Abstract
Nowadays, there are several wastewater treatment systems for treating industrial wastewater, but most of them require big areas for the construction, complex operation and high energy, elevating the costs. The goal of this study was to evaluate the effectiveness of the hybrid carrousel reactor, a multi-modular system, for coupling several biological processes in the same reaction unit, in order to remove nitrogenous and carbonaceous compounds from synthetic and industrial wastewater. The industrial wastewater used was coming from the raw influent entering the Wastewater Treatment Plant located in Lerma, Mexico. The hybrid carrousel reactor was of 21.5 L composed mainly of 4 modules: activated sludge module, secondary settler module and two facultative-anaerobic modules. With industrial wastewater, two organic loading rates were evaluated, 611 and 1025 mg CODs/L-d, achieving 60–80% of ammonia removal. The biodegradability index of this kind of industrial wastewater was 0.5, so hybrid bioreactor was indeed effective for removing all the biodegradable organic matter. The kinetic studies showed that the presence of toxic compounds contained in the industrial wastewater strongly inhibited the nitrite-oxidizing step. The experimental results showed that the combination of aerobic, facultative and anaerobic modules was effective for the biotransformation of ammonia to N2 and organic matter to CH4. This novel hybrid bioreactor might be a feasible technology for coupling biological processes in order to treat complex industrial wastewaters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In various parts of the world, and particularly in developing countries, wastewater is discharged directly into water bodies such as rivers, lakes and seas. The wastewater that contains highly concentrated pollutants, including carbon and nitrogen compounds, can deteriorate the quality of aquatic environments and produce eutrophication (Zhao et al. 2013). For this reason is relevant to develop new technologies allowing the complete mineralization or biotransformation of toxic compounds. Chemical compounds that can be readily biodegraded to CO2 (mineralization) and N2 will not cause any harm to the environment (Rücker et al. 2018).
The wastewater is classified into municipal and industrial; the latter is the focus of interest due to the chemical complexity and the presence of toxic compounds that affect the water bodies (Chan et al. 2009). The aggregation of wastewaters of different sectors: foods, textiles, chemicals, among others, has complicated the treatment of this wastewater, becoming a challenge. In order to treat this kind of wastewater, use of bioreactors called “hybrids” has emerged as an alternative technology. Nowadays, there is not a clear definition for hybrid reactor; however, hybrid reactor has been associated with that configuration with connect different modules, in the same unit reaction, that allows the coupling of different biological processes. Other authors are calling hybrid reactors to the configuration that allows free and attached biomass at the same unit reaction. Hybrid reactors combining anaerobic, aerobic and anoxic zones are improving the removal of pollutants and decreasing operational and land costs (Chan et al. 2009). The coupling of biological processes in the same unit reaction allows the simultaneous biotransformation of organic matter and nitrogen compounds into N2, CO2 and CH4; diminishing the pollutants that harm the environment and human being (Chan et al. 2009).
The “carrousel” is a hybrid bioreactor normally used successfully for simultaneous nitrification and denitrification (SND), achieving the nitrogen removal efficiency from 60 to 70% (Liu et al. 2010; Zheng et al. 2015). A novel hybrid biological reactor with suspended and attached-growth biomass was developed by introducing porous materials into a regular activated sludge unit and used for the treatment of domestic wastewater, obtaining COD removal greater than 80% (Jianlong et al. 2000). Banu et al. (2006) in a hybrid bioreactor, which combines the fixed-film and up-flow anaerobic sludge blanket systems, reached COD removal from 67 to 72%. Akizuki and Toda (2018) combined denitrification and methanogenesis in the same unit reaction, achieving 96% of COD removal and 64% of nitrogen removal, with low methane production. Moving bed biofilm reactor and membrane bioreactor (MBBR–MBR) is a hybrid bioreactor in which biofilm grows on plastic carriers; this system has a great potential for the simultaneous removal of organic carbon and nutrients such as phosphorus and nitrogen (Pastorelli et al. 1999). Yang et al. (2009) observed that a biofilm reactor can form aerobic, facultative and anaerobic zones allowing the coupling of nitrification and denitrification. A hybrid sequencing batch reactor (HSBR) was operated for treating dairy wastewater achieving high removal efficiencies for COD and nitrogen, above 90% (Nasr et al. 2014). Hybrid UASF-aerobic bioreactor was used for biodegradation of acid yellow-36 in wastewater, showing the complete removal of the dye at a maximum rate of 100 mg/L-d (Ahmad et al. 2010). In this context can be seen the efficiency and flexibility of hybrid bioreactors for removing simultaneously carbonaceous compounds and nutrients from wastewaters.
