illustrated by the enhancement of nanoparticles which ensures a higher thermal transfer rate that increases with nanoparticle addition especially in the optimal wt% addition value as the specific heat is increased (Fang et al. 2010; Altohamy et al. 2015). Therefore, the addition of ZnO nanoparticles to paraffin wax to improve heat storage performance has a notable potential for solar water heating application.
The temperature gained and heat rate gained through SWH stored through PCM process are different because the amount of nanocapsules increases from 0.05 to 1 wt% (Fig. 5c, d). However, the maximum heat gained is obtained at the 0.3% ZSR addition to the PCM. The heat gained through the treated ZnO is 7.7 kJ/min compared to 6.0 kJ/min for the untreated ZnO. Thus, this means that the SiO2 shell-coated ZnO can improve the thermal properties of the microencapsulated paraffin PCM composites because of the synergistic effect between the PW and SiO2 (Teng and Yu 2012).
Overall heat comparison of TES systems
The overall heat gained from the process is calculated for all PCM systems and compared as illustrated in Fig. 9. The overall heat rate gained by the PCM is the heat transferred by the HTF and is calculated for the whole process by Eq. 2. The pure PW and PW augmented with uncoated nanorods and coated ones PCMs show that the dispersion of nanoadditives to the PW improves the overall heat rate gained from PCM system. According to the experimental results, the useful rate of heat gained is greater for composite ZnO nanorods-PW (80.3 kJ/min) than for pristine PW (7.4 kJ/min). Moreover, the capsulated ZnO nanorods–PW-PCM showed a pronounced effect than the heat gained (140 kJ/min) by uncoated ZnO (80.3 kJ/min). In fact, based on this comparison, the significant higher heat rate gained is due to the increase in the heat transfer, due to the higher thermal conductivity of the mixture, and the heat gained rate is more important than the single paraffin. This rate increases proportionately with the weight percentage of the added nanorods. The overall temperature difference during the discharging process and the volume of water stored are higher; thus, the useful heat gained from the PCM process is higher. These results are in agreement with that previously stated in the literature (Kaygusuz and Sari 2005 Altohamy et al. 2015).
From all of the above-obtained results, the use of capsulated ZnO PCM maximizes the storage performance of PCM based on the SWH gained from the investigated system. Thus, the overall storing efficiency of that system at different ZSR additions is compared and illustrated in Fig. 10. Based on the amount of heat gained from HTF (Eq. 2) and the heat gained from the PCM material (Eq. 3), the overall efficiency of the system (η) is calculated in Eq. 4 which is the useful energy gained from the HTF to the heat gained from the PCM (Narayanan et al. 2017).
where \(m\) is the mass of PCM (kg), Cp is the specific heat capacity of PCM (kJ/kg.K), \(T_{\text{PCMi}}\) is the inlet PCM temperature, \(T_{\text{PCMo}}\) is the outlet PCM temperature from the tube of heat exchanger containing PCM and H is the latent heat of fusion of PCM (kJ/kg).
It is clear from Fig. 10 that the efficiency increased with ZSR addition and the maximum efficiency was observed for the 0.3 wt% addition. Thus, these results recommend and confirm that the presence of 0.3% ZSR in the PCM maximizes the efficiency and heat stored from the PCM system.
Conclusion
In this study, the performance of the solar collector efficiency is highly dependent on the solar radiation incident on the collector. The temperature and the useful energy gained from the collector are highly dependent on that collector efficiency. HTF heated from the collector is used for PCM for energy storage system for application on solar water heating.
The dispersion of two types of nanoparticles in PW was investigated. The results showed that the variation in the heat gained from the process, which is useful for solar water heating, is dependent on the amount of nanoparticles added and the type of dispersed material. Although the dispersion of nanoparticles to the phase change material have a pronounced effect on the system performance, the selection of the type and amount of this nanoadditive are important processes. The coated ZnO nanorods by SiO2 are more pronounced than the addition of the uncoated one. Moreover, the 0.3% nanoparticle addition to the paraffin PCM maximizes the heat gained from the system.
