Abstract
Sustainable leather processing is viable through conventional organic tanning processing that provides scopes for quality to match with the chrome-tanned leathers. Hence, there is a need to develop sustainable tanning technology for leather processing. Glyoxal alone and in combination with wattle extract has been tried to establish a new tanning system. Glyoxal as the solo-tanning agent, optimized at the level of 5%, can produce shrinkage temperature up to a level of 72 °C, whereas the combination tanning system with the help of wattle extract yielded leather with shrinkage temperature of 92 °C. The tanned leather was further processed into crust leather, and organoleptic properties are evaluated. Thermogravimetric analyses revealed better thermal stability at 113.52 °C, with a residue of 86.94%, in comparison with the leather tanned with combination tanning system using wattle extract showing stability at 110 °C and residue of 87.29%. The visual assessment results showed that the leathers produced from experiments were soft, with smoother grain, uniform dyeing properties and overall comparable strength properties. The plausible mechanism for present tanning systems has been elucidated. The results of scanning electron microscopic analysis revealed that leather produced from glyoxal tanning was of empty type where the fibers are tightly bounded, however, having less angle of weave as compared to control sample. The reduction in Bio-chemical oxygen demand and Chemical oxygen demand values from effluent stream in the case of solo tanning showed 64–70% and 68–71%, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tanning process involves organic and inorganic tannages out of which the chrome tanning comes under the latter category that gives better thermal and hydrothermal stability with desirable organoleptic properties. However, tanning industry is under threat from environmental regulatory bodies due to pollution and carcinogenicity related to the hazardous Cr(VI) (Kanagaraj et al. 2008). As an alternate, the tanning industry is to rely on organic tanning systems.
Organic tanning system comprises vegetable tanning, aldehyde (gluteraldehyde, formaldehyde), oxazolidine, oil tanning and syntans. Organic tanning system, especially vegetable tanning, is ecologically safer than chrome tanning. It is employed with natural materials (e.g., bark, wood, pods) for tanning the pelt. It gives unique properties such as fullness, solidity, compactness and good abrasion resistance (Covington and Shi 1998; Madhan et al. 2002; Lu et al. 2003). The vegetable tanning is composed of polymeric polyphenolic molecules covering a wide range of molecular mass ranging from 500 to 3000 D units. The tanning action of polyphenols depends on the molecular mass (particle size) and the number of phenolic hydroxyl groups (Haslam 1997). The disadvantage of the vegetable tanning is that it employs huge quantity of vegetable tannin as raw feed which causes severe pollution problem in terms of biochemical oxygen demand (BOD), chemical oxygen demand (COD), total dissolved solids (TDS) and total suspended solids (TSS) and sludge problems. Other than the vegetable tanning, there are reports on use of other tanning systems, like formaldehyde. A large number of aldehyde has been tried for tanning the pelt, but only formaldehyde shows good tanning property and the unsaturated aldehydes like acrolein and crotonaldehyde show a fair degree of tannin potency (Fein and Filachione 1957; Dasgupta 1977; Khanbabaee and Van Ree 2001). The ‘formaldehyde’ uses a biological specimen where the shrinkage temperature can be raised to 85 °C. Here, the cross-linking is inefficient and imposes health–safety implication that is harmful and hence banned. It contains polyhydroxy groups that are responsible for cross-linking and produces spongy and hydrophilic leather. In other words, gluteraldehyde tanning is widely used that gives shrinkage temperature of 80–85 °C maximum. Its polymeric structure can interact with peptide of collagen through H-bonding through alicyclic oxygen giving rise to spongy and hydrophilic leather (Fathima et al. 2004; Covington and Shi 1998). Glutaraldehyde is used as one of the practiced tanning methods to obtain leather with soft, white color, spongy and hydrophilic leathers. Recently, oxazolidine tanning has gained importance because of the fixation of more amount of chromium from chrome tanning. This tannin consists of alicyclic derivatives like amino alcohol and formaldehyde under hydrolytic conditions where the rings can open, to form an N-hydroxymethyl compound which can react with one or more amino sites, in an effective way through acridly odiferous tannage. An important aspect of this tanning is their influence on the chrome tanning reaction, promoting the fixation; it is not known whether this is a function of the chemistry analogous to ethanolamine. It gives hydrophobic leather and exhibits Ewald effect showing heat shrunk at 70 °C but retains 90% of area in cold water. These tanning agents can give shrinkage temperature up to 85 °C. Other organic tanning uses oil as tannin. Oil tanning used to make familiar chamois leather. In this case, the preferred oil is cod liver oil containing fatty acids, oils with free of glycerides. Other organic tanning systems comprising syntans, either in the form of combination or replacement, are also in use. They are the products made through sulfonation followed by polymerization by formaldehyde (Covington and Shi 1998). They have weak tanning power and can be applied after main chrome tanning for modifying handling properties of leather. This tanning method cannot give high shrinkage temperature but can produce the leather with spongy type that can be used for filtration of petrol, cleaning spectacles and water absorption application.