Therefore, the goal of this study was to investigate the feasibility of a novel hybrid carrousel bioreactor, which connects aerobic, facultative and anaerobic modules, in the same unit reaction, for the simultaneous removal of COD and nitrogen from industrial wastewater of chemical complexity. The experimental work was carried out in the Wastewater Treatment Laboratory of the Autonomous Metropolitan University-Lerma, Mexico, from 2016 to 2017.
Materials and methods
The hybrid carrousel bioreactor description
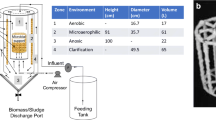
The geometry of the carrousel hybrid reactor is shown in Fig. 1. The module 1 (activated sludge) was designed to carry out nitrification and heterotrophic activity; module 2 is the secondary settler; modules 3 and 4 are facultative/anaerobic systems for carrying out denitrification and methanogenesis, respectively. In Fig. 2 is described the fate of nitrogen and carbon compounds of each module, being the main end products N2 and CH4.
Hybrid carrousel bioreactor setup
A multi-modular bioreactor, called hybrid carrousel bioreactor (HCBR), was used for treating synthetic wastewater and industrial wastewater in a continuous mode. The operational volume of the reactor was 21.5 L, and it was operated at a hydraulic retention time (HRT) of 24 h. The amount of volatile suspended solids (VSS) in the activated sludge module was 3000 mg/L; solids retention time (SRT), 20 days; and oxygen-dissolved concentration, 5.6 ± 0.3 mg/L. Bar diffusers in the bottom of the activated sludge module were used for the aeration, allowing an adequate mixing of the culture medium. The production of N2 and CH4 was measured by liquid displacement using an inverted a 0.1-L glass serum flask filled with saturated NaCl solution (300 g/L).
The HCBR was evaluated with synthetic water at loading rate of 1000 ± 50 mg CODs/L-d (Stages I and II). The synthetic water was composed of (g/L): C6H12O6 (1), NH4+-N (0.075), CaCl2 (0.1), NaHCO3 (1), KH2PO4 (0.35), K2HPO4 (0.27) and 0.5 mg/L of trace elements (Pérez-González et al. 2012). Industrial wastewater was taken from an Industrial Wastewater Treatment Plant (IWTP) located in Mexico, which treats a wastewater that is a mixture of 200 industrial effluents. Two loading rates were evaluated with the industrial wastewater, 611 ± 53 mg CODs/L-d (Stage III) and 1025 ± 67 mg CODs/L-d (Stage IV). The HCBR was inoculated with activated sludge taken from IWTP, and anaerobic modules were inoculated with anaerobic sludge taken from the Wastewater Treatment Plant installed in the “Costeña” Food Company, Mexico. In the influent, pH was adjusted at 7.0 ± 0.2, while in the output experimentally was of 7.38 ± 0.2.
Nitrifying batch cultures
The nitrifying kinetic assays were undertaken in the activated sludge module using synthetic water and industrial wastewater. Batch cultures were spiked with 1 g CODs/L (glucose) and/or 75 mg NH4+-N/L. The batch cultures contained 12 L of liquid medium and were inoculated with activated sludge at 2.85 ± 0.51 g VSS/L. The pH of the liquid medium was controlled at 7.43 ± 0.2 by the addition of sodium bicarbonate. The liquid was continuously aerated in order to provide an oxygenated culture to maintain dissolved oxygen concentration at 6.5 ± 0.2 mg/L. The experimental batch cultures were carried out by duplicate at a temperature of 20 °C. The kinetics of the activated sludge was evaluated through the following response variables: removal efficiencies [\(E_{{{\text{NH}}_{4}^{ + } }} \;{\text{or}}\; E_{\text{COD}} ;\) (mg substrate consumed/mg initial substrate) × 100], nitrate production yield or nitrifying yield (\(Y_{{{\text{NO}}_{3}^{ - } }} ,\) mg NO3−-N produced/mg NH4+-N consumed), nitrite production yield (\(Y_{{{\text{NO}}_{2}^{ - } }} ,\) mg NO2−-N/mg NH4+-N consumed), NH4+-N specific consumption rate \((q_{{{\text{NH}}_{4}^{ + } }} )\), NO3−-N specific production rate \((q_{{{\text{NO}}_{3}^{ - } }} )\) and NO2−-N specific production rate \((q_{{{\text{NO}}_{2}^{ - } }} )\). Average specific rates [q, mg substrate or product/g VSS d] were calculated by using the Gompertz model, using the analysis program Origin 8.0 (OriginLab, Inc. ®), as were previously reported by González-Blanco et al. (2012). The coefficient of determination (R2) was higher than 0.95 for all specific rates calculated.
