Abstract
The use of commercial adsorbents for the removal of dye from aqueous environment is expensive. Anthill could serve as alternative and cheap adsorbent in treating coloured effluents. However, this study focused on adsorption of dye from aqueous solution by anthill via batch mode process. The anthill was thermally activated at 900 °C for 2 h and then characterized using various techniques. The 2k factorial experimental design in Design Expert Software version 11 was employed for the optimization of adsorption process variables, which include initial dye concentration, contact time, adsorbent dosage and pH. Experimental data were evaluated using Langmuir and Freundlich models. The main effect analysis showed that adsorbent dosage contributes significantly to the adsorption of CR as much as 38.48%, while initial dye concentration contributes the least to the process as low as 0.00066%. The obtained data revealed that the maximum dye uptake was achieved under the optimized factor combination of adsorbent dosage of 0.5 g, contact time of 120 min, medium pH of 4 and initial CR concentration of 50 mg/L. Equilibrium adsorption isotherm and kinetic analyses revealed that Freundlich isotherm and pseudo-second-order model fitted well to the experimental data. The value of Freundlich exponent (n = 1.11) indicated that the adsorption process was favourable. The work showed that anthill material is a promising adsorbent for removing dyes from aqueous solution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In industries such as textiles, plastics, rubbers, cosmetics and many more, dyes are frequently used to provide colour to their products (Sharma and Janvega 2008). In Nigeria, wastes emanating from these industries pose serious environmental threats due to inability of dye to be decolorized once it is released into the river. However, no adequate measures are put in place to curb indiscriminate disposal of wastewater containing dyes. Many dyes are carcinogenic and usually yield toxic organic compounds when they biodegrade (Arumugam and Saravan 2015). Besides, exposure of aquatic species to dye has been known to be disastrous as it reduces the dissolved oxygen; hence, there is need to adopt a holistic approach in removing dyes from industrial effluent before it is being discharged into the environment.
Many treatment processes have been employed for the removal of dyes from industrial effluents. These treatment techniques include ultrafiltration (Lakdioui et al. 2017), ion exchange membrane (Ran et al. 2017), electrochemical degradation (Morsi et al. 2011), photocatalytic degradation (Alahiane et al. 2017) and adsorption process (Arumugam and Saravan 2015). Among these methods, adsorption of dye onto activated carbon has been most viable technique (Hameed et al. 2009). However, due to the exorbitant cost of commercial activated carbon and other industrially produced adsorbents, present research has geared towards rummage for cheap, eco-friendly and alternative adsorbents.
Anthill is a collection of sand, earth and other materials formed by the group of ants at the entrance of their colony (Henne 2009; Sharma and Sumbali 2013). It is of two types, namely type I and type II. Type I is small in diameter, and its nest is less noticeable in the ant territory, because it is small in size, characterized by waste deposition and easily influenced by erosion (Sharma and Sumbali 2013), while Type II is huge, often sticks together, sometimes surrounded by vegetation and persist for many years (Henne 2009). Researches have proven that anthill possesses different oxides of metal which have been used in their commercial forms for different purposes (Akinwekomi et al. 2012). As also reported by Gupta (2008), anthill is a form of siliceous fire clay which possesses high silica (SiO2) and alumina (Al2O3) contents. However, clay materials have been used as adsorbent in treating water and wastewater. Vimonses et al. (2009) investigated the adsorption of dye from aqueous solution using clay material, while Wang and Wang (2007) also removed dye through adsorption using chitosan/montmorillonite clay composite as an adsorbent. Recently, Mohamed et al. (2016) removed copper(II) ion from textile effluent using clay as an adsorbent. Some of the main reasons for selecting anthill for this study are that of its intrinsic pore structure and its availability. Meanwhile, there is a dearth of literature on the use of anthill as an adsorbent for the removal of dye from aqueous environment.
Thus, present study focused on investigation of adsorption efficacy of anthill as an adsorbent for the removal of Congo red (CR) dye from aqueous environment and various characterization techniques such as X-ray florescence (XRF), Brunauer–Emmett–Teller (BET) surface area measurement, scanning electron microscopy (SEM) and Fourier transform infrared (FTIR) spectroscopy were employed to determine the adsorbent properties. The statistical design of experiments was employed to check the influence of process parameter on the adsorption of CR onto anthill. Equilibrium adsorption isotherms and kinetics studies were also carried out and discussed in details.