Tanning by different aldehydes, e.g., formaldehyde, gluteraldehyde, glyoxal and crotonaldehyde, was studied by Fathima et al. (2004) where the thermal stability of the product was reported to be 77–86 °C. The order of shrinkage temperature for various aldehydes was found to be in the order formaldehyde > gluteraldehyde > glyoxal > crotonaldehyde. It was found that they had high degree of stability against collagenase without altering the secondary structure and triple helical conformation of collagen. In a separate study, with pre-treated acrylic polymer followed by the treatment with wattle extract (Acacia Mollissima) showed enhancement in shrinkage temperature up to 25 °C where the product showed improvement in stabilization of collagen (Madhan et al. 2002)
All the above-said organic tanning systems also possess some negative attributes, such as non-ecofriendly environmental implications, lacking desired leather properties (like perspiration and washing resistance with better fullness and density) and poor-stabilization of collagen, posing serious challenges to our continued reliance on it. In the present investigation, tanning with glyoxal has been attempted for producing sustainable leather as compared to other organic systems. Since glyoxal has potential to produce leather with better organoleptic properties, systematic investigations are required. Glyoxal links a wide range of polymers, e.g., starch, cellulose, proteinaceous material, polyacrylamide and polyvinyl alcohols (Deng et al. 2014). Chursin and Obolenskaya (2011) presented a composite tanning agent based on glyoxal where the aldehyde and sulfuric acid retarded reduction of Cr(VI). Also it is established fact that glyoxal as a biodegradable material does not impose environmental contamination (Ramires et al. 2010; Chursin and Obolenskaya 2011).
The present tanning technique has overall benefit in achieving the leather properties such as perspiration and washing resistances with better fullness and density in addition to providing desired shrinkage temperature. Besides, to that, combined tanning system has also been tried with glyoxal and wattle extract for producing leather with increased shrinkage temperature and organoleptic properties. Here, the mechanisms of glyoxal and combination tanning have been explored for improving leather properties in the present work.
Materials and methods
Materials
Glyoxal, LR grade (40%), procured from SD fine chemicals Ltd., India, wet-salted goat skins weighing 1 kg/skin, purchased from CLRI, Chennai, wattle extract (Acacia mearnsii, containing 35.6% polyphenol, Folin Denis Method), glycerol (LR grade, 98%) from Sigma-Aldrich, LR grade of sodium formate, sodium bicarbonate, fatliquoring, retanning agents, dyes, and potassium bromide from SD fine chemicals Ltd, India, were collected.
Methods
Preparation of wattle extract (Acacia mearnsii)
The bark is dried and chopped to desired size at first. It is made ready for stripping for extraction in a counter-current mode in autoclave under pressure and temperature above 100 °C. The extract is then concentrated in evaporator. The thick, hot and viscous liquor is spray-dried to a natural powder and is packed in bags for storage. The polyphenol content is found to be 35.6% in the extract.
Determination of particle size
The particle sizes of glyoxal, wattle extract separately, glyoxal combined with wattle extract powder were measured using Malvern Zetasizer Nano ZS ZEN 3600 (4 mW He–Ne laser, 633 nm), UK, adopting standard procedure. Particle size analysis was performed at a fixed scattering angle of 90°. The samples were filtered using 0.45-micron filter paper before measurements.
Solo and combination tanning systems with glyoxal
Two experiments were carried out: one with solo tanning with glyoxal (5%) and second experiment with glyoxal (5%) and wattle extract (5%). The control experiment had been carried out with wattle extract (15%).
The depickled pelt (pH 5.5) was treated with different concentrations of glyoxal (4, 5, and 6% based on pelt weight) and agitated in a drum for 120 min to tan the pelt. The glyoxal was fixed with formic acid 1%, and the tanned leather was produced. This is solo-tanning system. In another experiment, the tanned leather was obtained as above and was subsequently tanned with wattle extract powder (5%) and then fixed with formic acid. The leather produced was said to be combination-tanned leather, and the tanning was called combination tanning system. The control experiment was also carried out with the help of wattle extract (15%) on the pelt which was later fixed with formic acid. The resultant liquors and leathers were collected and analyzed for various tests.
