Abstract
In this paper, dairy wastewater treatment was investigated by Fenton and electro-Fenton (EF) processes in respect of removal efficiencies of chemical oxygen demand (COD), orthophosphate, suspended solid (SS), and color. The response surface methodology (RSM) approach using Box–Behnken design was carried out to develop mathematical model and to optimize process parameters. Experimental data were analyzed by the analysis of variance (ANOVA) to identify the interaction mechanism between the process variables and the dependent variables. According to ANOVA results of Fenton process, COD removal increased with an increase in H2O2/COD ratio and reaction time but decreased with increased H2O2/Fe2+ ratio and initial pH. Opposing to that, in the EF process, COD removal increased with an increase in H2O2/Fe2+ ratio and reaction time but decreased with an increase in H2O2/COD ratio and initial pH. The COD removal efficiencies were 65.5 and 72% under the optimum conditions for Fenton (H2O2/COD ratio 1.9, H2O2/Fe2+ ratio 5, pH 4 and reaction time 10 min) and electro-Fenton (H2O2/COD ratio 2, current density 32 mA/cm2, pH 2.4 and reaction time 45 min) processes, respectively. No significant removal differences for orthophosphate, SS and color were determined between the two processes because the removal efficiencies were over the 88% for each process configuration where P value was greater than 5.6 * 10−5 with 99% confidence level and greater than 1.7 * 10−3 with 95% confidence level for all responses for Fenton and EF processes, respectively).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dairy industries, similar to other agro-industries, generate enormous volume of wastewater up to 0.2–10 L per liter of processed milk (Vourch et al. 2008) primarily from the cleaning and washing operations in the milk-processing plants (Ramasamy et al. 2004), and the wastewater has been characterized by its non-stable pH characteristic due to the use of acid and alkaline cleaners and sanitizers in dairy industry (Baskaran et al. 2000), high COD, biochemical oxygen demand (BOD), and nutrients such as nitrogen, phosphorus, and potassium concentrations (Ayhan Şengin and Özacar 2006; Banu et al. 2008), and high levels of dissolved or suspended solids including fats, oils, and grease (Farizoglu and Uzuner 2011; Praneeth et al. 2014). Uncontrolled discharge of dairy wastewater along with high organic matter and nutrients causes serious pollution problems in water bodies and biodiversity such as algae and bacteria growth, resulting in oxygen depletion and, eventually, suffocating the rivers leading to the gradual disappearance of fish. Therefore, the need to treat highly polluted dairy effluents by various processes is indispensable (Perle et al. 1995; Banu et al. 2008; Deshpande et al. 2012).
The dairy wastewater can be treated using biological and physicochemical methods. Conventional anaerobic treatment requires high energy for aeration (Wheatley 1990), and aerobic treatment needs additional treatment to achieve discharge limits that are often used for treating such wastewater (Ayhan Şengin and Özacar 2006; Banu et al. 2008; Kushwaha et al. 2010a; Karadag et al. 2015; Dabrowski et al. 2017). On the other hand, physicochemical processes, such as coagulation–flocculation (Kushwaha et al. 2010a; Loloei et al. 2014), electrochemical treatment (Ayhan Şengin and Özacar 2006; Tchamango et al. 2010; Kushwaha et al. 2010b; Bazrafshan et al. 2013), nanofiltration (Turan 2004; Luo et al. 2012; Andrade et al. 2014; Chen et al. 2018), and reverse osmosis (Turan 2004; Balannec et al. 2005; Vourch et al. 2008), are used for removal of colloidals and SS in dairy wastewater. Fenton and EF processes are also alternative physicochemical treatment processes to treat dairy wastewater (Yavuz et al. 2011; Davarnejad and Nikseresht 2016).
