Abstract
Triclosan, a commonly available pesticide, has emerged as a ubiquitous pollutant posing a major threat to the environment. Here we have isolated a wastewater microorganism, Pseudomonas aeruginosa KS2002, capable of converting triclosan to 2,4-dichlorophenol within 96 h of incubation. The confirmation of the end product was done using Fourier transform infrared spectroscopy and mass spectroscopy. Different minimal media were investigated to establish a suitable media supporting maximum triclosan degradation. Spectral analysis showed that this bacterial isolate degraded 99.89% ± 0.3 of 2 g/L of triclosan spiked in an M9 minimal salt medium. This isolate utilized fructose and glycerol as a co-substrate to enhance degradation process. The cell-free extract of Pseudomonas aeruginosa KS2002 showed the activity of catechol 2,3-dioxygenase enzyme (specific enzyme activity = 0.161 U/mg). In the presence of 3-fluorocatechol, a meta-cleavage enzyme inhibitor, triclosan degradation was ceased suggesting a meta-cleavage pathway for triclosan degradation. Keeping in view the observations recorded, we proposed a pathway for partial triclosan degradation using this isolate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Triclosan surfaced the market as a most commonly used antimicrobial ingredient in a wide variety of household and medical goods. Excessive use of triclosan in the past few decades has raised an alarm of its persistence in our environment (Hovander et al. 2002; Balmer et al. 2004; Miller et al. 2008). Triclosan adversely affects the structural and functional aspects of algal communities. There are reports revealing the toxicity of triclosan in the aquatic environment specifically targeting aquatic microflora and has also been found to affect photosynthesis in diatom algae. Algae are considered as “first-step producers,” and any harm to algal life will hamper the ecological balance adversely (Wilson and Salyers 2003). Additive and synergistic effects of triclosan are seen when it combines with other contaminants in waterways making triclosan way more toxic to other aquatic organisms (Mathew et al. 2017). Triclosan has been found to disturb thyroid homeostasis (James et al. 2010). Triclosan toxicity has also been reported in different cells including human liver and cancerous cells (Olaniyan et al. 2016). This compound has been found to weaken cardiac and skeletal muscles contractility such that it negatively affects the muscle health (Cherednichenko et al. 2012).
Triclosan specifically targets the synthesis of fatty acid with the suppression of FabI gene coding for a carrier protein enoyl-acyl reductase in several microorganisms (McMurry et al. 1998). Triclosan mimics the natural substrate (enoyl-acyl reductase-NAD+ complex) of FabI gene, thereby acting as a potentially irreversible inhibitor of the same gene, and this has been reported to be slow and competitive (Heath et al. 1999). There are several other microbes that inherited resistance toward higher levels of triclosan, by the virtue of possessing FabK, an enoyl-acyl carrier protein reductase that remains unaffected by triclosan (Heath et al. 2000). Various efflux mechanisms and membrane destabilization processes often result in inducing triclosan resistance among microorganisms (Schweizer 2001).
Several microorganisms have been reported to biotransform triclosan. Aspergillus versicolor has recently been reported as being capable of triclosan degradation to 2,4-dichlorophenol and release of chloride ions at pH 5 and 7 (Tastan and Donmez 2014). A green alga, Nannochloris sp., was found capable of 100% removal of triclosan within 7 days of incubation by an unknown mechanism (Bai and Acharya 2017). Trametes versicolor and Pycnoporus cinnabarinus, two well-known white rot fungi, were found capable of biotransforming triclosan via conjugating to sugar moieties, thereby reducing the toxicity of the compound (Hundt et al. 2000). In another report, two different strains Pseudomonas putida TriRY and Alcaligenes xylosoxidans subsp. denitrificans TRI were found capable of utilizing triclosan as a sole carbon and energy source available in M9 minimal salt media (Meade et al. 2001). Triclosan-degrading bacterial consortia were isolated, and one of its members was found to be a Sphingomonas (Mulla et al. 2016). Nitrosomonas europea, an important nitrifying bacterium, was reported to be a potent triclosan degrader via co-metabolism (Zhao 2006). Majority of products containing triclosan, after use, are discharged into residential drains. Triclosan persists in the environment as the majority of wastewater treatment plants are unable to remove triclosan.