Analytical methods
Ammonium was analyzed by selective electrode (HANNA HI 4101). Soluble chemical oxygen demand (CODs) was determined by the closed reflux method (Pittwell 1983). Nitrite and nitrate were analyzed by high-performance liquid chromatography (HPLC) (PerkinElmer series 200), using an ion exchange column and a UV detector at 214 nm. N2 and CH4 were measured by gas chromatography with thermal conductivity detector. Temperatures for the column, injector and detector were 50, 100 and 110 °C, respectively. Helium was used as carrier gas at a constant flow rate of 16 ml/min (GOW-MAC 580). The dissolved oxygen concentration was determined by selective electrode (Hanna, HI 98186). The volatile suspended solids (VSS) were determined according to standard methods for the examination of water and wastewater (APHA/AWWA/WEF 2005).
Results and discussion
Bioreactor performance
Continuous hybrid carrousel bioreactor
The hybrid carrousel reactor was operated for more than 250 days in four stages (I, II, III and IV). In Stage I (synthetic medium), organic matter was fed at 1000 ± 50 mg CODs/L-d and nitrogen source at 140 ± 50 mg NH4+-N/L-d. The COD/N value was 100/14 (≈ 7). In terms of CODS removal efficiency, the steady state was reached at day 60 (Fig. 3). The global CODS removal efficiency was 98 ± 2%. One fraction of CODS was removed in the activated sludge module, and the other fraction was removed in the facultative/anaerobic modules. In the headspace of the anaerobic modules, at steady state, N2 and CH4 were detected; this means that denitrification and methanogenesis took place. At the end of the carrousel hybrid bioreactor was not detected neither nitrite nor nitrate, achieving global ammonium removal efficiency of 50 ± 7%. The ammonium removal was low; it might be due to the presence of organic matter, which could be inhibiting the nitrification biological process. For example, Hanaki et al. (1990) observed in a nitrifying reactor that increment of CODs up to 1000 mg/L diminished the ammonium oxidation to nitrite, increasing the ammonium concentration in the effluent.
In the experimental work, a bulking phenomenon was observed; this behavior is associated with the excessive growth of filamentous bacteria. Bulking of activated sludge has become a common problem of many wastewater treatment plants, due to the floating of the sludge (Aonofriesei and Petrosanu 2007). The optimal formation of flocs is essential to settle to the bottom in the secondary clarifier and to be recycled to the activated sludge module (Wanner 1994). Several factors might be stimulating bulking production such as nutrient deficiency, F/M (food/microorganism) ratio, oxygen concentration, aeration basin design. For instance, Peng et al. (2003) showed in an activated sludge system that sludge settled properly at an influent BOD5/N ratio of 100/4 (≈ 25); when the value of BOD5/N was 100/3 (≈ 33), the number of filamentous bacteria began to reduce and simultaneously an excessive growth of viscous Zoogloea with high percentage of moisture was observed and non-filamentous activated sludge bulking occurred. At BOD/N value of 100/2 (≈ 50), the excessive growth of filamentous microorganism could not be observed. These experimental results are pointing out that bulking phenomenon might be controlled limiting the nitrogen source.
In Stage II, the nitrogen loading rate was diminished, being 70 ± 3 mg NH4+-N/L-d. The organic loading rate was 1032 ± 43 mg CODs/L-d, having a CODs/N ratio of 14. The increment of CODs/N ratio reduced the bulking, improving the sludge settling. Nonetheless, global nitrogen and organic matter removal efficiencies did not significantly change regarding the first stage. The ammonium removal efficiency was 52 ± 5%, while the CODs removal efficiency was 96 ± 2%. In this stage, also at the end of the hybrid carrousel bioreactor was not detected neither nitrite nor nitrate.