Abbreviations
- PCM:
-
Phase change material
- PIP:
-
Pipe-in-pipe
- PW:
-
Paraffin wax
- ZR:
-
Uncoated ZnO nanorods
- ZSR:
-
Coated ZnO nanorods with SiO2
- SWH:
-
Solar water heating
- HTF:
-
Heat transfer fluid
- T a :
-
Air temperature (°C)
- T ab :
-
Ambient temperature (°C)
- T ω :
-
Charging temperature of PCM (°C)
- T α :
-
Discharging temperature of PCM (°C)
- \(T_{\text{wic}}\) :
-
Inlet collector water temperature (°C)
- \(T_{\text{woc}}\) :
-
Outlet water temperature from the collector (°C)
- T β :
-
Temperature gained from discharging PCM (°C)
- \(\xi\) :
-
Collector efficiency (%)
- η :
-
Overall efficiency from the PCM storing system (%)
- \(Q_{\text{uc}}\) :
-
Useful heat obtained from collector (W)
- \(Q_{\beta }\) :
-
Rate of useful heat gained from HTF (KJ/min)
- \(Q_{\text{PCM}}\) :
-
Heat gained from PCM (KJ/min)
- \(I_{\varepsilon }\) :
-
Intensity of solar radiation (W/m2)
- \(A_{c}\) :
-
Area of collector absorber (m2)
- Cp :
-
Specific heat capacity of water 4.18 kJ/kg K (Zelzouli et al. 2012
- Cp :
-
Specific heat capacity of paraffin wax 2.1 kJ/kg K (Sharma et al. 2009)
- \(m^{.}\) :
-
Water mass flow rate (kg/s)
- TES:
-
Thermal energy storage
- H:
-
Latent heat of fusion of paraffin wax 190 kJ/kg (Sharma et al. 2009)
References
Altohamy AA, Abd Rabbo MF, Sakr RY, Attia AA (2015) Effect of water based Al2O3 nanoparticle PCM on cool storage performance. Appl Therm Eng 84:331–338
Ashour EA, Tony MA, Purcell PJ (2014) Use of agriculture-based waste for basic dye sorption from aqueous solution: kinetics and isotherm studies. Am J Chem Eng 2(6):92–98
Canbazoglu S, Sahinaslan A, Ekmekyapar A, Akosy YG, Akarsu F (2005) Enhancement of solar thermal energy storage performance using sodium thiosulfate pentahydrate of a conventional solar water heating system. Energy Build 37:235–242
Chaichan MT, Kamel SH, Al-Ajeely AN (2015) Thermal conductivity enhancement by using nano-material in phase change material for latent heat thermal energy storage systems. Saussurea 5(6):48–55
Chieruzzi M, Miliozzi A, Crescenzi T, Torre L, Kenny JM (2015) A new phase change material based on potassium nitrate with silica and alumina nanoparticles for thermal energy storage. Nanoscale Res Lett 10:273
Dwivedi VK, Tiwari P, Tiwari S (2016) Importance of phase change material (pcm) in solar thermal applications: a review. In: International conference on emerging trends in electrical, electronics and sustainable energy systems (ICETEESES–16), March 11–12
El-Khayat MM, Ameen EE (2010) Renewable energy in egypt—challenges and prospects. In: Thermal Issues in Emerging Technologies, Cairo, Egypt, December 19–22
Fang G, Chen Z, Li H (2010) Synthesis and properties of microencapsulated paraffin composites with SiO2 shell as thermal energy storage materials. Chem Eng J 163:154–159
Fang X, Fan L, Ding Q, Wang X, Yao XL, Hou JF, Yu Z, Chen G, Hu Y, Cen K (2013) Increased thermal conductivity of eicosane-based composite phase change materials in the presence of graphene nanoplatelets. Energy Fuels 27(7):4041–4047
Farahat MA, Mousa MM, Mahmoud NH (2016) Solar distiller with flat plate collector and thermal storage. In: 17th international conference on applied mechanics and mechanical engineering, Military Technical College, Cairo, Egypt, pp 19–21
Goldemberga J, Coelh ST (2004) Renewable energy—traditional biomass vs. modern biomass. Energy Policy 32:711–714
Hajare VS, Gawali BS (2015) Experimental study of latent heat storage system using nano-mixed phase change material. Int J Eng Technol Manag Appl Sci 3(8):37–44
Hajare VS, Narode AR, Gawali BS, Bamane SR (2014) Experimental investigation of enhancement in heat transfer using nano-mixed PCM. Int J Eng Res Technol 3(11):843–848
Harikrishnan S, Deenadhayalan M, Kalaiselvam S (2014) Experimental investigation of solidification and melting characteristics of composite PCMs for building heating application. Energy Convers Manage 86:864–872
Hematian A, Ajabshirchi Y, Behfar H, Ghahramani H (2012) Designing, construction and analysis of speed control system of the fan with PV feeding source in an air solar collector. Mod Appl Sci 6(1):136–144
Ho CJ, Gao JY (2009) Preparation and thermophysical properties of nanoparticle-in-paraffin emulsion as phase change material. Int Commun Heat Mass Transf 36:467–470
Karunamurthy K, Murugumohan K, Suresh S (2012) Use of Cuo nano-material for the improvement of thermal conductivity and performance of low temperature energy storage system of solar pond. Digest J Nanomater Biostruct 7(4):1833–1841