In the present study, wastewater has been employed as a source for isolation of microorganisms capable of showing higher tolerance toward triclosan. Different media have been checked for maximum triclosan degradation. Further, the enzymes involved in triclosan degradation were investigated and the pathway involved in its degradation mechanism was also elucidated. Sampling was carried out in summer 2015 at Sadar Hospital situated in Ranchi, Jharkhand. The isolation and further degradation experiments started in 2015 at Birla Institute of Technology, Mesra, Ranchi, and ended in 2017. Data analysis was carried out in 2017 and finished in 2018 at Birla Institute of Technology, Mesra, Ranchi.
Materials and methods
Chemicals
Irgasan ≥ 97% (CAS: 3380-34-5) and 3-fluorocatechol 99% (CAS: 363-52-0) were purchased from Sigma-Aldrich (Bangalore, India). 2,4-Dichlorophenol ≥ 98% (CAS: 120-83-2) was supplied by HiMedia Laboratories Pvt. Ltd. (Mumbai, India). BSA protein estimation kit was procured from GeNei Laboratories (Bangalore, India). All other media supplements were purchased from HiMedia Laboratories Pvt. Ltd. (Mumbai, India). The stock solutions were prepared in 95% ethanol (v/v) and were kept under the refrigerated condition at 4 °C. The media were supplemented with appropriate volumes of stock solutions.
Isolation and growth conditions of triclosan-degrading bacteria
Wastewater samples were collected from Sadar Hospital, Ranchi, Jharkhand. Bacterial isolates capable of effectively degrading triclosan were isolated from wastewater samples by serial dilution in 0.9% (w/v) saline solution and plating 100 µL of the sample on 0.2% triclosan test agar plates (0.2% TTA; tryptone soy agar plates supplemented with triclosan (2 g/L) as an additional carbon source), followed by incubation at 37 °C for 48 h. The colonies obtained were purified by streaking them onto the nutrient agar plates and slants and kept at 4 °C for further experimental analysis. The process conditions for isolation of bacterial isolate KS2002 are summarized in Table 1.
Optimization of screening medium for effective triclosan degradation
Preparation of starter culture
The bacterial isolates obtained were further investigated for their ability to degrade triclosan. In order to prepare starter cultures of purified bacterial colonies, a loopful culture was inoculated into 25 mL decontaminated nutrient broth and incubated at 37 °C and 120 rpm in a shaker incubator (CLASSIC C24, New Brunswick Scientific Co., Edison, New Jersey, USA) for 16 h.
Optimization of screening medium
Minimal salt medium with yeast (MSMY) was selected as the first growth medium which was supplemented with 0.2% triclosan as a source of nutrient in it. This medium comprised of KH2PO4 (1.7 g/L), (NH4)2SO4 (2.69 g/L), CaCl2 (0.2 g/L), MgSO4 (0.03 g/L), yeast extract (1.6 g/L) and triclosan (2 g/L). Minimal salt medium without yeast extract was the second medium that was used for degradation studies. The composition of this medium was similar to that of the first medium without yeast extract. The third medium used was ammonium mineral salts (AMS) medium supplemented with 0.2% triclosan in it. The ingredients were K2HPO4 (0.7 g/L), KH2PO4 (0.54 g/L), MgSO4.7H2O (1.0 g/L), CaCl2.2H2O (0.2 g/L), FeSO4.7H2O (4 mg/L), NH4Cl (0.5 g/L), ZnSO4.7H2O (100 mcg/L), MnCl2.4H2O (30 mcg/L), H3BO3 (300 mcg/L), CoCl2.6H2O (200 mcg/L), CuCl2.2H2O (10 mcg/L), NiCl2.6H2O (20 mcg/L), Na2MoO4.2H2O (60 mcg/L) and triclosan (2 g/L) (Tastan et al. 2016). Another medium that was used for screening of isolates was M9 minimal salts medium (M9 MSM). The components of this medium were Na2HPO4 (33.9 g/L), KH2PO4 (15 g/L), NH4Cl (5 g/L), NaCl (2.5 g/L) and triclosan (2 g/L) (Meade et al. 2001). Each medium had a pH of 7 adjusted by addition of 1 M sodium hydroxide solution.