Method of producing crust leather from tanned leather
The tanned leathers were neutralized to pH 5.5 and subsequently post-tanned. Retanning, dyeing and fatliquoring were carried out for making the crust leather. In the retanning stage, composition of (Basyntan DI—5%, Relugan RE—5%, Basyntan FB6—4%) was added. Then, brown dye—2% and fat liquor (Lipoderm Liquor SA—5%, Lipoderm Liquor 2FB—3%, Lipoderm Liquor SAF—5%) were added and agitated in the drum for a period of 4 h, and later the chemicals were fixed with formic acid (1%). The ultimate aim of the process is to make leather for shoe upper. The leathers were further dried, staked and processed as per standard conventional procedures.
Determination of shrinkage temperature
The shrinkage temperature of the leather sample was determined with a Theis shrinkage meter. The sample of dimensions 20 × 3 mm was taken and hooked in the shrinkage meter. The samples were then immersed in a glycerol–water solution. The temperature at which the specimen starts to shrink was noted as the shrinkage temperature of the particular leather.
Optimization studies
Effect of tanning process was studied under different pHs, temperatures and initial concentrations of glyoxal. Experimental results are used to find optimal parameters (pH, temperature and concentration) using a software written in Matlab V 12. Response surface methodology was used to find optimal operating parameters.
FT-IR analysis
The controls and experimental samples after tanning were collected and dried in the water bath. They were mixed with potassium bromide (1:20; 0.02 g of sample with KBr at a final weight of 0.4 g) and sintered. The samples were then ground, desorbed at 60 °C for 24 h and pressed to obtain IR-transparent pellets. The absorbance FT-IR spectra of the samples were recorded using an FT-IR spectrum 2000 PerkinElmer spectrophotometer. The spectra were collected within the scanning range of 400–4000 cm−1 (Corrales et al. 2002).
BOD and COD generated in tanning processes
The spent liquor from control and experimental samples after tanning processes was collected and analyzed for the pollution loads such as BOD, COD, TDS and TSS using the standard method (Eaton and Franson 2005).
Thermogravimetric analysis (TGA)
TGA was performed to determine the transition temperatures like glass transition temperature (Tg), melting temperature (Tm) and crystallization temperature (Tc) of the tanning agent. TGA of the sample was carried out using a Q50 TA Instruments, with ramp of 15 °C/min from room temperature to 600 °C in a nitrogen atmosphere. Weight loss of these materials as a function of temperature was recorded using this study (Corrales et al. 2002).
SEM studies
The control and experimental tanned leather samples obtained from experiments were subjected to JEOL JSM-5300 SEM for the analysis. The micrographs for the sample were obtained by operating the SEM at an accelerating voltage of 20 kV with different lower and higher magnification levels. The sample that showed clear views was presented in the present investigation (Echlin 1971).
Physical testing
Samples for various physical tests from experimental and control crust leathers were obtained as per IULTCS method. Specimens were conditioned at 80° ± 4 °F and 65 ± 2%. Over a period of 48 h, physical properties such as tensile strength, % elongation at break, tear strength and grain crack were examined for both experimental and control leather samples.
Results and discussion
Organic tanning systems are considered to be very ecofriendly due to employment of natural plant materials, oils, syntans and aldehydes. Most tanning agents are polymeric in nature (either lesser or greater extent) conferring to some positive enthalpic effect that do not provide strong cross-linking through crystallinity effect as chromium. On the other hand, vegetable and other organic tanning agents provide stabilizing effect by multiple hydrogen (bond) and hydrophobic interactions. In contrast, glyoxal and dialdehyde react covalently, in solution, to create extended cross-linking (Bickley 1992). The present tanning system was carried out with glyoxal and glyoxal plus wattle extract (combination tanning systems), and the behavioral aspects of glyoxal have been studied. The mechanistic aspects of reactant glyoxal with collagen and combination tanning system with wattle extract have been studied in this particular work. In order to study the distribution of tanning agent around the leather substrate, it is necessary to know the particle size distribution of the tanning agent.