In Fenton process, hydrogen peroxide is catalyzed by ferrous ions to produce hydroxyl radicals (OH·) where the OH· is involved in the breakdown of organic matters in the wastewater (Fenton 1896; Zhang et al. 2006; Bautista et al. 2008). Oxidation reactions initiated by hydroxyl radical lead to the ultimate decomposition of organic molecules into CO2 and H2O, which makes these processes “environmental-friendly” processes (Weast 1969; Ayoub et al. 2010; Cheng et al. 2016). The EF process is an indirect electrochemical oxidation that employs OH radical generated by the Fenton reaction to oxidize organic compounds. The process is based on the electrochemical in situ production of the Fenton’s reagent, either or both of H2O2 and Fe2+ (Oturan et al. 2000). The EF increases the degradation of organic matters in a highly strong wastewater (Qiang et al. 2003; Chang et al. 2004; Davarnejad and Nikseresht 2016). However, there are only a few studies which investigated the treatment of dairy wastewater using Fenton and EF processes so far. Yavuz et al. (2011) investigated treatment of dairy wastewater by electro-coagulation (EC) and EF processes and succeeded 79.2% COD removal at optimum conditions. On the other hand, Davarnejad and Nikseresht (2016) treated dairy wastewater by EF process results with 93.9% COD and 97.3% color removal efficiencies.
The present study investigates dairy wastewater treatment by Fenton and EF processes and aims: (1) to develop mathematical model and to optimize operating conditions on COD, orthophosphate, color and SS removal, (2) to evaluate the effects and interactions of process variables: H2O2/COD ratio, H2O2/Fe2+ ratio, initial pH, reaction time for Fenton process and H2O2/COD ratio, current density, initial pH, and reaction time for EF process. Optimizations of Fenton and EF processes were carried out by RSM approach using BBD to develop a mathematical model and to study the interactive effects of studied parameters.
Materials and methods
Dairy wastewater
The dairy wastewater was taken from wastewater treatment plant of a dairy factory in Istanbul where milk, yogurt, and butter are the main products. The characterization of raw dairy wastewater is given in Table 1.
Samples were stored in containers and kept at 4 °C until Fenton and EF applications. All dairy wastewater samples were preserved and analyzed according to the standard methods (APHA 2005).
Experimental setup and procedure
The schematic view of Fenton and EF systems are presented in Fig. 1. In Fenton oxidation process, 35% H2O2 solution with a density of 1.13 kg/L and 10 g/L stock solution of Fe2+ by dissolving FeSO4∙7H2O in pure water were prepared, and 500 mL of wastewater was used for each experimental test. In the first step of Fenton oxidation process, pH of dairy wastewater was adjusted to the desired value by addition of 6 N H2SO4 or 6 N NaOH. The necessary amount of the FeSO4∙7H2O was supplemented from the stock solution, and then desired volume of H2O2 solutions was added to initiate Fenton reaction. After this step, jar test apparatus was used for a rapid mix at 200 rpm for 5 min, and then samples were gently stirred at 20 rpm for a desired amount of reaction time. To improve sludge settling rates, pH was adjusted to 7.0 and around by adding 6 N NaOH solution, leading to the precipitation of residual Fe2+ ions. Then, samples were settled for 60 min in a graduated settling column, about 200 mL of supernatant was collected, and pH of supernatant samples was adjusted to 10 and mixed at 70 °C for 10 min to eliminate residual H2O2 to prevent any interference during COD measurements (Erkan and Apaydin 2015; Gotvajn et al. 2011). COD, orthophosphate, SS, and color were analyzed in final supernatant samples by only using analytical grade chemicals.