The degradation medium was supplemented with 0.2% of additional carbon source along with triclosan in the medium. A total of seven additional carbon sources such as glucose, sucrose, maltose, lactose, fructose, glycerol and starch were investigated in order to study their effect individually on triclosan degradation.
Detection of triclosan degradation percentage
The starter cultures (4%) were inoculated in 50 mL of different screening media prepared in a 250-mL Erlenmeyer flask. The flasks were then incubated under static condition (Simeco Instruments Private Limited, West Bengal, India) at 37 °C. The flasks were periodically removed and centrifuged at 10,000 rpm for 20 min at an interval of 24 h. The supernatant was collected, and its spectrophotometric analysis was carried out at 280 nm using the UV-1800 UV–Visible spectrophotometer (Shimadzu, Japan). Triclosan concentration was determined by comparing the absorbance to a standard curve of triclosan solubilized in 95% (v/v) ethanol. The percentage biodegradation of triclosan was calculated by using Eq. (1) (Tastan et al. 2016).
where C0 is the initial triclosan concentration (mg/L); C1 is the final triclosan concentration (mg/L).
Cell growth of the microorganisms was observed by recording the optical density of the bacterial culture at a wavelength of 600 nm using the UV-1800, UV–Visible spectrophotometer (Shimadzu, Japan) for the determined set of growth conditions. All the experiments were performed in triplicate.
Phenotypic and genotypic characterization of the screened isolate
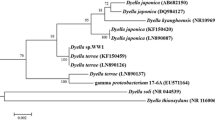
An efficient triclosan degrader was selected and further identified using morphological, biochemical and molecular characterization. Phenotypic characterization of the isolate was done based on its Gram reaction, shape, size and motility. Several biochemical tests were performed such as oxidase test, glucose fermentation test, indole production test, nitrate reduction test, triple sugar iron test, lactose fermentation test, hydrogen sulfide production test, methyl red test, amylase, protease, gelatinase and Voges–Proskauer test. The genus of the screened isolate was identified based on Bergey’s Manual of Determinative Bacteriology (Holt et al. 1994). Species identification of the selected isolate was carried out using 16S rRNA gene sequencing performed at Xcelris Labs, Ahmedabad, India. The alignment of consensus sequences along with the sequence of similar strains was done using Clustal W. The percentage homology in the sequences was determined by BLAST of National Centre for Biotechnology Information (NCBI). The phylogenetic tree was constructed with the use of the Molecular Evolutionary Genetics Analysis (MEGA) software version 6.0 with the help of the neighbor-joining (NJ) method (Tamura et al. 2013).
Detection of enzymes involved in triclosan degradation
Cell-free extract preparation
A spectrophotometric method was employed to detect the activity of catechol 1,2-dioxygenase or catechol 2,3-dioxygenase. Cells of the screened isolates were allowed to grow on 0.2% triclosan and were harvested by centrifugation at 10,000 rpm for 10 min. The pellet was redissolved in phosphate buffer (50 mM; pH 7.0) after treating the pellet twice with the same buffer. Sonication of the cell suspension was carried out using a cell disruptor (Sonics and Materials Inc., USA) providing alternate bursts (22 kHz) of 30 s each for 5 min followed by centrifugation at 15,000 rpm for 20 min at 4 °C. The supernatant collected was further used for enzymatic activity and protein content estimation (Lee et al. 2012).
Detection of Ring cleavage activity
The reaction mixture comprised of 20 mg/L catechol (1.9 mL) and 0.1 mL of the collected supernatant to make a volume of 2 mL. For the detection of the type of ring cleavage (ortho or meta ring cleavage), optical activity of the products cis–cis-muconic acid and 2-hydroxymuconic was determined at 260 nm and 375 nm, respectively. A standard plot of BSA was obtained to estimate the protein content. Enzymatic activity was expressed as a function of micromole of product formed per minute per milligram of protein.