The particle size distribution of samples is given in Table 1. Tanning agents, namely glyoxal and wattle extract, show particle size distribution of 157.9 and 256.4 nm as an independent compound. However, the wattle extract in glyoxal solutions showed particle size distribution of 495.8 nm. The distributions of the wattle extract, glyoxal and glyoxal plus wattle extract in the collagen fibril have different orientations. It has been found that tropo-collagen molecule is staggered by D region which is one of many possible forms of assembly with offset as multiples (Pizzi 1979). The gap and overlap zones represent 0.6 (40 nm) and 0.4 (27 nm) for D region, respectively. However, the particle size distribution of glyoxal and glyoxal plus wattle extract is unable to penetrate in the D region due to its bigger size distribution of more than 67 nm. The wattle extract containing size distribution of 256.4 nm will stabilize the basic structure of collagen molecule called tropo-collagen owing to 4.4 D or 290 nm of collagen. The combination tanning mixture glyoxal plus wattle extract showed size distribution of 495.8 nm, however, was unable to stabilize the tropo-collagen molecule and cross-link in the gap region owing to bigger particle size distribution leading to stabilization of fiber studies of the collagen. In overall, particle size of glyoxal helps to penetrate in the gap region providing better cross-links with collagen, whereas the combination tanning system with wattle extract provides filling effect to the leather. Besides particle size analysis, shrinkage temperature is an important factor for deciding efficacy of the tanning agent.
Shrinkage temperature of leather
Shrinkage temperature is important property to rate the performance of tanning agent used in tanning process since it gives a measure of endothermic reaction resulting in the cleavage of hydrogen bonds in the polypeptide chain of collagen. Shrinkage temperature for the tanned leather produced with glyoxal and combination tanning system with wattle extract had been carried out separately for which results are presented in Table 1. The leathers produced with tanning agent glyoxal gave shrinkage temperature of 72 °C, whereas combination tanning system made with glyoxal and wattle extract showed shrinkage temperature of 92 °C. The results showed that the leather produced with glyoxal yielded a shrinkage temperature of 72 °C, whereas other organic tanning systems such as syntan, aldehyde and other vegetable tanning agents can produce similar results. The binding force of glyoxal with collagen is due to dialdehyde which is weakened due to sharing by hydrogen bonding. However, the cross-links may be due to covalent bonds formed between dialdehyde and collagen that helped to tan the leather (RamasamI 2001).
On the other hand, the leather produced with combination tanning system gave shrinkage temperature of 92 °C which was due to multipoint hydrogen bonds and covalent cross-links due to the functional groups of dialdehyde of glyoxal and polyphenols originating from wattle extract. As the composition of combination tannage was different, the cross-link with polyphenolic groups was different from the solo ones. From Table 1, it is seen that the shrinkage temperature of glyoxal–wattle extract-tanned leather shows a higher shrinkage temperature when compared to solo glyoxal-tanned leather. The results indicate that combination tanning with glyoxal lead to significant improvement in hydrothermal stability. Similar results have been obtained by other researchers (Kanagaraj et al. 2015a; Zhan et al. 2016; Fuchs et al. 1993) too.
Optimization of the tanning systems
In order to find optimal operating condition, theoretical studies have been carried out using formulated model and Matlab software (version 2013). The effect of pH, temperature and duration on the shrinkage temperature for the solo tanning with glyoxal has been provided in Fig. 1. In Fig. 1a, with changes in pH and duration of tanning, the shrinkage temperature varies and forms a curve where the surface of the curve shows an optimal minima of pH 5.5 and Temp = 38 °C. The maximum shrinkage temperature becomes 72 °C. Similarly, in Fig. 1b, the optimal curved surface shows pH 5.5 and concentration of glyoxal as 5 g/L for a shrinkage temperature of about 71.6 °C. In a similar way, the optimal curve for the effect of concentration of glyoxal and temperature on shrinkage temperature is shown in Fig. 1c. The maximum shrinkage temperature obtained is about 72 °C for an optimal concentration of glyoxal at 5.5 g/L and temperature of 38 °C. These data are in close agreement with experimental observations obtained during tanning using glyoxal as tanning agent.
The combination tanning had been tried with glyoxal and wattle extract to study shrinkage temperature and improvement in organoleptic properties of leather. The optimal curves are studied to find operating points that are shown in Fig. 1d–f. Figure 1d represents effect of pH and temperature on shrinkage temperature. The maximum shrinkage temperature obtained is 94 °C for an optimal pH of 5.5 and temperature of 38 °C. The observable thing is that the addition of wattle extract has remarkable effect on leather in improving the shrinkage temperature to a quite high. Similarly, Fig. 1e represents effects of pH and concentration of glyoxal with wattle extract on the shrinkage temperature. A maximum shrinkage temperature of 94 °C (as compared to experimental value of 92 °C) was achieved for an optimal pH of 5.5 and concentration of combined tanning system using glyoxal with wattle extract as 5:15 (representing 5 g/L glyoxal mixed with 15 g/L wattle extract).