In the facultative/anaerobic modules was quantified N2 and CH4 production in continuous, being 6 ± 1.5 mg CH4/L-d and 20 ± 2 mg N2/L-d. The low methane production indicated that most of biodegradable organic matter was removed via heterotrophic activity in the activated sludge module, while the other organic fraction was mineralized by denitrifying process. In the following equation is shown the global nitrogen balance:
Approximately 54% of ammonium consumed was used for N2 production in the facultative/anaerobic modules, indicating that the other 46% could have been used for the anabolic pathways, for example, for growth and exopolymers production. The exopolymers production linked to the activated sludge activity is unavoidable. For instance, Sponza (2003) observed in activated sludge systems that protein was the predominant exopolymeric substance followed by polysaccharides and deoxyribonucleic acid (DNA).
In Stage III, HCBR was fed with industrial wastewater of chemical complexity with organic loading rate of 611 ± 35 mg CODs/L-d and 28 ± 12 mg NH4+-N/L-d, having a COD/N ratio of 21. At steady state, global CODs removal efficiency was 51 ± 2%, and global ammonium removal efficiency was 81 ± 8%. The biodegradability index (BOD5/COD) of this industrial wastewater was 0.5; this means that only 50% of the CODs can be removed via biological process. In according to this biodegradability index, HCBR was able to remove all biodegradable organic compounds. The other 50% of CODs is not biodegradable, and it is corresponding to recalcitrant compounds that must be removed via physicochemical processes. In the following equation is shown the nitrogen balance in the activated sludge module:
The nitrogen balance pointed out that ammonium consumed was partially oxidized up to nitrite. This means that the nitrite oxidation step to nitrate could have been inhibited. In the present work, the industrial wastewater fed to the HCBR is chemically complex, so it is possible to find organic matter with high degree of toxicity. For instance, Pérez-Alfaro et al. (2013) studied the effect of 2-chlorophenol in the nitrification biological process; the authors observed that this phenolic compound inhibited the nitrite oxidation step. Regarding the organic matter, in the activated sludge module, CODs removal efficiency was 35%, indicating that two biological processes were carried out in this module: partial nitrification and heterotrophic activity.
In the following equation is now shown the nitrogen balance in the facultative/anaerobic modules:
The nitrogen balance showed that 39 mg NTotal/L-d was fed to facultative/anaerobic modules, and in the effluent was recovery 28.5 mg NTotal/L-d. Twenty-seven percent of nitrogen not recovered in the effluent might have been used for anabolic pathways. The nitrogen balance also showed that nitrite and nitrate reduction was coupled to CODS oxidation, producing as the end product N2. The global CODs removal efficiency was 51%; 35% was removed in the activated sludge module, whereas the other 16% was oxidized in the facultative module. Besides, methane was not detected, suggesting that nitrite or another chemical compound could have inhibited the methanogenesis. For instance, Klüber and Conrad (1998) observed that the addition of nitrite in methanogenic assays did not change the redox potential, but nevertheless inhibited completely the methane production.
In Stage IV, HCBR was fed at organic loading rate of 1025 ± 67 mg CODs/L-d and 46 ± 9 mg NH4+-N/L-d, having a COD/N ratio of 22. At steady state, global CODS removal efficiency was 53 ± 6%. Nonetheless, global ammonium removal efficiency diminished, being 60 ± 7%. In this stage, the biodegradable organic compounds also were removed, according to the biodegradability index (BOD5/COD = 0.5). In the following equation is shown the nitrogen balance in the activated sludge module:
The nitrogen balance indicated that 97% of ammonium consumed was partially oxidized up to nitrite. The increment of CODs loading rate increased the partial nitrification. In this module, CODs removal efficiency via heterotrophic activity was 35%. These experimental results indicated the partial nitrification and heterotrophic activity in the activated sludge module. This is the advantage of aerobic consortium that can be removed simultaneously ammonium and organic carbon; for instance, Pérez-González et al. (2012) observed the simultaneous removal of p-cresol, phenol, p-hydroxybenzoate and ammonium using a nitrifying continuous-flow reactor.
In the following equation now is shown the nitrogen balance in the facultative/anaerobic modules:
The mass balance pointed out that nitrate and nitrite reduction was coupled to CODs oxidation, producing N2 as the end product. The global CODs removal efficiency was 53%; 35% was removed in the activated sludge module, whereas the other 17.65% was oxidized in the facultative module. In this last stage, methane was not detected either, similar behavior was observed in the Stage III, suggesting that nitrite or another chemical compound could have inhibited the methanogenesis biological process.
