Abstract
Fish diseases are a major obstacle to the development of the fisheries industry. Aeromonas sobria is an infectious waterborne bacterium that causes ulcers, tail rot and hemorrhagic septicemia in fishes and resistant to many existing antibiotics. In this context, A. sobria-AgNPs were synthesized by A. sobria using AgNO3. A. sobria-AgNPs were characterized using UV–Vis spectroscopy, and a peak was obtained at a range of 420–480 nm. A. sobria-AgNPs were evaluated for antibacterial activities against different fish pathogens. The highest antibacterial activity was observed against A. hydrophila, E. cloacae and E. coli. The lower activity was found against C. braakii and E. hermannii, but against H. alvei, P. rettger and M. morganii subsp. sibonii no zone of inhibition was recorded. The results indicated that the A. sobria-AgNPs can be used to develop antibacterial agent and as a therapeutic agent in the fishing industry and water disinfection. The antibacterial efficacy against the fish pathogen A. hydrophila of silver nanoparticles is a hope for possible application as a disinfectant or antimicrobial agent for better fish health management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, there has been an increase in the fishing industry. Fish is a healthy food because it is rich in quality protein and fatty acids (Tacon and Metian 2013). For this reason, fish can provide an important food source for humans. In addition, it also provides added value to livelihoods, job creation, income generation and, most importantly, global food security (Gustafson 2013). With appropriate policies, it is necessary to ensure that both fish farming and aquaculture production are sustainable. Therefore, governments need to manage ecological and social impacts in aquaculture. In this sector, in order to grow healthy fish, it must first be purified from pathogenic microorganisms. Fish diseases are the cause of great economic losses in fish farming. The global economic impact of diseases causes serious economic losses in finfish aquaculture estimated between 0.16 and 4.0 billion USD per year (Kubitza 2005; Martins et al. 2008). Aeromonas are isolated from food, clinical and environmental samples and fish (Beaz-Hidalgo et al. 2013). In this environment, A. sobria virulent strains are a possible source of infection (Janda and Abbott 2010; Rathore et al. 2005). In order to grow and feed healthy fish, it is necessary to remove fish pathogens such as Aeromonas from the environment where fish lives. Since bacteria have developed resistance against antibiotics in recent years, alternative new treatment methods are needed. For this reason, nanotechnology research has gained worldwide momentum in recent years (Glisovic et al. 2017). Metal nanoparticles are increasingly being used for biological and environmental safety. Recently, noble metal nanoparticles (Ag, Zn, Mn) have become the focus of attention with their new applications in biotechnology, catalysis, electronics, environment and optics (Dastafkan et al. 2015; Ahadi et al. 2016). Although chemical and physical approaches from basic methods are used for nanoparticle production, plant extracts and microorganisms have been actively pursued in recent years as an alternative method (Singh and Prasad 2017). Nanoscience and nanotechnology, especially nanomedicine, silver–bacteria interaction, have found application areas. AgNPs can be used as antifungal, antiviral, anti-inflammatory, anti-angiogenic and anticancer agents as a result of biosynthesis with microorganisms (Zhang et al. 2016). They are commonly used in diagnostic and therapeutic products, wound dressings, air and water treatment, paint and food packaging (Li et al. 2014). In addition, silver nanoparticles (AgNPs) are used as alternative antimicrobial agents in antibiotics for the treatment of infections caused by highly resistant bacteria in humans, fish and other animals (Akram et al. 2006). Therefore, antibacterial characteristics of nanoparticle synthesized by environmentally friendly microorganisms known as small nanofactories have been used against the bacterial fish diseases in aquaculture (Raut et al. 2009). Due to the increasing demand for nanoparticles in treatment of fish pathogens, several studies are carried out for the synthesis of nanoparticles from different microorganisms (Whitesides 2003; Fortin and Beveridge 2000). Extracellular biosynthesis of AgNPs from E. coli, S. aureus, P. aeruginosa, Morganella sp. and A. hydrophila was carried out by several researchers (Gurunathan et al. 2009). There is no study of silver nanoparticle synthesis from A. sobria, which is the cause of disease in fishes such as A. hydrophila.
The aim of this work was to study the synthesis and characterization of AgNPs using A. sobria isolated from organs of fish of Sıdıklı Küçükboğaz Dam Lake. Their antibacterial activity was also evaluated against isolated fish pathogens.