This experimental work was conducted at the Department of Chemical and Petroleum Engineering, Afe Babalola University, Ado-Ekiti, Nigeria, between the month of February 2017 and August 2017.
Materials and methods
Adsorbate
The anionic dye used as adsorbate was Congo red (CR) bought from Nizochem Laboratory Limited (Lagos, Nigeria). CR has molecular formula and weight of C32H22N6Na2O6S2 and 696.66 g/mol, respectively. H2SO4 and NaOH were used to adjust the pH of solution. 1000 mg/L of dye solution was prepared by adding 1.0 g of CR in 1000 mL of distilled water, and solutions required for the experimental study were prepared by diluting the CR stock solution to various initial adsorbate concentrations.
Preparation and characterization of adsorbent
The anthill adsorbent was synthesized by subjecting the harvested anthill material to a calcination process. Typically, 150 g of anthill was gently ground into powder with the aid of mortar and pestle and sieved to required particle size of 100–200 µm. The resulting powder was thereafter thermally treated in a muffle furnace at 900 °C for 2 h. The chemical composition of the raw anthill was examined by XRF analysis. The textural properties (surface area and pore sizes) of the thermally activated anthill were evaluated using Quantachrome adsorption analyser (model 11.03, USA) operated at − 196 °C using inert gas (nitrogen) as a carrier gas. The morphological features of the activated anthill before and after adsorption process were examined using a scanning electron microscope (JEOL-JSM 7600F). In order to identify the functional groups on the surface of the prepared anthill adsorbent before and after loading of dye, Fourier transform infrared radiation (FTIR) spectrophotometer (Model IRAffinity-1S, Shimadzu, Japan) was employed.
Experimental design
In this current study, simple 2k factorial design in Design Expert Software version 11 was applied in order to establish the optimum condition for the adsorption of CR onto thermally treated anthill. However, four process parameters, initial CR concentration, contact time, adsorbent dosage and pH were evaluated at two levels (maximum and minimum) with the required responses being the removal percentage and Congo red uptake. The minimum initial CR concentration was 50 mg/L, and the maximum was 300 mg/L. The maximum contact time was 120 min, while the minimum was 30 min. The minimum value for adsorbent dosage was 0.3 g, and the maximum was 0.5 g. The maximum pH was 9.0, while the minimum was 4.0.
Batch adsorption experiments
The apparatus used in the adsorption studies consists of the set of 250-mL conical flasks and a temperature controlled water bath shaker (SearchTech Instrument). An exact amount (0.3 or 0.5 g) of calcined anthill as indicated by the factorial design (Table 3) was charged into 250-mL conical flasks containing 50 mL of CR solution with initial concentration in the range of 50–300 mg/L. The mixtures were thoroughly agitated in the water bath shaker operated at temperature and stirring speed of 35 °C and 150 rpm, respectively. At the end of the equilibrium adsorption, the treated dye solutions were filtered and the concentration of the CR in the filtrate was measured at 495 nm (maximum absorption wavelength) by using a UV–Vis spectrophotometer, UV-1920 Jenway, UK. Each experimental run was conducted according to the experimental design (Table 3). The present dye removal percentage, \(E_{\text{A}}\) (%) and amount of CR dye adsorbed at equilibrium, \(q_{\text{e}}\) (mg/g), were evaluated as follows:
where \(C_{\text{o}}\) and \(C_{\text{e}}\) (mg/L) are the initial CR concentration and concentration at equilibrium, respectively. V (L) is the volume of the dye solution, and m (g) is the mass of calcined anthill.
After the optimum adsorption process variables were obtained, the equilibrium isotherms and kinetics studies were conducted by considering different adsorbate concentrations from 50 to 300 mg/L at fixed contact time, adsorbent dosage and pH. The dye uptake at time t, \(q_{t}\) was thus calculated as follows (Hameed et al. 2009).