In a similar study, as shown in Fig. 1f, shrinkage temperature is obtained as 94 °C for an optimal value of temperature of 38 °C and combined concentration ratio of tanning agent as 5:15. Thus, the effect of wattle extract along with glyoxal has been established as very effective tanning system that can be suggested as replacement for chrome tanning system. In this combination tanning system, gain in shrinkage temperature with grain softness properties could be achieved where the new tanning system helps for sustainable leather production. The present tanning systems, comprising solo and combination tanning, show different behaviors which can be further analyzed by mechanism.
Mechanism of glyoxal tanning
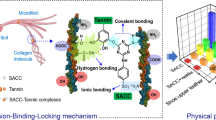
The mechanism of solo tanning and combination tanning using glyoxal and wattle extract is elucidated very clearly. Glyoxal, a tanning agent, is employed to tan the leather where glyoxal at the level of 5% is sufficient to tan the leather. The leather obtained by this tanning also gave the shrinkage temperature of 72 °C. Here, various approaches such as calorimetry, hydrogen bond, hydrophobic-bond breaking and chemical transformations of collagen were employed to know the mechanism of tanning systems (Rossouw et al. 1980; Hemingway and Karchesy 2012; Grenier-Loustalot et al. 1996). The cross-link options are wider for glyoxal tanning system than other tanning systems such as with simple aldehydes (Scholnick et al. 1992). Glyoxal, a starch dialdehyde compound is predominantly present in polymerized form. The terminal hydroxyl groups of the polymer are active and capable of reacting with amino groups of collagen that forms cross-links. The polymer itself can interact with the collagen peptide links through hydrogen bonding via alicyclic oxygens yielding very soft, spongy and hydrophilic leather in nature (Santiago-Medina et al. 2017; Ollé Otero et al. 2016). The mechanism of cross-linking of glyoxal with collagen is shown in Fig. 2a, b.
In the combination tanning of glyoxal and wattle extract, aldehyde primarily reacts with amino groups of collagen which later establishes cross-link between collagen and wattle extract provided there are nucleophilic sites in tannin molecules. It can be interpreted that the synergistic effect of the combination tanning increases hydrothermal stability of collagen fibers where the cross-links of polyphenol and dialdehyde increase rapidly, primarily through multiple hydrogen bonding and covalent cross-links. This results in increased shrinkage temperature in the leather for combination tanning system using glyoxal and wattle extract. The higher hydrothermal stability of aldehyde wattle in combination tannage is attributed due to formation of cooperating units mainly from covalent cross-links of aldehyde and polyphenols (wattle extract). It is therefore believed by researchers that the functional groups of tannin form hydrogen bonds with the functional groups of collagen. When one tannin molecule forms hydrogen bonds with the functional groups of different polypeptide chains, a new cross-link is formed and therefore the hydrothermal stability of leather is increased rapidly. According to an alternate theory, the electron-donating groups like hydroxyl and carboxyl attached to aromatic tannin molecules, push/increase electron density in the ortho- and para-positions of benzene rings. Naturally, the tannin molecules behave like dipoles and get attached to –CONH– bonds of collagen. Due to these carbamino links of collagen, tannage also becomes electrically charged due to uneven distribution of electrons and resonance between oxygen and nitrogen atoms which results in higher shrinkage temperature requiring more melting energy.
TGA analyses
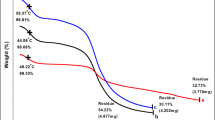
TGA has been carried out to study the decomposition pattern and thermal stability of the tanning agent. TGA results are presented in Fig. 3a, b. One can see that there are three stages in the curve. The compound decomposes in TGA analysis of glyoxal tanning showing glass transition temperature starts at 92.58 °C, with melting temperature at 327.61 °C and crystallization temperature at 404 °C with a residue left over with 31.76%. In the case of combination tanning, it showed glass transition temperature starting at 96.37 °C, with melting temperature at 323.82 °C and crystallization temperature at 403.43 °C with a residue left over with 33.01%. This reveals that stability is better in the case of combination tanning system. The weight losses in this stage are because of loss in moisture. The second and third parts of the curve represent the maximum weight loss due to the thermal degradation of the sample. In order to confirm involvement of further functional groups in tanning, FTIR is necessary to be carried out.