Triclosan degradation mechanism was investigated by addition of 3-fluorocatechol (50 mg/L) in the degradation broth (Toyama et al. 2010). Catechol 2,3-dioxygenase is the enzyme involved in meta-cleavage reactions for which 3-fluorocatechol acts as an inhibitor. Thus, the absence of triclosan degradation in the presence of this inhibitor would, thereby, propound the fact that triclosan degradation advances through a meta-cleavage pathway.
Identification of degradation products
The degraded products formed were concentrated in a rotary vacuum evaporator at 37 °C to dryness after solvent extraction using ethyl acetate. Dried samples were dissolved in 95% ethanol (v/v). The metabolites formed were confirmed using mass spectroscopy (MS Thermo Scientific™ LTQ XL™) and Fourier transform infrared spectroscopy (FTIR). For FTIR characterization, the liquid samples were mixed with finely powdered spectroscopic grade KBr in an agate mortar. The mixture was pressed into pellets applying manual pressure. The FTIR spectra were recorded on Shimadzu’s FTIR Prestige-21 (Japan) in the frequency range 4000–500 cm−1 (Trivedi et al. 2015). Mass spectra were generated in both positive (EI+) and negative (EI−) ionization modes under following conditions: ionization energy, 30 eV; source temperature, 80 °C; and mass range, m/z to 150–400.
Results and Discussion
Isolation and screening of triclosan-degrading strain
A total of seven morphologically distinct colonies were isolated from wastewater samples grown on agar plates supplemented with 0.2% triclosan. Since the plates were supersaturated with triclosan, a zone of clearance (Fig. 1a) was observed around the colonies which expanded over time. The most possible explanation for the appearance of a clearing zone is the conversion triclosan to a more soluble form within the agar medium (Meade et al. 2001). The isolated strains were further investigated in four different types of screening medium (data not shown) in order to establish a suitable medium for degradation studies. An M9 minimal salt medium was found to be the best suited for the degradation of triclosan. The isolates which were found capable of utilizing 2 g/L of triclosan as a sole source of carbon were further examined for triclosan degradation in an M9 minimal salt medium. The bacterial strain designated as KS2002 was found capable of degrading 87.240% ± 0.4 of triclosan at a concentration of 2 g/L within 6 days of incubation (Fig. 1b). The concentration of triclosan kept on reducing with respect to time with an increase in cell density (Fig. 2). Within 6 days of incubation, triclosan was reduced up to a concentration of 25.52 ± 0.4 µg/mL. When fructose and glycerol were used as an additional source in the screening medium, triclosan degradation enhanced up to 99.89% ± 0.3 within 4 days of incubation. It can be concluded that the presence of fructose and glycerol individually as additional carbon source in the culture medium increased the tolerance of organism toward triclosan by acting as a co-substrate readily metabolizable to support cell growth (Lakshmi et al. 2009). The process parameters for effective triclosan removal are listed in Table 2. Each experiment was performed in triplicate, and the values are expressed as a mean of the values with standard deviation.
Characterization of strain KS2002
Among seven morphologically distinct triclosan-degrading bacterial colonies, one isolate (white-mucoid), designated as KS2002, was capable of maximum degradation. Strain KS2002 is a Gram-negative rod-shaped bacterium. Other culture characteristics are listed in Table 3. Based on the information obtained from its 16S rRNA gene sequence, strain KS2002 is a member of the genus Pseudomonas. As shown in Fig. 3, strain KS2002 shows 99% similarity to Pseudomonas aeruginosa strain DSM 50071. The 16S rRNA gene sequence has been submitted to GenBank under the accession number MG561870.