Adsorption equilibrium isotherm
Langmuir isotherm
Langmuir isotherm is a model with two parameters which operates on the principle that the maximum adsorption corresponds to a saturated monolayer of adsorbate molecules on the adsorbent surface (Hameed et al. 2009). Langmuir model equation in its linear form is given as:
where \(q_{{\rm max} }\) (mg/g) is the maximum adsorption capacity and b (L/mg) is the Langmuir equilibrium constant.
The favourability of the adsorption process was verified by expressing the Langmuir isotherm in a dimensionless form as written in Eq. (5).
where (RL) is the separation factor and Co (mg/L) the highest initial concentration of adsorbate. The value of RL describes the nature of isotherm either to be unfavourable (RL > 1), favourable (0 < RL < 1), irreversible (RL = 0) or linear (RL = 1).
Freundlich isotherm
The experimental data were further analysed by considering another two-parameter isotherm called Freundlich model. This model is an empirical type that is meant for multilayer and heterogeneous adsorption sites (Yusuff et al. 2017), and it is expressed according to Eq. (6).
where \(k_{\text{F}}\) (mg/g(L/mg)1/n) indicates the adsorption capacity of the adsorbent and n is an adsorption intensity. The linear form of Eq. (6) is given by:
Adsorption kinetics
In order to analyse the kinetic data obtained at different adsorbate concentrations, three adsorption kinetic models, namely pseudo-first-order, pseudo-second-order and intraparticle diffusion models, were applied to evaluate the extent of utilization of the adsorption capacity with respect to contact time between the CR dye (adsorbate) and the calcined anthill (adsorbent). The linearized forms of the pseudo-first-order, pseudo-second-order and intraparticle diffusion models are expressed in Eqs. (8), (9a) and (10), respectively, as follows:
where \(q_{t}\) and \(q_{\text{e}}\) are the amount of CR adsorbed (mg/g) at time t in min and at equilibrium, respectively. \(k_{1}\) (min−1) and \(k_{2}\) (g mg−1 min−1) are the rate constants for pseudo-first-order and pseudo-second-order, respectively. h is the initial sorption rate (mg g−1 min−1). \(k_{\text{D}}\) is the intraparticle diffusion rate constant (mg g−1 min0.5), and C is the intercept.
Results and discussion
Adsorbent characterization
The mineral constituent of raw anthill sample was analysed with the XRF technique. The analysis result is shown in supplementary material (Table SM1), and it showed that the anthill material has silicon oxide (SiO2, 57.9%), aluminium oxide (Al2O3, 13.4%), zirconium oxide (ZrO2, 13.31%) and iron(III) oxide (Fe2O3, 9.78%) as the major contents. This result is in trend with the findings reported by Sharma and Sumbali (2013) for fire clay samples and Akinwekomi et al. (2012) for anthill clay. It was also found to contain rare earth and transition metal oxides like zinc oxide (ZnΟ), tin oxide (SnO2) as well as ornamental oxides such as silver oxide (Ag2O) and gold oxide (Au2O3) (Table SM1), which are hardly detected in natural clay and thus indicates a good discovery.
However, metal oxides such as CaO, TiO2, Fe2O3, Al2O3 and SiO2 have been applied individually and combined form as adsorbents in adsorption process as documented by some researchers (Lim et al. 2015; Fisli et al. 2017). As reflected in the literature, mixed rare earth oxides have been used as adsorbent in removing arsenic from wastewater (Raichur and Panvekar 2002). Therefore, these metal oxides in anthill could discourage the use of commercial metal oxides in making composite adsorbents.
Textural characteristic analysis
The specific surface area, total pore volume and pore diameter of the as-synthesized adsorbent were estimated by Brunauer–Emmett–Teller (BET), t-plot and Dubinin–Radushkevich (DR) techniques, respectively. As shown in Table 1, the analysis result revealed that activated anthill has better textural properties. Most especially, its surface area and total pore volume were obtained as 48.12 m2/g and 0.0227 cm3/g. Thus, these values indicate that there is a better possibility for dye adsorption (Hameed et al. 2009). In addition, the value of BET surface area of thermally activated anthill obtained in this present study could be said to be relatively large when compared with wood shaving bottom ash (21.60 m2/g) (Leechart et al. 2009) and activated rice husk fly ash (39.0 m2/g) (Aworn et al. 2008). Meanwhile, the high BET surface area obtained for activated anthill is due to the removal of volatile components and attached gases which block the available pores for adsorption (Yusuff et al. 2017). This is achieved at higher calcination temperature as affirmed by SEM image of activated anthill (Fig. 1a). The average pore diameter of the activated anthill as can be seen in Table 1 is 12.46 Å. Therefore, this value indicates that the as-synthesized thermally activated anthill can be classified as mesoporous material because its pore diameter is between 2 and 50 Å.