FTIR analyses
FTIR spectral data for glyoxal tanning and combination tanning were carried out and are presented in Table 2 and Fig. 3c, d. From these figures, it is found that peak at 3431 cm−1 is responsible for O–H bond stretching. At 1634 cm−1, the band is due to extended stretching of hydrogen-bonded C=O functional group. At 1416 cm−1 and 1071.26 cm−1, bands are due to antisymmetrical stretch-vibrational absorption spectra of saturated –CH3 group and C–O stretching vibration, respectively. This indicates that glyoxal exists mainly in the hydrated form in aqueous solution. This result is in line with our previous reports since glyoxal is present in aqueous solution (Pizzi and Stephanou 1993; Tondi and Petutschnigg 2015; Kanagaraj et al. 2015b). Owing to the influence of hydrogen bonding, the adsorption peak of C=O shifts to lower wave number.
An FTIR spectrum for the combination tanning case is shown in Fig. 3d. In the combination tanning case (with glyoxal and wattle extract), band at 3314 cm−1 represents presence of O–H functional group which is shifted due to hydrogen bonding. Besides, C=O stretching occurs at 1614 cm−1 which may be due to dialdehyde that plays major role as an activated functional group to promote better cross-linking that increased shrinkage temperature as well as organoleptic properties.
Dialdehyde functional groups are found in activated position of flavonoid producing diolic (–CH(OH)–CH(OH)–) cross-link or in enol form (–CH=C(OH)– ↔ –CH2(–C=O)–). There may be a possibility of glyoxal-polymerised tannin at higher concentration (125 ppm) of glyoxal, whereas at lower concentration (75 ppm) it favors keto-enolic equilibrium. Similar results were also reported by other researchers (Kain et al. 2014; Roux et al. 1975).
SEM analyses
The leathers produced by solo and combination tanning were analyzed for SEM analysis. The objective of the analyses was to study morphology and spatial orientation of collagen fibers. The samples have been prepared at different concentrations and are subjected to different magnifications. Experimental sample obtained by treating tanning agents (at various concentrations) were put under magnification 300 µm 200 × , 300 µm 250 × , 100 µm 500 ×, respectively, to obtain clear morphology with better resolution. Results are presented in Fig. 4a–d and Supp. Figures 1 and 2a, b with different magnifications. When tanned with glyoxal, empty type of leather was produced, where the fibers are tightly bounded and angle of weave is less which clearly shows less filling effects, whereas in the case of leather produced with combination tanning, the collagen fibers are compactly aligned with fillers from excess wattle extract. It is more of filling type compared to empty types of fiber-glyoxal. Angle of weave is also found to be higher in combination tanning compared to solo one. Similarly, in the case of crust leather, crust leather obtained was subjected to SEM analyses with magnification 100 µm 500 × , 300 µm 200 ×, respectively, to obtain morphology. It was found from supp. figures that similar results were observed from crust leather surface. However, fibers are very compact in solo tanning as compared to layered thickened patches of collagen fibers in the combination tanning.
Pollution reduction
The effluent from tanning operation is subjected to BOD and COD analysis, and results are presented in Table 3. The reduction of BOD and COD for glyoxal-tanned leather over combined tanned leather has been presented. It can be seen from the tables (Table 3) that BOD and COD data in the case of combination tanning are higher compared to that of solo (glyoxal) tanning. This can be attributed to the fact that concentration of glyoxal is less as compared to higher concentration of dialdehyde and phenolic compounds present in combination tanning. It can be found that use of 1–5% of solo-tanning agent (glyoxal) could remove 64–70% of BOD as compared to combination tanning where 15% of wattle extract (fixed amount) was used with 1–5% of glyoxal. Similarly, using solo-tanning agent, COD could be reduced up to 68–71%, whereas using combinational agent, the increase in COD level may be attributed to unabsorbed moiety present in the effluent. Thus, the reduction in pollution load may be attributed to the presence of reactive groups of dialdehyde that play major role in establishing cross-linking between tanning agent and the collagen. The biodegradability of both these systems was also reported (Theis and Grohe 2002).
Organoleptic properties of the leather
The experimental tanned leathers were made into crust upper leather and visually assessed for organoleptic properties such as grain softness, grain tightness, fullness and dyeing properties. The results are presented in Fig. 5. It is seen from the figure that the grain softness property is better in experiment E1 (experiment carried out with solo-tanning glyoxal 5%) as compared to experiment E2 (combination tanning) and control process. The reason may be due to the fact that dialdehyde splits the fibers into fibrils and provides better softness in the case of experiment E1. The grain tightness becomes better in experiment E2 and control process than in experiment E1. Fullness is found to be better in the cases of experiment E-2 and control process than in experiment E1, while the dyeing property is very good in experiment E1 than in experiment E2 and control process. Overall, the general appearance and feel was very good in experimental E1 process.