Enzymes involved in triclosan degradation
Batch culture experiments were conducted in a 250-mL Erlenmeyer flask under static conditions to examine the effect of a meta-cleavage inhibitor, 3-fluorocatechol on triclosan biodegradation. As shown in Fig. 4, triclosan degradation terminated after the addition of 3-fluorocatechol, implying that isolate KS2002 might follow a meta-cleavage pathway for triclosan degradation. Moreover, the cell-free extract of isolate KS2002 flourished on triclosan shows catechol 2,3-dioxygenase activity (specific enzyme activity = 0.161 U/mg). On the basis of the above results and in regard to the absence of catechol 1,2-dioxygenase from the cell-free extract of KS2002, it is evident that the meta-cleavage pathway is involved in triclosan degradation by isolate KS2002. Similar results were obtained in case of Sphingopyxis strain KCY1 isolated from wastewater which followed a meta-cleavage pathway for triclosan degradation (Lee et al. 2012).
Identification of triclosan degradation products
During triclosan biodegradation by isolate KS2002, one end product was formed and identified as 2,4-dichlorophenol (2,4-DCP) along with a certain amount of chloride ions released in minimal media. Confirmation of 2,4-dichlorophenol was done using authentic standard. The conditional identification of the end product formed is based on its FTIR spectra, mass spectra and the fragmentation pattern. The FTIR spectra of samples obtained after 24 h of incubation showed similar band vibrations as that of triclosan standard (Fig. 5a). For triclosan, the stretching of the carbon–halogen bond is the main reason behind the strong absorption of halogenated hydrocarbons. In the spectra of aromatic compounds, the most prominent and informative bands fall in a lower frequency range of 900–600 cm−1. The aromatic C-H stretching bands were detected in the range of 3000–2800 cm−1 resulting from out-of-plane bending. The in-plane bending bands of C-H bonds appeared in the range of 1300–1000 cm−1. C–C stretching involving skeletal vibrations is absorbed in the range of 1610–1585 cm−1 and 1500–1400 cm−1. The absorption bands of C–Cl were absorbed in the range of 722–570 cm−1. The band stretching obtained in the range of 1300–1150 cm−1 corresponds to strong CH2 wagging bands for a CH2Cl group (Orhan 2012). 2,4-DCP was detected in samples obtained after 96 h of incubation showing similar band vibrations in FTIR spectra as that of its authentic standard (Fig. 5b). For 2,4-DCP, the C–C aromatic stretching bands were observed in the range of 1400–1300 cm−1 in both the standard and the sample. Both the standard (2,4-DCP) and the sample showed a strong band vibration of the C–Cl band in the frequency range of 700–500 cm−1. A ring stretching vibration was observed at 1597 cm−1 in the standard and at 1635 cm−1 in the sample (Trivedi et al. 2015).
The FTIR spectra were supported by the results obtained using mass spectroscopy. The mass spectrum of triclosan is characterized by a sharp peak at m/z 287 representing its molecular peak. A similar characteristic fragment (m/z 287) was observed in the sample obtained after 24 h of incubation (Fig. 6a). The mass spectrum obtained from the sample collected after 96 h of incubation showed a characteristic fragment of m/z 202 which is similar to that of the peak observed in the standard of 2,4-DCP (Fig. 6b). The sample showed the absence of fragment m/z 287, thereby, confirming bioconversion of triclosan to 2,4-DCP. The conversion of triclosan to 2,4-DCP is very well supported by the literature. A small amount of 2,4-DCP was formed in case of Trametes versicolor as reported by Hundt et al. 2000. During triclosan degradation involving Sphingopyxis strain KCY1, one of the metabolites formed was 2,4-dichlorophenol (Lee et al. 2012). In the case of Aspergillus versicolor, 2,4-DCP was detected as one of the products formed during triclosan conversion (Tastan and Donmez 2014). Based on the results recorded from enzyme activity assays and inhibition of its activity by 3-fluorocatechol, we hereby propose that triclosan degradation by isolate KS2002 is likely to follow a meta-cleavage pathway. Since the organism was not capable of degrading 2,4-DCP any further, thereby suggesting a pathway capable of partial degradation of triclosan up to 2,4-DCP (Fig. 7).