Scanning electron microscope (SEM)
Figure 1a, b displays the SEM images and EDX spectra of the anthill adsorbent before and after loading of dye, respectively. Figure 1a reveals that the activated anthill has undefined surface with different layer of pores through which dye could percolate. However, the SEM image of the anthill adsorbent loaded with dye (Fig. 1b) showed that the adsorbent structure changed and the pores seen on its surface before adsorption were blocked. By comparing EDX result of the as-synthesized adsorbent before and after adsorption, there was increase in compositions of carbon (C) from 4.4 to 7.9 wt% and sodium (Na) from 2.6 to 2.9 wt%, which are the main components of Congo red. This indicates that CR is adsorbed onto the activated anthill. Meanwhile, the decrease in compositions of silicon (Si) and oxygen (O) implies that silica (SiO2) is one of the active ingredients in the adsorbent. This observation was also affirmed by Fisli et al. (2017) who reported that the silica (SiO2) could serve as an adsorbent, while its presence in composites plays a key role in the adsorption process.
Fourier transform infrared spectroscopy (FTIR)
Figure 2 and Table 2 display the FTIR spectra of the prepared adsorbent before and after loading of dye. There were shift in peaks and formation of new peaks after adsorption process. The change observed in the spectra is as a result of involvement of surface functional groups during the adsorption of CR on anthill.
Statistical analysis of experimental data
The removal of CR from aqueous solution by anthill via adsorption process was carried out using a 2k factorial experimental design as shown in Table 3. Sixteen experimental runs were conducted at different levels of process variables studied. It was revealed that run 1 which was conducted at 50 mg/L initial concentration, 120 min contact time, 0.5 g adsorbent dosage and pH of 4 resulted in 98.87% of CR removal. However, the lowest percentage removal of 49.9% was provided by run 4, which was conducted at 50 mg/L initial CR concentration, contact time of 30 min, adsorbent dosage of 0.3 g and pH of 9. These results indicate that in order to obtain highest percentage removal of adsorbate, an acidic solution containing 50 mg/L of CR would be treated with 0.5 g dose of treated anthill for 120 min. More so, next to the run 1 in terms of percentage removal of adsorbate is run 16 which resulted in 90.88% of CR uptake. This run was conducted using 0.5 g dose of anthill to adsorb CR from acidic solution for contact time of 120 min. Comparing runs 1 and 16, it can be concluded that higher adsorbent dosage, higher contact time and acidic solution containing adsorbate regardless its concentration favour CR uptake. The regression model equation which correlates the percentage removal of CR and the process variables (C, t, d and p) studied in terms of coded factor is given in Eq. (11).
where C, t, d and p are the initial CR concentration, contact time, adsorbent dosage and pH, respectively. These are the main effects, while Ct, Cd, Cp, td, tp and dp represent interaction effects.
Analysis of variance (ANOVA) for CR uptake
The fitness of the developed model was examined using ANOVA. Table SM2 presents the results of the ANOVA analysis for 2k factorial design. The model F-value of 95.52 with a probability value (prob > F) of 0.0104 confirms the fitness of the model. Additionally, three of the linear terms (t, d and p) and three of the interactive terms (Ct, Cd and td) are the significant terms of the model. This is because they have p values less than 0.0500. However, the value of correlation coefficient (R2) was found to be 0.9984, which indicates that the model accounts for 99.84% of the total variation in the experimental CR uptake.