Physical strength properties
The tanned leathers were made into crust leather and were analyzed for the physical strength properties by standard methods, and the results are provided in Table 4. Physical strength properties such as tensile strength, elongation at break, tear strength, load at grain crack and distension at grain crack were measured. Leathers produced from the combination tanning using glyoxal and wattle extract showed better strength properties as that of leather produced with the solo tanning using glyoxal alone. The improvement may be attributed to the fact that improved cross-links of collagen with dialdehyde and polyphenols of wattle extract played major role in enhancing the properties.
Conclusion
A new organic tanning system using glyoxal as solo-tanning agent and glyoxal plus wattle extract as combination tanning agent, has been explored in this work. The particle size of the solo agent was 157.9 nm, whereas the same for combination tanning system gave rise to particle size of 495.8 nm. The reactive functional group of glyoxal, namely dialdehyde, tans the leather satisfactorily. Therefore, it can be preserved for sufficient time. The leather produced with new tanning system showed shrinkage temperature of 72 °C compared to 92 °C for the combination tanning system. It was found that the optimal operating value of pH was about 5.5, whereas temperature of tanning was 38 °C, with an initial concentration of glyoxal as 5 g/L. The plausible mechanism for improved tanning performance of glyoxal was due to the multipoint hydrogen bonding by the presence of –OH functional groups with active sites of collagen. The increase in shrinkage temperature for solo tanning may be attributed to a starch dialdehyde compound predominantly present in polymerized form, where the terminal hydroxyl groups of the polymer are active and capable of reacting with amino groups of collagen, whereas in combination tanning reaction between functional groups from polyphenol (of water extract) and dialdehyde increases rapidly, primarily through multiple hydrogen bonding and then covalent cross-links improving hydrothermal stability of collagen fibers. FTIR investigation confirms participation of dialdehyde groups in the solo-tanning process. TGA analysis of glyoxal-tanned and combination-tanned leather showed overall better stability but a slight increase in the case of combination tanning system. The results of SEM analysis revealed that crust leather obtained from solo as well as combination tanning produced different surface morphology with different orientations of collagen fibers. In the case of combination tanning, wattle extract acted as a filling agent so that comparative smooth morphology was observed. The reduction in BOD and COD values from effluent stream in the case of solo tanning showed 64–70% and 68–71%, respectively. Physical strength properties of leather were slightly better in combination tanning as compared to solo-tanning system. The above results relating to pollution reductions, improved organoleptic and stability properties yield to sustainable leather processing.
References
Bickley JC (1992) Vegetable tannins and tanning. J Soc Leath Technol Chem 76:1–5
Chursin VI, Obolenskaya KV (2011) Use of glyoxal in production of a composite chromium tanning agent. Russ J Appl Chem 84:2083–2087
Corrales T, Catalina F, Peinado C, Allen NS, Fontan E (2002) Photooxidative and thermal degradation of polyethylenes: interrelationship by chemiluminescence, thermal gravimetric analysis and FTIR data. J Photo Chem Photobiol A Chem 147:213–224
Covington AD, Shi B (1998) High stability organic tanning using plant polyphenols, part I—the interactions between vegetable tannins and aldehyde cross linker. J Soc Leath Technol Chem 82:64–71
Dasgupta S (1977) Oxazolidines—a new class of tanning agent. J Soc Leather Technol Chem 87:203–208
Deng S, Pizzi A, Du G, Zhang J, Zhang J (2014) Synthesis, structure, and characterization of glyoxal- urea-formaldehyde cocondensed resins. J Appl Polym Sci 131(21):41009–41010
Eaton AD, Franson MAH (2005) Standard methods for the examination of water and wastewater, 22nd edn. The American Public Health Association (APHA), Washington, DC
Echlin P (1971) In: Heywood VH (ed) Scanning electron microscopy. Systematic and evolutionary applications, vol 4. Academic Press, London, p 307
Fathima NN, Balaraman M, Rao JR, Nair BU, Ramasami T (2004) Interaction of aldehydes with collagen: effect on thermal, enzymatic and conformational stability. Int J Biol Macromol 34(4):241–247