a Mass spectra of triclosan standard (A) and sample (B) obtained from isolate KS2002 after 24 h of incubation. Both show a characteristic peak of m/z 287. b Mass spectra of 2,4-dichlorophenol standard (A) and sample (B) obtained from isolate KS2002 after 96 h of incubation. Both show a characteristic peak of m/z 202
Conclusion
In this study, a triclosan-resistant strain Pseudomonas aeruginosa MG561870 isolated from wastewater showed the ability to convert triclosan within 96 h of incubation. The maximum triclosan biodegradation was achieved as 99.89% ± 0.3 at a much higher concentration of 2 g/L by strain KS2002. The strain was capable of utilizing fructose and glycerol as a co-substrate individually, thereby suggesting that we can employ fruit industry waste to enhance triclosan degradation. Our results also depict the involvement of catechol 2,3-dioxygenase and meta-cleavage in triclosan degradation. To the best of our knowledge, this is the first report on Pseudomonas aeruginosa KS2002 capable of triclosan degradation at such a high concentration. The results suggest that this strain can be used as a potent triclosan degrader in wastewater systems to effectively remove triclosan. Future studies are required to investigate other process parameters that can affect triclosan degradation and the possibility to genetically engineer this strain to reduce triclosan toxicity at a faster rate.
References
Bai X, Acharya K (2017) Algae-mediated removal of selected pharmaceutical and personal care products (PPCPs) from Lake Mead water. Sci Total Environ 581–582:734–740. https://doi.org/10.1016/j.scitotenv.2016.12.192
Balmer ME, Poiger T, Droz C, Romanin K, Bergqvist PA, Muller MD, Buser HR (2004) Occurrence of methyl triclosan, a transformation product of the bactericide triclosan, in fish from various lakes in Switzerland. Environ Sci Technol 38:390–395. https://doi.org/10.1021/es030068p
Cherednichenko G, Zhang R, Bannister RA (2012) Triclosan impairs excitation—contraction coupling and calcium ions dynamics in striated muscle. PNAS 109:14158–14163. https://doi.org/10.1073/pnas.1211314109
Heath RJ, Rubin JR, Holland DR, Zhang E, Snow ME, Rock CO (1999) Mechanism of triclosan inhibition of bacterial fatty acid synthesis. J Biol Chem 274:11110–11114. https://doi.org/10.1074/jbc.274.16.11110
Heath RJ, Su N, Murphy CK, Rock CO (2000) The enoyl-[acyl-carrier-protein] reductases FabI and FabL from Bacillus subtilis. J Biol Chem 275:40128–40133. https://doi.org/10.1074/jbc.M005611200
Holt JG, Kreig NR, Sneath PHA, Staley JT, Williams ST (1994) Bergey’s manual of determinative bacteriology, 9th edn. Williams and Wilkins, Baltimore
Hovander L, Malmberg T, Athanasiadou M, Athanassiadis I, Rahm S, Bergman A, Wehler EK (2002) Identification of hydroxylated PCB metabolites and other phenolic halogenated pollutants in human blood plasma. Arch Environ Contam Toxicol 42:105–117. https://doi.org/10.1007/s002440010298
Hundt K, Martin D, Hammer E, Jonas U, Kindermann MK, Schauer F (2000) Transformation of triclosan by Trametes versicolor and Pycnoporus cinnabarinus. Appl Environ Microbiol 66:4157–4160. https://doi.org/10.1128/AEM.66.9.4157-4160.2000
James MO, Li W, Summerlot DP, Rowland-Faux L, Wood CE (2010) Triclosan is a potent inhibitor of estradiol and estrone sulfonation in sheep placenta. Environ Int 36:942–949. https://doi.org/10.1016/j.envint.2009.02.004
Lakshmi MVVC, Sridevi V, Neharika E, Beena CH, Rao MN, Swamy AVN (2009) Effect of temperature and carbon source on phenol degradation by Pseudomonas aeruginosa (NCIM 2074) and Pseudomonas desmolyticum (NCIM 2028) and their comparison. Int J Chem Sci 7:2591–2601
Lee DG, Zhao F, Rezenom YH, Russell DH, Chu KH (2012) Biodegradation of triclosan by a wastewater microorganism. Water Res 46:4226–4234. https://doi.org/10.1016/j.watres.2012.05.025