Kaviarasu C, Prakash D (2016) Review on phase change materials with nanoparticle in engineering applications. J Eng Sci Technol Rev 9(4):26–386
Kaygusuz K, Sari A (2005) Thermal energy storage system using a technical grade paraffin wax as latent heat energy storage material. Energy Sources 27:1535–1546
Kurtbash I, Durmush A (2004) Efficiency and exergy analysis of a new solar air heater. Renew Energy 29(9):1489–1501
Lin C, Li Y (2009) Synthesis of ZnO nanowires by thermal decomposition of zinc acetate dihydrate. Mater Chem Phys 113:334–337
Mallika AN, Reddy AR, Reddy KV (2015) Annealing effects on the structural and optical properties of ZnO nanoparticles with PVA and CA as chelating agents. J Adv Ceram 4(2):123–129
Mote VD, Purushotham Y, Dole BN (2102) Williamson-Hall analysis in estimation of lattice strain in nanometer-sized ZnO particles. J Theor Appl Phys 6(1):6–14. https://doi.org/10.1186/2251-7235-6-6
Muller K, Bugnicourt E, Latorre M, Jorda M, Sanz YE, Lagaron JM, Miesbauer O, Bianchin A, Hankin S, Bolz U, Perez G, Jesdinszki M, Lindner M, Scheuerer Z, Castello S, Schmid M (2017) Review on the processing and properties of polymer nanocomposites and nanocoatings and their applications in the packaging automotive and solar energy fields. Nanomaterials 7(4):74
Narayanan S, Kardam SA, Kumar V, Bhardwaj N, Madhwal D, Shukla P, Kumar A, Verma A, Jain VK (2017) Development of sunlight-driven eutectic phase change material nanocomposite for applications in solar water heating. Resour Eff Technol 3(3):272–279
Otanicar TP, Golden JS (2009) Comparative environmental and economic analysis of conventional and nanofluid solar hot water technologies. Environ Sci Technol 43:6082–6087
Pise AT, Waghmare AV, Talandage VG (2013) Heat transfer enhancement by using nanomaterial in phase change material for latent heat thermal energy storage system. Asian J Eng Appl Technol 2(2):52–57
Ramasamy M, Das M, An SN, Yi DK (2014) Role of surface modification in zinc oxide nanoparticles and its toxicity assessment toward human dermal fibroblast cells. Int J Nanomed 9:3707–3718
Sharma A, Tyagi VV, Chen CR, Buddhi D (2009) Review on thermal energy storage with phase change materials and applications. Renew Sustain Energy Rev 13:318–345
Skovajsa J, Kolacek M, Zalesak M (2017) Phase change material based accumulation panels in combination with renewable energy sources and thermoelectric cooling. Energies 10:152
Teng T, Yu C (2012) Characteristics of phase-change materials containing oxide nano-additives for thermal storage. Nanoscale Res Lett 7:611
Tony MA, Tayeb A (2011) The use of solar energy in a low-cost drying system for solid waste management: concept, design and performance analysis. In: Eurasia waste management symposium, 14–16 November
Tony MA, Tayeb A (2016) Response surface regression model in optimization of alum sludge drying facility: solar-fenton’s reagent dewatering. Int J Chem Eng Appl 7(5):331–335
Tony MA, Tayeb A, Zhao YQ (2016) An alternative arrangement for the alum sludge management: minimising waste with low-cost solar techniques. Am J Chem Eng 4(2):30–37
Wang J, Xie H, Xin Z, Li Y, Chen L (2009) Thermal properties of paraffin based composites containing multi-walled carbon nanotubes. Thermochim Acta 488:39–44
Wen BF, Wang X, Xu Y, Zhang D, Yang M (2011) The effect of encapsulation of nano zinc oxide with silica on the UV resistance of polypropylene. Polym Plast Technol Eng 50:1375–1382
Xu J, Shi S, Zhang X (2013) Structural and optical properties of (Al, K)-co-doped ZnO thin films deposited by a sol–gel technique. Mater Sci Semicond Process 16:732–737
Zelzouli K, Guizani A, Sebai R, Kerkeni C (2012) Solar thermal systems performances versus flat plate solar collectors connected in series. Engineering 4:881–893
Zeng RX, Wang W, Xiao Y, Zhang Q, Di H (2010) Thermal performance of phase change material energy storage floor for active solar water-heating system. Front Energy Power Eng Chin 4(2):185–191
Acknowledgements
There is no acknowledgment that the authors can include.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility M. Abbaspour.
Rights and permissions
About this article
Cite this article
Tony, M.A., Mansour, S.A. Sunlight-driven organic phase change material-embedded nanofiller for latent heat solar energy storage. Int. J. Environ. Sci. Technol. 17, 709–720 (2020). https://doi.org/10.1007/s13762-019-02507-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-019-02507-z