Nitrifying batch cultures
Nitrifying respiratory activity of an activated sludge collected from the HCBR at steady state was evaluated in the presence of ammonium, ammonium plus CODs and industrial wastewater. Biomass sample was taken from Stages II, III and IV; the biomass taken from Stage II was used to evaluate the nitrifying kinetic, with synthetic water, in the presence of ammonium (S-II (N)) and ammonium plus CODs (S-II (N + C)). On the other hand, activated sludge sample taken from Stages III and IV was used to evaluate the nitrifying respiratory activity with synthetic water in the presence of ammonium (S-III or S-IV (N)) and industrial wastewater (S-III or S-IV (N + C)). The time course of ammonium consumption and nitrate/nitrite production is shown in Fig. 4.
The batch cultures [S-II (N)] showed ammonium consumption profile during all the incubation period (Fig. 4). Ammonia consumption efficiency was 91%, with nitrifying yield of 0.79 ± 0.02 (Table 1). Nitrite is an intermediate compound in the nitrifying biochemical pathway, and it was produced from the beginning, reaching nitrite yield of 0.21 ± 0.01. Ammonia was consumed at specific rate of 84 mg NH4+-N/g VSS d, while nitrate was produced at 88 mg NO3−-N/g VSS d (Table 1). In batch cultures spiked with ammonia plus CODs [S-II (N + C)], ammonia consumption efficiency and nitrifying yield diminished were 76% and 0.62 ± 0.02, respectively. Nitrite was also produced from the beginning, but in this case, nitrite yield increased up to 0.38 ± 0.01. The ammonia consumption specific rate was 72 mg NH4+-N/g VSS d, while nitrate was produced at 75 mg NO3−-N/g VSS d. The presence of organic matter affected the kinetics and metabolism of the nitrification, since specific rates for ammonia and nitrate, as well as the nitrifying yield, diminished regarding the batch cultures in S-II (N) [Table 1]. It is well known that in biological systems fed with ammonia and organic matter, heterotrophs and nitrifiers can compete for the oxidizing source (oxygen), affecting in some cases the catabolism or anabolism of the nitrifying process (Hanaki et al. 1990). In the present work, either metabolism or nitrifying kinetic was affected for the presence of organic matter, suggesting an inhibition phenomenon. Another important factor to consider is that original sludge was taken from industrial wastewater treatment plant, which was operated at HRT of 4 h. For example, Metcalf and Eddy (2014) indicated that HRT suitable for carrying out nitrification and heterotrophic activity must be above 9 h; this is because the growth of nitrifying bacteria is very slow compared to heterotrophic bacteria. The doubling time for different species of Nitrobacter could be longer and varies between 10 and 140 h (Bock et al. 1991). So, at HRT of 4 h, hydraulic conditions are unfavorable for nitrifying growth. In this context and taking account of the physiology behavior observed in the nitrifying activities might be justifying the low ammonium removal efficiency observed in the hybrid carrousel bioreactor, in the activated sludge module.
In the batch cultures [S-III (N)], ammonia was oxidized up to nitrite and nitrate, with consumption efficiency of 90%. Nitrate and nitrite yields were 0.72 ± 0.03 and 0.30 ± 0.01, respectively. Ammonia was consumed at the specific rate of 41 mg NH4+-N/g VSS d, while the nitrate production rate was 31 mg NO3−-N/g VSS d (Fig. 5). The nitrifying metabolism was not significantly affected regarding the S-II (N), since nitrite was the main end product (Table 1). Nonetheless, the nitrifying kinetic was significantly affected, since ammonium consumption specific rate and nitrate specific production rate diminished around 50 and 64%, respectively, regarding the batch cultures in S-II (N). This metabolic and kinetic behavior suggested that the sludge in contact with industrial wastewater provoked a certain damage or structural changes in the nitrifying bacteria. The industrial wastewater treated in the HCBR, in the continuous mode, is chemically complex, since it is a mixture of several industries like food, chemical, pharmaceutical, textile. For instance, phenol is known to damage the cell membrane. However, there are reports of microorganisms that have developed mechanisms to consume high phenol concentrations, modifying the rigidity of the cell membrane (van Schie and Young 2000). On the other hand, Kim et al. (2008) worked with Cokes wastewater, which is one of the most toxic industrial effluents; although the activated sludge process has been acclimated to treat this kind of wastewater, serious inhibitory effects have occasionally upset the nitrification.