Fein ML, Filachione EM (1957) Tanning studies with aldehydes. J Leath Chem Assoc 51:17–23
Fuchs K, Kupfer R, Mitchell JW (1993) Glyoxalic acid: an interesting contribution to clean technology. J Am Leather Chem Assoc 88:402–409
Grenier-Loustalot MF, Larroque S, Grenier P, Bedel D (1996) Phenolic resins: 4. Self-condensation of methylolphenols in formaldehyde-free media. Polymer 37:955–964
Haslam E (1997) Vegetable tannage: Where do the tannins go? J Soc Leath Technol Chem 81:45–51
Hemingway RW, Karchesy JJ (2012) Chemistry and significance of condensed tannins. Springer, Berlin
Kain G, Güttler V, Barbu MC, Petutschnigg A, Richter K, Tondi G (2014) Density related properties of bark insulation boards bonded with tannin hexamine resin. Eur J Wood Wood Prod 72:417–424
Kanagaraj J, Chandra Babu NK, Mandal AB (2008) Recovery and reuse of chromium from chrome tanning waste water aiming towards zero discharge of pollution. J Clean Prod 16(16):1807–1813
Kanagaraj J, Panda RC, Sumathi V (2015a) Water soluble graft copolymer synthesized from collagenous waste and PEG with functional carboxylic chains: a highly efficient adsorbent for chromium (III) with continuous recycling and molecular docking studies. Ind Eng Chem Res 54:7401–7414
Kanagaraj J, Senthilvelan T, Panda RC, Kavitha S (2015b) Eco-friendly waste management strategies for greener environment towards sustainable development in leather industry: a review. J Cleaner Prod 89:1–17
Khanbabaee K, Van Ree T (2001) Tannins: classification and definition. Nat Prod Rep 18:641–649
Lu Z, Liao X, Shi B (2003) The reaction of vegetable tannin—aldehyde—collagen: a further understanding of vegetable tannin—aldehyde combination tannage. J Soc Leather Technol Chem 87:173–178
Madhan B, Muralidharan C, Jayakumar R (2002) Study on the stabilisation of collagen with vegetable tannins in the presence of acrylic polymer. Biomaterials. 23(14):2841–2847
Ollé Otero L, Díaz J, Casas C, Bacardit Dalmases A (2016) Low carbon products to design innovative leather processes. Part IV: manufacture of automotive leather using Tara. J Am Leath Chem Assoc 111(5):171–205
Pizzi A (1979) Phenolic and tannin-based adhesive resins by reactions of coordinated metal ligands. II. Tannin adhesive preparation, characteristics and application. J Appl Polym Sci 24:1257–1268
Pizzi A, Stephanou AA (1993) Comparative C13 NMR study of polyflavonoid tannin extracts for phenolic polycondensates. J Appl Polym Sci 50:2105–2113
RamasamI T (2001) Approach towards a unified theory for tanning: Wilsons dream. J Am Leath Chem Assoc 96:290–304
Ramires EC, Megiatto JD, Gardrat C, Castellan A, Frollini E (2010) Biobased composites from glyoxal–phenolic resins and sisal fibers. Bioresour Technol 101:1998–2006
Rossouw DDT, Pizzi A, McGillivray G (1980) The kinetics of condensation of phenolic polyflavonoid tannins with aldehydes. J Polym Sci Polym Chem Ed 18:3323–3343
Roux DG, Ferreira D, Hundt HK, Malan E (1975) Structure, stereochemistry, and reactivity of natural condensed tannins as basis for their extended industrial application. Appl Polym Symp 28:335–353
Santiago-Medina FJ, Pizzi A, Basso MC, Delmotte L, Celzard A (2017) Polycondensation resins by flavonoid Tannins reaction with Amines. Polymers. 9:37
Scholnick F, Liao LL, Brown EM, Feair SH (1992) Crosslinking of collagen with di-carboxilic acid. J Am Leather Chem Assoc 87:333–338
Theis M, Grohe B (2002) Biodegradable lightweight construction boards based on tannin/hexamine bonded hemp shaves. Holz Roh Werkst 60:291–296
Tondi G, Petutschnigg A (2015) Middle infrared (ATR FT-MIR) characterization of industrial tannin extracts. Ind Crop Prod 65:422–428
Zhan K, Ejima H, Yoshie N (2016) Antioxidant and adsorption properties of bioinspired phenolic polymers: a comparative study of catechol and gallol. ACS sustain. Chem Eng 4:3857–3863
Acknowledgements
The authors would like to thank CSIR, New Delhi, India, for carrying out the research under the Mission Mode Projects CLRI/SIP/2018/05260
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Gobinath Ravindran.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kanagaraj, J., Panda, R.C. & Jayakumar, G.C. Interaction of glyoxal with collagenous matrix and its behavioral aspects for non-toxic and sustainable tanning system. Int. J. Environ. Sci. Technol. 17, 879–890 (2020). https://doi.org/10.1007/s13762-019-02327-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-019-02327-1