Analysis of main effects
The contribution of each of the variables studied to the adsorption of dye by anthill was investigated, and the results are presented in Table SM3 and Fig. SM1. It was revealed that adsorbent dosage contributes the most to the adsorption process as much as 34.476%. Adsorbent dosage has been found to affect adsorption process just as is the case with other process parameters. This can be explained from the experimental results by selecting runs 1 and 2, runs 4 and 9, runs 8 and 10 or runs 3 and 16. For each of these set of runs, it was observed that the maximum CR dye uptake was attained at maximum value of adsorbent dosage (0.5 g) compared to that of minimum dosage value (0.3 g) under the same operating conditions. For instance, runs 8 and 10 exhibited the adsorbent loading effect. It was observed that at run 8 where 0.5 g anthill was used at 300 mg/L initial dye concentration, 120 min contact time and medium pH of 9 a removal efficiency of 73.50% was achieved. At run 10, a removal percentage of 57.91% was achieved at 0.3 g all other variables remained the same as that of run 8, this is as a result of high adsorbent loading effect. Similar effects were also observed among other set of runs earlier stated just as above. This finding revealed that increasing the adsorbent dosage would provide enough adsorption sites for the adsorbate and also increase the contact between the solute and adsorbent.
Contact time is the second factor that favours the adsorption of CR by anthill with percentage contribution of 25.335% as shown in Table SM3 and Fig. SM1. In this present study, two different contact times (30 min and 120 min) were considered and maximum contact time was found to have a positive effect on CR uptake as confirmed in most of the runs. It was observed that at the maximum contact time (120 min), the removal percentages of CR dye were mostly above those recorded at the minimum contact time (30 min). For example, 98.87% of dye was adsorbed after experimental run (run 1) at 120 min, 50 mg/L initial dye concentration, 0.5 g adsorbent dosage and pH of 4. Although, initial concentration of dye was at minimum value and adsorbent dosage was at its maximum, it can be deduced that contact time has a great influence in batch adsorption study since an identical run (run 12) that proceeded at 30 min with other variables being the same as run 1, resulted in a 66.75% removal efficiency which is relatively low. Moreover, it was observed that the maximum loading capacity of anthill was attained at maximum value of contact time than those at minimum. This observation is attributed to the fact that the adsorption capacity of dye on anthill increased with increasing contact time until equilibrium was attained (Chiban et al. 2009).
From both the effect list and Pareto chart shown in Table SM3 and Fig. SM1, respectively, it was found that pH was ranked as third among the factors that contributes significantly to the adsorption of CR as much as 19.709%. Since initial pH of wastewater or aqueous solution has impact on the surface characteristics as well as the degree of ionization of the dye molecules (Sahu 2015), it becomes imperative to investigate the effect of pH on the adsorption. This can be explained from the experimental results (Table 3), where pH 4 and 9 were studied, the lower pH was observed to exhibit positive effect on removal percentage and loading capacity of anthill as confirmed in some of the runs like run 1, 2, 3, 5, 6, 12, 13 and 16. This shows that lower pH enhanced adsorption of CR onto anthill. The same finding was reported for the adsorption of CR onto orange peel (Sahu 2015). The maximum loading capacity of anthill and removal efficiency of CR dye attained at lower pH might be attributed to the interactions between the dye molecules and the active sites on the anthill adsorbent (Chiban et al. 2009). In particular, silica surface is acidic in nature, which can form surface silanol (SiOH) groups when it reacts with water. In this regard, at low pH the silica surface is positively charged and negatively charged at high pH (Mohapatra et al. 2009).
Initial CR concentration contributes the least to the adsorption of dye onto anthill as low as 0.00066% according to the effect list and Pareto chart of effect presented in Table SM3 and Fig. SM1, respectively. It was noticed that CR uptake increased when initial dye concentration increased from 50 to 300 mg/L as shown in Table 3. For example, 19.77 mg/g (run 1) and 109.06 mg/g (run 16) were obtained as CR uptakes when the initial dye concentrations were 50 mg/L and 300 mg/L, respectively, at the same contact time of 120 min, adsorbent dosage of 0.5 g and pH of 4. This finding showed that loading capacity of anthill increased with an increase initial dye concentration and also affirmed the fact that the initial contaminant concentration is one of determining factors in adsorption process. Meanwhile, reverse trend was recorded in case of the dye removal percentage. It decreased from 98.87% (run 1) for low level initial concentration to 90.88% (run 16) for high level initial concentration. Nevertheless, there was no much difference in the removal percentage of dye considering the result obtained for minimum and maximum values of initial dye concentration with other variables being the same as earlier stated. The result obtained herein indicates that there is availability of same adsorption surface sites of anthill for the increasing initial dye concentration that resulted in the saturation of active bonding sites on the adsorbent (Mohamed et al. 2016). However, anthill exhibited a better performance in removing dye from wastewater as it shows high adsorption capacity.