Nitrifying cultures were carried out using the industrial wastewater [S-III (N + C)] (Fig. 4). Ammonium was consumed throughout the incubation period, achieving a removal efficiency of 97%. The nitrate production was low, with nitrifying yield of 0.19 ± 0.01. In these environmental conditions, nitrite was the main end product, with the nitrite yield of 0.75 ± 0.01. The ammonium consumption specific rate was 31 mg NH4+-N/g VSS d, while the nitrate production rate was 8.7 mg NO3−-N/g VSS d (Fig. 5). In this study, ammonium removal was higher; however, the main product was nitrite instead of nitrate. The experimental results suggested that the presence of toxic compounds in the industrial wastewater inhibited the nitrite oxidation step to nitrate. These kinetic studies supported the results observed in the hybrid carrousel bioreactor, in the continuous mode, where partial nitrification took place in the activated sludge module, nitrite being the main product of nitrification.
In the batch cultures [S-IV (N)], ammonia was oxidized up to nitrite, mainly, with consumption efficiency of 95% and nitrate was not produced (Table 1). At the end of batch cultures, nitrite yield was of 0.95 ± 0.02, indicating that ammonium consumed was oxidized only to nitrite. Ammonia was consumed at the specific rate of 41 mg NH4+-N/g VSS d, while nitrite was produced at 40 mg NO2−-N/g VSS d (Fig. 5, Table 1). These experimental results showed that the high organic loading rate fed to the HCBR increased the damage on the activated sludge, since kinetics and metabolism of nitrifying activity were altered, because nitrate was not produced. In batch cultures using the industrial wastewater (S-IV (N + C)), the ammonium removal was of 95%, nitrite being the main end product, with the yield of 0.91 ± 0.03. Nitrate also was not produced. The ammonium consumption specific and nitrite specific production rates were similar to the batch cultures in S-IV (N). These kinetic studies showed clearly that the high organic matter concentration and chemical complexity of the industrial wastewater evaluated in the present work inhibited strongly the nitrite oxidation step to nitrate.
Conclusion
The new findings from this study are the following: (1) The novel hybrid carrousel reactor showed the potential capability of coupling several biological processes for the simultaneous removal of organic matter and ammonia, (2) the biodegradation of COD and ammonia was in multi-steps, depending on the environmental conditions given in each module, and (3) chemical composition of the wastewater is influencing the biological processes involved; with synthetic wastewater ammonia and COD were removed via nitrification, heterotrophic activity, denitrification and methanogenesis; however, with industrial wastewater ammonia and COD were removed via partial nitrification, heterotrophic activity and denitrification. Finally, the use of a multi-modular hybrid carrousel reactor might be the feasible technology for treating complex industrial wastewaters.
References
Ahmad R, Mondal PK, Usmani SQ (2010) Hybrid UASFB-aerobic bioreactor for biodegradation of acid yellow-36 in wastewater. Bioresour Technol 101:3787–3790
Akizuki S, Toda T (2018) An anaerobic-aerobic sequential batch process with simultaneous methanogenesis and short-cut denitrification for the treatment of marine biofoulings. Waste Manag 74:168–176
Aonofriesei F, Petrosanu M (2007) Activated sludge bulking episodes and dominant filamentous bacteria at waste water treatment plant Constanţa Sud (Romania). Proc Rom Acad Ser B 9:83–87
APHA, AWWA and WEF (2005) Standard methods for the examination of water and wastewater. American Public Health Association, Alexandria
Banu JR, Kaliappan S, Beck D (2006) Treatment of sago wastewater using hybrid anaerobic reactor. Water Qual Res J 41:56–62
Bock E, Koops HP, Harms H, Ahlers B (1991) In variations in autotrophic life. In: Barton JM (ed) The biochemistry of nitrifying organisms. Academic Press, San Diego, pp 171–200