Materials and methods
Isolation and identification of bacterial strain
The bacteria in this study were isolated from the organs of Cyprinus carpio L. and Tinca tinca L. collected from Sıdıklı Küçükboğaz Dam Lake. Nutrient agar was plated with fish organs (gill, intestine) and incubated at 37 °C for 72 h. Bacteria were identified using the VITEK 2 system. Thirty-seven isolated bacteria were purified and identified at species level. A. sobria was selected from the bacteria isolated from fishes and investigated in this study.
Synthesis of AgNPs
For AgNPs synthesis, the culture A. sobria was freshly inoculated into nutrient broth and incubated for 72 h on shaker at 120 rpm at 30 °C. The culture of A. sobria was centrifuged at 12,000 rpm for 10 min, and the supernatant was used for the synthesis of AgNPs. The four test tubes, the first containing AgNO3 (Sigma, USA, 99.9% purity) without the supernatant, the second containing only the media and the third and fourth containing the supernatant and AgNO3 solution in 250 ml aliquots at concentrations of 0.5 mM and 1 mM, respectively, were incubated at 30 °C for 18, 24 and 72 h. The absorption spectrum of the sample was recorded on the range of 420–480 nm using a UV-1800 spectrophotometer (Kumar and Mamidara 2011).
UV–Visible spectroscopic analysis
UV–Vis spectrum analysis was performed in the range of 420–480 nm using a UV-1800 spectrophotometer. The reduction of Ag+ ions was measured at different time intervals: 18, 24 and 72 h. AgNPs were visually examined by the color change in the culture medium from light brown to brown, and nanoparticles were confirmed by measuring AgNPs with UV–vis spectrum.
Determination of antibacterial activity
Disk diffusion test
The antibacterial activity of the synthesized AgNPs was assessed by well diffusion method against pathogenic organisms such as Aeromonas veronii, Hafnia alvei, Aeromonas hydrophila, Pseudomonas oleovorans, Citrobacter braakii, Escherichia hermannii, Aeromonas sobria, Providencia rettger, Morganella morganii subsp. sibonii, Enterobacter cloacae and Escherichia coli. Bacterial cultures were also grown in trypticase soy broth, and each strain was cultivated with individual trypticase soy agar using sterile cotton swabs. Then, wells of 6 mm diameter were made on trypticase soy agar plates using gel puncture. For the biosynthesized silver nanoparticle, sterile distilled water was taken as a control group and other 10 wells were loaded with 75 μL of silver nanoparticles of 5 μg/ml, 2.5 μg/ml, 1.25 μg/ml, 0.75 μg/ml, 0.37 μg/ml, 0.187 μg/ml, 0.09 μg/ml, 0.046 μg/ml, 0.023 μg/ml and 0.01 μg/ml concentrations of original nanoparticles solution. After incubation at 37 °C for 48 h, diameter (in mm) of the obvious clear zones was measured.
Minimum inhibitory concentration (MIC) determination
A broth microdilution method was used to determine the toxicity of AgNPs to pathogenic bacteria. Similar concentrations of AgNPs showed antimicrobial activity by agar well diffusion method. Bacterial growth was assessed in the presence and absence of AgNPs.
Statistical analysis
Agar well diffusion and MIC were performed in triplicate, and the results were expressed as means ± the standard deviation of the means. SPSS academic software was used for statistical calculations. P values lower than 0.05 were considered significant.
Results and discussion
Synthesis of AgNPs
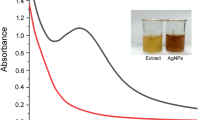
When the A. sobria is exposed to AgNO3, the color turns into dark brown within a few minutes after the reaction started, indicating the formation of AgNPs. The formation of the dark brown color intensity with the incubation time of the reaction was directly proportional. The concrete increase in absorbance with color intensity was revealed by periods of time of up to 72 h, as shown in Fig. 1. The formation of brown color at 30 min in the solution (bacteria with silver nitrate solution) reveals the reduction of silver ions into silver nanoparticles. The maximum color intensity was attained after 24 h. The color change was exhibited due to the oscillation of electron in the silver nanoparticles (Duran et al. 2007).
UV–visible spectroscopic analysis
The AgNPs were characterized by UV–Vis spectrophotometry. The reaction in A. sobria was determined at different times (18, 24 and 72 h). The UV–Vis spectrum is graphically shown in Fig. 1. In the biosynthesis of AgNPs, the nanoparticle production was monitored by color change and then optically measured by UV–Vis spectrophotometer; this analysis showed an absorbance peak shifted from 480 to 420 nm. This band shift shows that the synthesis process of the particles increases when the incubation time increases (Fig. 1). This increase in absorbance due to color intensity can be attributed to the increase in the number of silver nanoparticles over time (Bhainsa and D’Souza 2006). In the biosynthesis of metal nanoparticles, metal ions are completely reduced by bacteria and fungi for 24–124 h (Korbekandi et al. 2009). Together with the intensity of color, the increase in absorbance was revealed with time periods of 72 h. Our work is comparable to other studies.