For EF process, a laboratory-scale plexiglass reactor with 9 cm diameter and 13 cm height was manufactured. One anode and one cathode iron electrodes (comprised of two monopolar (MP) plates) with 6 cm width × 11.5 cm height, 0.1 cm thickness, and 46.2 cm2 effective area were placed 2 cm apart from each other. A valve was installed at the bottom of the reactor to discharge the precipitated material through a sludge chamber. For each test, 500-mL wastewater sample was used. Before each run, electrodes were washed with acetone and the impurities on the aluminum electrode surfaces were removed by dipping in a solution freshly prepared by mixing 100 cm3 35% HCl solution and 200 cm3 2.8% hexamethylenetetramine aqueous solution for 5 min (Gengec et al. 2012). The EF experiments were initiated by supplying a current density between 4 and 32 mA/cm2 by a DC power supply. At the end of each run, the floated and precipitated materials were collected, and the clarified effluent sample was pipetted out from the reactor and then allowed to settle for a few hours in a polyethylene flask. Finally, the clarified supernatant liquid was collected and preserved according to the Standard Methods (APHA 2005) and stored for characterization. All analyses were performed in accordance to the Standard Methods (APHA 2005). All chemicals used were analytical reagent grade.
Design of experiments and data analysis
In this study, the BBD based on RSM was used to design the set of experiments for Fenton and EF processes. RSM is fundamentally a particular set of mathematical and statistical methods for designing experiments, buildings models, determining the effect of variables, and investigating optimum operating conditions (Körbahti 2007). Statgraphics Centurion XVI.I software program was used for the statistical design of experiments and data analysis. The four operational parameters: H2O2/COD ratio (X1), H2O2/Fe2+ ratio (X2), initial wastewater pH (X3), and reaction time (X4) were optimized for Fenton process, whereas H2O2/COD ratio (X1), current density (X2), initial wastewater pH (X3), and reaction time (X4) were optimized for EF process in dairy wastewater treatment. Each independent factor was coded at three levels in the range of − 1 and + 1 determined by preliminary experiments (Table 2).
RSM makes possible to represent independent process parameters in quantitative form as:
where Y is the response (dependent parameter), f is the response function, ε is the experimental error, and X1, X2, X3,…, Xn are independent variables. In the optimization process, the responses can be related to independent factors by linear or quadratic models. A quadratic model which includes the linear model is given in Eq. (2).
where β is set of regression coefficients: the intercept (β0), linear (β1, β2, β3), interaction (β12, β13, β23), and quadratic coefficients (β11, β22, β33). The experiment sets are presented in Table 3 for Fenton and EF processes.
Analysis of variance (ANOVA) was used to obtain the interaction between the independent variables and the responses. The quality of the fit polynomial model was evaluated by R2, and its statistical significance was checked by the Fisher F test in the same program. Model terms were utilized by the P value (probability) with 95% confidence level.
Results and discussion
Statistical analysis of Fenton and EF processes
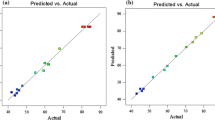
The Fenton and EF batch experiments were designed using RSM in order to understand the effect of the variables (H2O2/COD ratio, H2O2/Fe2+ ratio or current density, pH and reaction time) on four important process responses (COD, orthophosphate, color, and SS). All the factors and responses are shown in Tables 2 and 3. The experimental results for all responses analyzed by ANOVA are summarized in Table 4. As can be seen in Table 4, R2 values were over 91% indicating high coefficient of determination for actual and predicted values for COD, orthophosphate, SS, and color removal. For the Fenton process, F values of 10.91, 10.91, 12.06, and 20.52 implied significant models for COD, orthophosphate, SS, and color removal efficiencies, respectively. On the other hand, F values of COD, orthophosphate, SS, and color removal efficiencies were found 24.98, 9.31, 9.76, and 11.17 for EF process, respectively. The large F value represents a high significance of the corresponding term. The values of Prob. > F less than 0.05 imply that the model terms are significant, whereas the values greater than 0.1 indicate that the model terms are insignificant (Körbahti and Rauf 2008; Arslan-Alaton et al. 2009). Prob. > F values less than 0.0001 indicates that the terms are highly significant in all the models except orthophosphate removal for EF process.