Chan YJ, Chong MF, Law CL, Hassell DG (2009) A review on anaerobic–aerobic treatment of industrial and municipal wastewater. Chem Eng J 155:1–18
González-Blanco G, Beristain-Cardoso R, Cuervo-López F, Cervantes FJ, Gómez J (2012) Simultaneous oxidation of ammonium and p-cresol linked to nitrite reduction by denitrifying sludge. Bioresour Technol 103:48–55
Hanaki K, Wantawin C, Ohgaki S (1990) Effects of the activity of heterotrophs on nitrification in a suspended-growth reactor. Water Res 24:289–296
Jianlong W, Hanchang S, Yi Q (2000) Wastewater treatment in a hybrid biological reactor (HBR): effect of organic loading rates. Process Biochem 36:297–303
Kim YM, Park D, Lee DS, Park JM (2008) Inhibitory effects of toxic compounds on nitrification process for cokes wastewater treatment. J Hazard Mater 152:915–921
Klüber HD, Conrad R (1998) Effects of nitrate, nitrite, NO and N2O on methanogenesis and other redox processes in anoxic rice field soil. FEMS Microbiol Ecol 25:301–318
Liu Y, Shi H, Xia L, Shi H, Shen T, Wang Z, Wang Y (2010) Study of operational conditions of simultaneous nitrification and denitrification in a Carrousel oxidation ditch for domestic wastewater treatment. Bioresour Technol 101:901–906
Metcalf E, Eddy M (2014) Wastewater engineering: treatment and resource recovery. McGraw-Hill, New York
Nasr M, Elreedy A, Abdel-Kader A, Elbarki W, Moustafa M (2014) Environmental consideration of dairy wastewater treatment using hybrid sequencing batch reactor. Sustain Environ Res 24(6):449–456
Pastorelli G, Canziani R, Pedrazz L, Rozzi A (1999) Phosphorus and nitrogen removal in moving-bed sequencing batch biofilm reactors. Water Sci Technol 40:169–176
Peng Y, Gao C, Wang S, Ozaki M, Takigawa A (2003) Non-filamentous sludge bulking caused by a deficiency of nitrogen in industrial wastewater treatment. Water Sci Technol 47:289–295
Pérez-Alfaro JE, Buitrón G, Gomez J, Texier AC, Cuervo-López FM (2013) Kinetic and physiological evaluation of ammonium and nitrite oxidation processes in presence of 2-chlorophenol. Appl Biochem Biotechnol 169:990–1000
Pérez-González D, Gómez J, Beristain-Cardoso R (2012) Biological removal of p-cresol, phenol, p-hydroxybenzoate and ammonium using a nitrifying continuous-flow reactor. Bioresour Technol 120:194–198
Pittwell L (1983) Standard COD. Chem Br 19:907
Rücker C, Mahmoud WM, Schwartz D, Kümmerer K (2018) Biodegradation tests of mercaptocarboxylic acids, their esters, related divalent sulfur compounds and mercaptans. Environ Sci Pollut Res 25:18393–18411
Sponza DT (2003) Investigation of extracellular polymer substances (EPS) and physicochemical properties of different activated sludge flocs under steady-state conditions. Enzyme Microb Technol 32:375–385
Van Schie PM, Young LY (2000) Biodegradation of phenol: mechanisms and applications. Bioremediat J 4:1–18
Wanner J (1994) Activated sludge: bulking and foaming control. CRC Press, Boca Raton
Yang S, Yang F, Fu Z, Lei R (2009) Comparison between a moving bed membrane bioreactor and a conventional membrane bioreactor on organic carbon and nitrogen removal. Bioresour Technol 100:2369–2374
Zhao YJ, Cheng P, Pei X, Zhang H, Yan C, Wang SB (2013) Performance of hybrid vertical up- and downflow subsurface flow constructed wetlands in treating synthetic high-strength wastewater. Environ Sci Pollut Res 20:4886–4894
Zheng M, Tian Y, Liu T, Ma T, Li L, Ahma M, Chen Q, Ni J (2015) Minimization of nitrous oxide emission in a pilot-scale oxidation ditch: generation, partial variation and microbial interpretation. Bioresour Technol 179:510–517
Acknowledgements
O. Velasco-Garduño received a Ph.D. fellowship from CONACyT-México.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: Hari Pant.
Rights and permissions
About this article
Cite this article
Velasco-Garduño, O., Mendoza-Reséndiz, A., Fajardo-Ortiz, C. et al. Simultaneous ammonia and organic matter removal from industrial wastewater in a continuous novel hybrid carrousel bioreactor. Int. J. Environ. Sci. Technol. 16, 3429–3436 (2019). https://doi.org/10.1007/s13762-018-2017-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-018-2017-z