Antibacterial assay
The effect of AgNPs on bacteria was performed using disk diffusion method. Biologically synthesized silver nanoparticles showed great antibacterial activity against A. sobria. The biosynthesized nanoparticles showed antimicrobial activity against most bacteria at concentrations of 0.37 μg/mL, 0.187 μg/mL and 0.023 μg/mL.
It is observed that the synthesized silver nanoparticles exhibited antibacterial activity against A. sobria by both the well diffusion method and minimal inhibitory concentration (MIC) (Table 1).
The antimicrobial activity of AgNPs was investigated against various bacteria such as A. veronii, H. alvei, A. hydrophila, P. oleovorans, C. braakii. E. hermannii, A. sobria, P. rettger, M. morganii subsp. sibonii, E. cloacae and E. coli using well diffusion method. The highest antibacterial activity was observed against A. veronii, A. hydrophila, P. oleovorans, A. sobria, E. cloacae and E. coli. The lower activity was found against C. braakii and E. hermannii, but against H. alvei, P. rettger and M. morganii subsp. sibonii no zone of inhibition was recorded. The mean of three replicates of the diameter of inhibition zones (in millimeters) around each well with AgNPs solution is represented in Table 1.
Shaffiey et al. (2014) reported that synthesized AgNPs exhibited moderate activity toward A. hydrophila. In one study, AgNPs were reported to exhibit good antibacterial activity against E. coli and B. subtilis (Anandalakshmi et al. 2016). Because phytosynthesized AgNPs are entirely of natural origin, they can be administered as an alternative to antibiotics and biocides and therapeutic agents against A. hydrophila induced diseases in aquatic animals (Mahanty et al. 2013). The antibacterial efficacy against the fish pathogen A. hydrophila of silver nanoparticles is a hope for possible application as a disinfectant or antimicrobial agent for better fish health management (Sarkar et al. 2012). All test bacteria (about 108 cells/mL) were produced for 24 h at AgNPs concentrations (5 μg/mL). The MIC values of AgNPs are presented in Table 1. The results show the effectiveness of AgNPs against A. sobria with low MIC (0.023 μg/ml). The biosynthesized nanoparticles showed antimicrobial activity against most bacteria at concentrations of 0.37 μg/mL, 0.187 μg/mL and 0.023 μg/mL. Similarly, Haytham (2015) examined the MIC of B. subtilis, S. aureus, P. aeruginosa and E. coli against the silver nanoparticle at different concentrations (6.8, 5.1, 1.70 and 3.4 mg/ml). For the first time, this study shows the synthesis of silver nanoparticles and antibacterial activity against fish pathogens using A. sobria-AgNPs at different time intervals (18 h, 24 h and 72 h) with UV spectrum.
Conclusion
AgNPs presented the best antimicrobial activity test against A. veronii, A. hydrophila, P. oleovorans, A. sobria E. cloacae and E. coli. AgNPs can be used as an alternative to antibiotics in fisheries and other aquaculture production. For this reason, further in vivo studies are needed.