The approximating functions for Fenton and EF processes are presented in Eqs. 3–10. The first-order terms (X1, X2, X3 or X4) represent the effects of the linear main factor; the interaction effects terms (X1X2, X1X3, X1X4, X2X3, X2X4 or X3X4) and the second-order terms (X 21 , X 22 , X 23 or X 24 ) represent the interaction between the two factors and the quadratic effects in these equations, respectively. The positive sign in front of the coefficients indicates a synergistic effect, whereas the negative sign indicates an antagonistic effect (Kim 2016).
On the basis of the coefficients in Eq. (3), it can be said that COD removal efficiencies increase with an increase in H2O2/COD ratio and reaction time, but the COD removals decrease with increased H2O2/Fe2+ ratio and initial pH values in Fenton process. In the EF process (Eq. 7), the COD removal efficiencies increase with an increase in H2O2/Fe2+ ratio and reaction time but decrease with an increase in current density and initial pH. On the other hand, orthophosphate removal efficiencies in Fenton process increase with an increase in H2O2/COD ratio, H2O2/Fe2+ ratio, and initial pH, whereas the removals decrease with an increase in reaction time (Eq. 4). In the EF process, the orthophosphate removals increase with current density, initial pH, and the reaction time (Eq. 8). Color removal efficiencies increase with increase in H2O2/COD ratio, initial pH, and the reaction time in the Fenton process (Eq. 5). H2O2/Fe2+ ratio has a negative effect on color removal efficiencies in both processes.
ANOVA results and response surface plots for responses
The ANOVA tables obtained from the response surface quadratic models for COD removal with both process are shown in Table 5. As can be seen from Table 5, the comparison of the ANOVA results showed that there are highly significant interaction (P < 0.0001) between X1 (H2O2/COD ratio) and X2 (H2O2/Fe2+ ratio) within the experimental range for COD removal in Fenton process. On the other hand, X2 (current density) and X4 (time) have highly significant effect for COD removal in EF process.
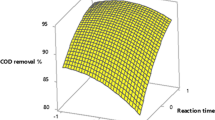
Response surface graphs were produced by varying two of the process variables within the experimental range while holding the other factors at their central values to visualize the effect of the response variables on the dependent ones. The response surface graphs for COD removal efficiencies are presented in Figs. 2 and 3. As can be seen from Fig. 2a, a slight increase in COD removal was observed with an increase in the H2O2/COD ratio at low H2O2/Fe2+ ratio in Fenton process. As an operational approach, low H2O2/Fe2+ ratio is suggested. It is remarkable that the influence of X1 and X2 were very significant. Figure 2b shows that the influence of H2O2/COD ratio and pH on COD removal efficiency was significant, and it was observed that high COD removal efficiencies were obtained at the low H2O2/Fe2+ ratio with an increase in pH. As higher pH values results with lower chemical consumption for pH adjustment, it can be said that pH about 4 could act more acceptable and much suitable. The main effective parameters were H2O2/COD ratio, H2O2/Fe2+ ratio, and pH. As it could be observed, time was not significant on any experimental set of Fenton process. In EF process, maximum COD removal efficiency was obtained high current density and long reaction time at low H2O2/COD ratio (Fig. 3a). The effect of pH was found similar to Fenton process. H2O2/COD ratio, current density, and time were found the main operating parameter in EF process. COD removal efficiencies varied between 23.9–56.6 and 32.6–67.0% for Fenton and EF processes, respectively.
The ANOVA results for orthophosphate removal are given in Table 6 for Fenton and EF processes. In terms of orthophosphate removal, the X2X3, X3X4 have highly significant effects on Fenton process (P < 0.0001), whereas the only X4 has highly significant effect on orthophosphate removal in EF process (P < 0.0001).