Adsorption equilibrium isotherms
The equilibrium adsorption data analysis was studied by applying the two-parameter adsorption model, namely Langmuir and Freundlich isotherm. The isotherm model that described the experimental data more accurately was selected based on value of correlation coefficient (R2). The closer the R2 value to unity, the more accurate the experimental data are described by the model
The linear plot of \(\frac{{C_{\text{e}} }}{{q_{\text{e}} }}\) against \(C_{\text{e}}\) which can be found in supplementary material (Fig. SM2) provided a slope of \(\left( {\frac{1}{{q_{{\rm max} } }}} \right)\) and \(\left( {\frac{1}{{q_{{\rm max} } b}}} \right)\) as intercept through which the values of \(q_{{\rm max} }\), b and R2 were obtained and presented in Table 5. The R2 value (0.9539) indicates that the experimental data could not be accurately predicted by the Langmuir isotherm. However, the value of RL (0.7227) at 35 °C obtained in this study implied that the adsorption of CR dye onto anthill was favourable.
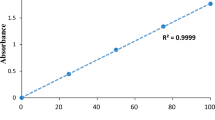
The Freundlich model parameters (\(k_{\text{F}}\) and n) which correspond to intercept and slope of the plot of \(\log q_{\text{e}}\) versus \(\log C_{\text{e}}\) (Fig. SM3) were determined and presented in Table 4. The R2 value (0.9999) is greater than value of R2 obtained in the case of Langmuir isotherm. This indicates that the experimental data are better described by the Freundlich isotherm, thus indicating that anthill would provide multilayer and heterogeneous adsorption sites. A similar result has been reported for removal of CR by zeolite (Vimonses et al. 2009) and adsorption of CR onto bottom ash (Saleh et al. 2012). In addition, Freundlich exponent value (n > 1) is an indication that the adsorption of CR on anthill is favourable.
Meanwhile, the anthill was found to exhibit a very high adsorption capacity of 714.29 mg/g (Table 4). This implies that it could be used as a promising adsorbent for the removal of dye of any kinds from wastewater, especially when compared with previously used adsorbents such as kaolin (5.44 mg/g) (Vimonses et al. 2009), montmorillonite (12.70 mg/g) (Wang and Wang 2007) and Ca-bentonite (107.41 mg/g) (Lian et al. 2009).
Adsorption kinetic
By plotting log(\(q_{\text{e}} - q_{t}\)) against t (Fig. 3), the pseudo-first-order parameters are determined and summarized in Table 5. The R2 values obtained for all the adsorbate concentrations studied are generally lower than 0.950 as shown in Table 5. This indicates that the experimental results were not well fitted to the pseudo-first-order kinetic model. Similar trends were reported by many researchers (Abbas and Trari 2015; Khaniabadi et al. 2017). Therefore, pseudo-second-order model was applied to analyse further the kinetic behaviour of the adsorption of CR onto anthill.
The values of pseudo-second-order model parameters (\(q_{\text{e}}\) and \(k_{2} )\) were evaluated from the plot of \(t/q_{t}\) versus t shown in Fig. 4, and the results are also presented in Table 5. The values of coefficients of correlation (R2) at various adsorbate concentrations were in the range of 0.9973–1.00 and high compared to those of pseudo-first-order model. The obtained results indicate that the adsorption of dye onto anthill could be well predicted by the pseudo-second-order model. Additionally, high linearity of Fig. 4 indicates that pseudo-order kinetic model corresponds to chemisorption as a rate determining step and it can be deduced that the role of the chemisorption in the rate controlling step cannot be ignored (Kamaraj et al. 2013). Furthermore, the rate of adsorption (h) was found to be higher at the initial CR concentration of 50 mg/L as can be seen in Table 5. This result indicates that at the lowest adsorbate concentration, the mass transfer of CR onto the surface of the anthill adsorbent was rapid.