Mathew J, Joy NS, Kuppuswamy S (2017) A review on “Triclosan a controversial antibacterial”. Int J Pharm Pharm Res 8:200–216
McMurry LM, Oethinger M, Levy SB (1998) Triclosan targets lipid synthesis. Nature 394:531–532. https://doi.org/10.1038/28970
Meade MJ, Waddell RL, Callahan TM (2001) Soil bacteria Pseudomonas putida and Alcaligenes xylosoxidans subsp. denitrificans inactivate triclosan in liquid and solid substrates. FEMS Microbiol Lett 204:45–48. https://doi.org/10.1111/j.1574-6968.2001.tb10860.x
Miller TR, Heidler J, Chillrud SN, Delaquil A, Ritchie JC, Mihalic JN, Bopp R, Halden RU (2008) Fate of triclosan and evidence for reductive dechlorination of triclocarban in estuarine sediments. Environ Sci Technol 42:4570–4576. https://doi.org/10.1021/es702882g
Mulla SI, Wang H, Sun Q, Hu Anyi YuCP (2016) Characterization of triclosan metabolism in Sphingomonas sp. strain YL-JM2C. Sci Rep 6:21965. https://doi.org/10.1038/srep21965
Olaniyan LWB, Mkwetshana N, Okoh AI (2016) Triclosan in water, implications for human and environmental health. SpringerPlus 5:1639–1656. https://doi.org/10.1186/s40064-016-3287-x
Orhan M (2012) Determination and characterization of triclosan on polyethylene terephthalate fibers. J Text Eng 19:27–30. https://doi.org/10.7216/130075992012198506
Schweizer HP (2001) Triclosan: a widely used biocide and its link to antibiotics. FEMS Microbiol Lett 202:1–7. https://doi.org/10.1111/j.1574-6968.2001.tb10772.x
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tastan BE, Donmez G (2014) Biodegradation of pesticide triclosan by A. versicolor in simulated wastewater and semi-synthetic media. Pest Biochem Physiol 118:33–37. https://doi.org/10.1016/j.pestbp.2014.11.002
Tastan BE, Ozdemir C, Tekinay T (2016) Effects of different culture media on biodegradation of triclosan by Rhodotorula mucilaginosa and Penicillium sp. Water Sci Technol 74:473–481. https://doi.org/10.2166/wst.2016.221
Toyama T, Momotani N, Ogata Y, Miyamori Y, Inoue D, Sei K, Mori K, Kikuchi S, Ike M (2010) Isolation and characterization of 4-tert-butylphenol-utilizing Sphingobium fuliginis strains from Phragmites australis rhizosphere sediment. Appl Environ Microbiol 76:6733–6740. https://doi.org/10.1128/AEM.00258-10
Trivedi MK, Branton A, Trivedi D, Nayak G, Singh R, Jana S (2015) Studies on physicochemical properties of biofield treated 2,4-dichlorophenol. Am J Environ Protect 4:292–299. https://doi.org/10.11648/j.ajep.20150406.15
Wilson BA, Salyers AA (2003) Is the evolution of bacterial pathogens an out-of-body experience? Trends Microbiol 11:347–350. https://doi.org/10.1016/S0966-842X(03)00179-3
Zhao F (2006) Biodegradation of triclosan by a triclosan-degrading isolate and an ammonia oxidizing bacterium. Dissertation, Texas A&M University. http://hdl.handle.net/1969.1/5966
Acknowledgements
The authors are extremely thankful to the Council of Scientific and Industrial Research (Scheme No. 24(0340)/16/EMR-II) for providing financial assistance for the research work. We would also like to acknowledge Department of Bio-Engineering and Central Instrumentation Facility (CIF) at Birla Institute of Technology, Mesra, for providing us with the infrastructure to conduct our research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: M. Abbaspour.
Rights and permissions
About this article
Cite this article
Kumari, R., Ghosh Sachan, S. Bioconversion of toxic micropollutant triclosan to 2,4-dichlorophenol using a wastewater isolate Pseudomonas aeruginosa KS2002. Int. J. Environ. Sci. Technol. 16, 7663–7672 (2019). https://doi.org/10.1007/s13762-018-2129-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-018-2129-5