Figures 4 and 5 show the effects of dependent variables on orthophosphate removal efficiencies. As seen from Fig. 4, H2O2/COD ratio was kept on a central value at high initial pH. On the other hand, orthophosphate removal efficiencies increased with an increase in reaction time, and H2O2/Fe2+ ratio did not have a remarkable influence on orthophosphate removal. In EF process, orthophosphate removal efficiencies increased with an increase of current density, pH and time, whereas the influence of H2O2/COD ratio was not significant, and it should be kept central point. Orthophosphate removal efficiencies were found higher than 94% for both processes.
For color removals, the ANOVA results showed that the X1X3, X1X4, and X3X4 have highly significant effects on color removal in Fenton process (Table 7), whereas X1 and X 21 have highly significant effects on color removal in EF process.
The effect of operational parameters on color removal is shown in Figs. 6 and 7 for Fenton and EF processes, respectively. From Fig. 6, maximum color removal efficiency could be obtained at high H2O2/COD ratio and H2O2/Fe2+ ratio for Fenton process. The pH value could hold central value. The effect of time was not considerable after a specific time, and there was no need for longer experimental time. In EF process, best color removal efficiency could be seen for central point of H2O2/COD ratio and low pH value. Current density and reaction time were not remarkably effective on color removal.
SS removal is one of the most important parameters for chemical and electrochemical treatment. For this reason, SS removal efficiencies were modeled for each experimental run. As seen from the ANOVA result in Table 8, X 21 and X1X4 have high significant effect on SS removal efficiencies for the Fenton process (P < 0.0001), whereas X 22 , X 23 , and X 24 have high significant effect on the SS for EF process.
The effects of variables on SS removal efficiencies are given in Figs. 8 and 9.
Process optimization
The optimum operating conditions obtained from ANOVA tests are presented in Table 9. Under the optimum conditions, the optimum COD removal efficiencies of model prediction were 70 and 75% for Fenton and EF, respectively. In order to control the optimization, the actual experimental processes were conducted at these optimum operating conditions, and COD removal efficiencies were obtained as 65.5 and 72% for Fenton and EF, respectively. The prediction of model for COD removal was in the confidence interval. The optimum orthophosphate, color, and SS removal efficiencies of model prediction were 100% for both Fenton and EF processes. At the end of the laboratory experiments, orthophosphate removal efficiencies were 90 and 88%, color removal efficiencies were 92.5 and 93%, and SS removal efficiencies were 95 and 92% for Fenton and EF process, respectively. The experimental results for orthophosphate, color, and SS removals were consistent with the predicted values.
Since pH leads to the production of hydroxyl radicals (∙OH) and the concentration of ferrous ions in the solution, it plays a vital role in both processes (Mohajeri et al. 2010). During the processes, H2O2 is catalyzed by ferrous ions to produce ∙OH for degradation of organic matters in the wastewater (Zhang et al. 2007; Mohajeri et al. 2010). On the other hand, the electrical current causes the dissolution of metal electrodes into wastewater in EF process. The dissolved metal ions, at an appropriate pH, can form wide ranges of coagulated species and metal hydroxides that destabilize and aggregate the suspended particles or precipitate and adsorb dissolved contaminants (Bazrafshan et al. 2013). In the literature, there are several studies related to dairy wastewater treatment by EC process using aluminum and iron electrode (Sengil and Ozacar 2006; Tchamango et al. 2010; Kushwaha et al. 2010b; Bazrafshan et al. 2013). As aforementioned, however, there are only a few studies for the treatment of dairy wastewater using Fenton and EF processes. Yavuz et al. (2011) investigated treatment of dairy wastewater by EC and EF processes and found 79.2% COD removal efficiency at current density of 15 mA/cm2, natural pH (6–7) and 3000 mg/L H2O2 concentration. On the other hand, Davarnejad and Nikseresht (2016) studied dairy wastewater treatment using EF process and 93.9% COD removal efficiency and 97.3% color removal efficiency were achieved at 55.1 mA/cm2 current density, 7.48 pH, 0.907 mL/L H2O2/Fe2+ molar ratio, and 86 min reaction time.