References
Ahadi M, Tehrani SM, Azar PA, Husain SW (2016) Novel preparation of sensitized ZnS nanoparticles and its use in photocatalytic degradation of tetracycline. Int J Environ Sci Technol 13(12):2797–2804
Akram FE, El-Tayeb T, Abou-Aisha K, El-Aziz A (2016) A combination of silver nanoparticles and visible blue light enhances the antibacterial efficacy of ineffective antibiotics against methicillin-resistant Staphylococcus aureus (MRSA). Ann Clin Microbiol Antimicrob 15(48):1–13
Anandalakshmi K, Venugoba J, Ramasamy V (2016) Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl Nanosci 6(3):399–408
Beaz-Hidalgo R, Martinez-Murcia A, Figueras MJR et al (2013) Reclassification of Aeromonas hydrophila subsp. Dhakensis Huys et al. 2002 and Aeromonas aquariorum Martínez-Murcia et al. 2008 as Aeromonas dhakensis sp. nov. comb nov. and emendation of the species Aeromonas hydrophila. Syst Appl Microbiol 36:171–176
Bhainsa KC, D’Souza SF (2006) Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf B 47:160–164
Dastafkan K, Sadeghi M, Obeydavi A (2015) Manganese dioxide nanoparticles-silver-Y zeolite as a nanocomposite catalyst for the decontamination reactions of O, S-diethyl methyl phosphonothiolate. Int J Environ Sci Technol 12(3):905–918
Duran N, Marcato PD, De Souza GIH, Alves OL, Esposito E (2007) Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment. J Biomed Nanotechnol 3(2):203–208
Fortin D, Beveridge TJ (2000). Biomineralization: from biology to biotechnology and medical application. In: Baeuerlein E (ed).Wiley-VCH, Weinheim
Glisovic S, Pesic D, Stojiljkovic E, Golubovic T, Krstic D, Prascevic M, Jankovic Z (2017) Emerging technologies and safety concerns: a condensed review of environmental life cycle risks in the nano-world. Int J Environ Sci Technol 14(10):2301–2320
Gurunathan S, Kalishwaralal K, Vaidyanathan R, Venkataraman D, Pandian SR, Muniyandi J, Hariharan N, Eom SH (2009) Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf B Biointerfaces 74:328–335
Gustafson DJ (2013) Rising food costs & global food security: key issues & relevance for India. Indian J Med Res 138(3):398–410
Haytham MMI (2015) Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J Rad Res Appl Sci 8:265–275
Janda JM, Abbott SL (2010) The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin Microbiol Rev 23(1):35–73
Korbekandi H, Iravani S, Abbasi S (2009) Production of nanoparticles using organisms. Crit Rev Biotechnol 29:279–306
Kubitza F (2005) Antecipando-se as doenc¸as na tilapicultura. PanorAquicultura 15:15–23
Kumar CG, Mamidyala SK (2011) Extracellular synthesis of silver nanoparticles using culture supernatant of Pseudomonas aeruginosa. Colloids Surf B Biointerfaces 84(2):462–466
Li CY, Zhang YJ, Wang M, Zhang Y, Chen G, Li L, Wu D, Wang Q (2014) In vivo real-time visualization of tissue blood flow and angiogenesis using Ag2S quantum dots in the NIR-II window. Biomaterials 35(1):393–400
Mahanty A, Mishra S, Bosu R, Maurya UK, Netam SP, Sarkar B (2013) Phytoextracts-synthesized silver nanoparticles inhibit bacterial fish pathogen Aeromonas hydrophila. Indian J Microbiol 53(4):438–446
Martins ML, Miyazaki DMY, Mourıño JLP (2008) Aeromonas caviae durante surto de mortalidade em tilápia do Nilo e suplementaçao com vitamina C na dieta. Bol Inst Pesca 34:585–590
Rathore G, Swaminathan TR, Abidi R, Mahanta PC, Kapoor D (2005) Isolation and characterization of motile aeromonads from aquatic environment. Indian J Fish 52(2):241–248
Raut R, Lakkakula J, Kolekar N, Mendhulkar V, Kashid S (2009) Phytosynthesis of silver nanoparticle using Gliricidia sepium (Jacq.). Curr Nanosci 5:117–122
Sarkar B, Mahanty A, Netam SP, Mishra S, Pradhan N, Samanta M (2012) Inhibitory role of silver nanoparticles against important fish pathogen Aeromonas hydrophila. Int J Nanomater Biostruct 2(4):70–74
Shaffiey SF, Shaffiey SR, Ahmadi M, Azari F (2014) Synthesis and evaluation of bactericidal properties of CuO nanoparticles against Aeromonas hydrophila. Nanomed J 1(3):199–205
Singh AS, Prasad M (2017) Nanotechnology and its role in agro-ecosystem: a strategic perspective. Int J Environ Sci Technol 14(10):2277–2300
Tacon AGJ, Metian M (2013) Fish matters: importance of aquatic foods in human nutrition and global food supply. Rev Fish Sci 21(1):22–38
Whitesides GM (2003) Th e ‘right’ size in nanobiotechnology. Nat Biotechnol 21:1161–1165
Zhang XF, Liu ZG, Shen W, Gurunathan S (2016) Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci 17(9):1–34
Acknowledgements
This work was supported by the Ahi Evran University Scientific Research Projects Coordination Unit. Project Number FEF.E2.17.038.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Dr. Iskender Akkurt.
Rights and permissions
About this article
Cite this article
Erdem, B., Dayangaç, A., Kıray, E. et al. Biosynthesis of silver nanoparticles from Aeromonas sobria and antibacterial activity against fish pathogens. Int. J. Environ. Sci. Technol. 16, 5125–5130 (2019). https://doi.org/10.1007/s13762-018-1944-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-018-1944-z