In order to further investigate the kinetic data obtained at different adsorbate concentrations and also evaluate the diffusion mechanism, the intraparticle diffusion model was employed. The plot of \(q_{t}\) versus \(t^{1/2}\) (the plot is not given) gave a straight line at different adsorbate concentrations from which \(k_{\text{D}}\) and R2 were evaluated. The computed values for intraparticle diffusion rate constant, \(k_{\text{D}}\) (mg/g min0.5) and R2 are also presented in Table 5. In this case, the intraparticle diffusion model cannot be regarded as rate-limiting step, because R2 values for intraparticle model are smaller than those of pseudo-second-order kinetic model as can be seen in Table 5. However, the plots (\(q_{t}\) versus \(t^{1/2}\)) were linear and this is an indication that intraparticle diffusion played a key role in the adsorption of dye onto activated anthill. In addition, it is evidence that both surface adsorption and intraparticle diffusion participated in the rate-limiting step (Lim and Lee 2015).
Conclusion
The adsorption capacity of anthill has been investigated and results revealed its potential as a naturally occurring and cheap adsorbent for removing basic dye from aqueous solution. Detailed studies on the effects of adsorption process parameters on CR uptake showed that the adsorbent dosage contributed massively to the adsorption of dye by anthill as much as 38.48%, while the initial dye concentration had the least contribution to the process as low as 0.00066%. Analysis of variance (ANOVA) showed a high coefficient of determination (\(R^{2} = 0.9984\;{\text{and}}\;{\text{Adj}} - R^{2} = 0.9879\)), thus indicating the significance of the model. The studied further showed that the optimized factor combination of 0.5 g adsorbent dosage, 120 min contact time, solution pH of 4 and 50 mg/L initial dye concentration resulted in maximum adsorption of CR. Equilibrium data obtained were well predicted by Freundlich isotherm model with the value of Freundlich exponent (n = 1.11) indicating favourable process. Pseudo-second-order kinetic model was found to be best fitted with the experimental data. Therefore, it can be concluded that thermally activated anthill is effective for the removal of anionic dye from aqueous solution. Meanwhile, on the accord of the highest removal efficiency obtained, calcination of anthill sample at 900 °C for 2 h is assumed as the best preparation conditions and thus should be used for the separation of anionic dye from natural wastewater or industrial effluents.
References
Abbas M, Trari M (2015) Kinetic, equilibrium and thermodynamic study on the removal of Congo red from aqueous solutions by adsorption onto apricot stone. Process Saf Environ Prot 98:424–436
Akinwekomi AD, Omotoyinbo JA, Folorunso D (2012) Effect of high alumina cement on selected foundry properties of anthill clay. Leonardo Electron J Pract Technol 1:37–46
Alahiane S, Sennaoui A, Safr F, Qourzal S, Dinne M, Assabbane A (2017) A study of the photocatalytic degradation of the textile dye reactive yellow 17 in aqueous solution by TiO2-coated non-woven fibres in a batch photoreactor. J Mater Environ Sci 8(10):3556–3563
Arumugam A, Saravanam M (2015) Adsorption of Congo red dye from aqueous solution onto a low-cost natural orange peel and groundnut shell powder. Der Pharmacia Lettre 7(12):332–337
Aworn A, Thiravetyan P, Nakbanpote W (2008) Preparation and characteristics of agricultural waste activated carbon by physical activation having micro- and mesopores. J Anal Appl Pyrolysis 82:279–285
Chiban M, Lehutu G, Sinan F, Carja G (2009) Arsenate removal by Withama frutescens plant from the south-western Morocco. Environ Eng Manag J 8:1377–1383
Fisli A, Krisnanandi YK, Gunlazuardi J (2017) Preparation and characterization of Fe2O3/SiO2/TiO2 composite for methylene blue removal in water. Int J Technol 1:76–84
Gupta OP (2008) Elements of fuels, furnaces, and refractories. Romesh Chander Khana Press, Jabalpur, pp 665–791