Conclusion
In this study, BBD and RSM were adopted to model and to optimize the performance of Fenton and EF processes, and COD, orthophosphate, SS, and color removal efficiencies by Fenton and EF process were determined. The RSM approach was also applied to find the optimum operating parameters for these responses. According to the ANOVA results, the RSM could be used to navigate the design space with high regression coefficient value above 91% for all the responses. COD removal efficiencies were 65.5 and 72% at the optimum operating conditions for Fenton and EF processes. The orthophosphate, SS, and color removal efficiencies were over 88, 92, and 92.5% for both processes, respectively. According to the overall results, it can be concluded that the EF process was found as a much suitable treatment method for dairy wastewater. Contrary to Fenton process, extra consumption of chemicals can be avoided by EF process. The results also indicated that the RSM was a powerful technique for optimizing the operational conditions of Fenton and EF processes for the removal of COD, orthophosphate, SS, and color from dairy industry wastewater.
References
Andrade LH, Mendes FDS, Espindola JC, Amaral MCS (2014) Nanofiltration as tertiary treatment for the reuse of dairy wastewater treated by membrane bioreactor. Sep Purif Technol 126:21–29
APHA (2005) Standard Methods for the Examination of Water and Wastewater, 21st edn. American Public Health Association/American Water Works Association/Water Environment Federation, Washington DC, USA
Arslan-Alaton I, Tureli G, Olmez-Hanci T (2009) Treatment of azo dye production wastewaters using Photo-Fenton-like advanced oxidation processes: optimization by response surface methodology. J Photochem Photobiol A: Chem 202:142–153
Ayhan Şengin İ, Özacar M (2006) Treatment of dairy wastewaters by electrocoagulation using mild steel electrodes. J Hazard Mater B137:1197–1205
Ayoub K, Van Hullebusch ED, Cassir M, Bermond A (2010) Application of advanced oxidation processes for TNT removal: a review. J Hazard Mater 178:10–28
Balannec B, Vourch M, Rabiller-Baudry M, Chaufer B (2005) Comparative study of different nanofiltration and reverse osmosis membranes for dairy effluent treatment by dead-end filtration. Sep Purif Technol 42(2):195–200
Banu JR, Anandan S, Kaliappan S, Yeom IT (2008) Treatment of dairy wastewater using anaerobic and solar photocatalytic methods. Solar Energy 82(9):812–819
Baskaran K, Palmowski LM, Watson BM (2000) Wastewater reuse and treatment options fort he dairy industry. Water Sci Technol 3:85–91
Bautista P, Mohedano AF, Casas JA, Zazo JA, Rodriguez JJ (2008) An overview of the application of Fenton oxidation to industrial wastewaters treatment. J Chem Technol Biotechnol 83:1323–1338
Bazrafshan E, Moein H, Mostafapour FK, Nakhaie S (2013) Application of electrocoagulation process for dairy wastewater treatment. J Chem. https://doi.org/10.1155/2013/640139
Chang PH, Huang YH, Hsueh CL, Lu MC, Huang GH (2004) Treatment of non-biodegradable wastewater by electro-Fenton method. Water Sci Technol 49(4):213–218
Chen Z, Luo J, Hang X, Wan Y (2018) Physicochemical characterization of tight nanofiltration membranes for dairy wastewater treatment. J Membr Sci 547:51–63
Cheng M, Zeng G, Huang D, Lai C, Xu P, Zhang C, Liu Y (2016) Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: a review. Chem Eng J 284:582–598
Dabrowski W, Zylka R, Malinowski P (2017) Evaluation of energy consumption during aerobic sewage sludge treatment in dairy wastewater treatment plant. Environ Res 153:135–139