Hameed BH, Krishni RR, Sata SA (2009) A novel agricultural waste adsorbent for the removal of cationic dye from aqueous solution. J Hazard Mater 162:305–311
Henne GA (2009) Anthill as a resource for ceramics. Published Ph.D. thesis, Faculty of fine art, Kwame Nkrumah University of Science and Technology, Ghana
Kamaraj R, Ganesan P, Vasudevans S (2013) Removal of lead from aqueous solutions by electrocoagulation: isotherm, kinetics and thermodynamic studies. Int J Environ Sci Technol 12:683–692
Khaniabadi YO, Mohammadi MJ, Shegerd M, Sadeghi S, Saeedi S (2017) Removal of Congo red dye from aqueous solutions by a low-cost adsorbent: activated carbon prepared from Aloe vera leaves shell. Environ Health Eng Manag J 4(1):29–35
Lakdioui T, Essamri A, El Harfi A (2017) Optimization study of ultrafiltration rate of a membrane based on polysulfone modified titanium dioxide on coloured water by indigo. J Mater Environ Sci 8(11):4052–4056
Leechart PW, Nakbanpote W, Thiravetyan P (2009) Application of waste wood-shaving bottom ash for adsorption of azo reactive dye. J Environ Manag 90:912–920
Lian L, Guo I, Guo C (2009) Adsorption of Congo red from aqueous solutions onto Ca-bentonite. J Hazard Mater 16(1):126–131
Lim S, Lee AYW (2015) Kinetic study on removal of heavy metal ions from aqueous solution by using soil. Environ Sci Pollut Res 22(13):10144–10158
Mohamed Z, Abdelkarim A, Ziat K, Mohamed S (2016) Adsorption of Cu(II) onto natural clay. Equilibrium and thermodynamic studies. J Mater Environ Sci 7(2):566–570
Mohapatra M, Khatum S, Anand S (2009) Adsorption behaviour of Pb(II), Cd(II) and Zn(II) on NALCO plant sand. Indian J Chem Technol 16:291–300
Morsi MS, Al-Sarawy AA, Shehab Eldein WA (2011) Electrochemical degradation of some organic dyes by electrochemical oxidation on a Pb/PbO2 electrode. Desalination Water Treat 26:301–308
Raichur AM, Panvekar V (2002) Removal of As(V) by adsorption onto mixed rare earth oxides. Sep Sci Technol 37:1095–1108
Ran J, Wu L, He Y, Yang Z, Wang Y, Jiang C, Ge L, Bakangura E, Xu T (2017) Ion exchange membranes: new developments and application. J Membr Sci 522(15):267–291
Sahu R (2015) Removal of Congo red dye from water using orange peel as an adsorbent. B.Tech. Thesis, National Institute of Technology, Rourkela, India
Saleh SM, Maarof HI, Rahim SNSA, Nasuha N (2012) Adsorption of Congo red onto bottom ash. J Appl Sci 12(11):1181–1185
Sharma J, Janvega B (2008) A study on removal of Congo red dye from the effluents of textile industry using rice husk carbon activated by steam. Rasyan J Chem 1(4):936–942
Sharma V, Sumbali G (2013) An overview of the symbiotic interaction between ants, fungi and other living organisms in ant-hill soil. Int J Environ Sci 4(3):432–443
Vimonses V, Lei S, Jin B, Chow C, Saint C (2009) Kinetic study and equilibrium isotherm analysis of Congo red adsorption by clay materials. Chem Eng J 148(2–3):354–364
Wang L, Wang A (2007) Adsorption characteristics of Congo red onto the Chitosan/montmorillonite nanocomposite. J Hazard Mater 147(3):978–985
Yusuff AS, Olateju II, Ekanem SE (2017) Equilibrium, kinetic and thermodynamic studies of the adsorption of heavy metals from aqueous solution by thermally treated quail eggshell. J Environ Sci Technol 10(5):246–257
Acknowledgments
We authors thank the head, Chemical and Petroleum Engineering Department, Afe Babalola University, Ado-Ekiti, Nigeria, for allowing us to make use of their research facilities. The authors also thank Mr. Paul Attah of the Department of Geology, University of Ibadan for the assistance he rendered in the adsorbent characterization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declared that no potential conflicts of interest exist.
Additional information
Editorial responsibility: Parveen Fatemeh Rupani.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yusuff, A.S., Adesina, O.A. Characterization and adsorption behaviour of anthill for the removal of anionic dye from aqueous solution. Int. J. Environ. Sci. Technol. 16, 3419–3428 (2019). https://doi.org/10.1007/s13762-018-1981-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-018-1981-7