Davarnejad R, Nikseresht M (2016) Dairy wastewater treatment using an electrochemical method: experimental and statistical study. J Electroanal Chem 775:364–373
Deshpande DP, Patil PJ, Anekar SV (2012) Biomethanation of dairy waste. Res J Chem Sci 2(4):35–39
Erkan HS, Apaydın Ö (2015) Final treatment of young, middle-aged, and stabilized leachates by Fenton process: optimization by response surface methodology. Desalin Water Treat 54:342–357
Farizoglu B, Uzuner S (2011) The investigation of dairy industry wastewater treatment in a biological high performance membrane system. Biochem Eng J 57:46–54
Fenton HJH (1896) Oxidation of tartaric acid in presence of iron. J Chem Soc 65:899–910
Gengec E, Kobya M, Demirbas E, Akyol A, Oktor K (2012) Optimization of baker’s yeast wastewater using response surface methodology by electrocoagulation. Desalination 286:200–209
Gotvajn AZ, Zagorc-Koncan J, Cotman M (2011) Fenton’s oxidative treatment of municipal landfill leachate as an alternative to biological process. Desalination 275:269–275
Karadag D, Köroğlu OE, Ozkaya B, Cakmakci M (2015) A review on anaerobic biofilm reactors for the treatment of dairy industry wastewater. Process Biochem 50(2):262–271
Kim SC (2016) Application of response surface method as an experimental design to optimize coagulation-flocculation process for pre-treating paper wastewater. J Ind Eng Chem 38:93–102
Körbahti BK (2007) Response surface optimization of electrochemical treatment of textile dye wastewater. J Hazard Mater 145:277–286
Körbahti BK, Rauf MA (2008) Response surface methodology (RSM) analysis of photo induced decoloration oftoludine blue. Chem Eng J 136:25–30
Kushwaha JP, Srivastava VC, Mall ID (2010a) Treatment of dairy wastewater by inorganic coagulants: parametric and disposal studies. Water Res 44:5867–5874
Kushwaha JP, Srivastava VC, Mall ID (2010b) Organics removal from dairy wastewater by electrochemical treatment and residue disposal. Sep Purif Technol 76:198–205
Loloei M, Alidadi H, Nekonam G, Kor Y (2014) Study of the coagulation process in wastewater treatment of dairy industries. Int J Environ Health Eng 3(1):12
Luo J, Ding L, Wan Y, Paullier P, Jaffrin MY (2012) Fouling behavior of dairy wastewater treatment by nanofiltration under shear-enhanced extreme hydraulic conditions. Sep Purif Technol 88:79–86
Mohajeri S, Aziz HA, Isa MH, Zahed MA, Adlan MN (2010) Statistical optimization of process parameters for landfill leachate treatment using electro-Fenton technique. J Hazard Mater 176:749–758
Oturan MA, Peiroten J, Chartrin P, Acher AJ (2000) Complete destruction of p-nitrophenol in aqueous medium by electro-Fenton method. Environ Sci Technol 34:3474–3479
Perle M, Kimchie S, Shelef G (1995) Some biochemical aspects of anaerobic degradation of dairy wastewater. Water Res 29(6):1549–1554
Praneeth K, Moulik S, Vadthya P, Bhargava SK, Tardio J, Sridhar S (2014) Performance assessment and hydrodynamic analysis of a submerged membrane bioreactor for treating dairy industrial effluent. J Hazard Mater 274:300–313
Qiang Z, Chang JH, Huang CP (2003) Electrochemical regeneration of Fe2+ in Fenton oxidation processes. Water Res 37(6):1308–1319
Ramasamy EV, Gajalakshmi S, Sanjeevi R, Jithesh MN, Abbasi SA (2004) Feasibility studies on the treatment of dairy wastewaters with upflow anaerobic sludge blanket reactors. Bioresour Technol 93(2):209–212
Sengil A, Ozacar M (2006) Treatment of dairy wastewaters by electrocoagulation using mild steel electrodes. J Hazard Mater 137:1197–1205
Tchamango S, Nanseu-Njiki CP, Ngameni E, Hadjiev D, Darchen A (2010) Treatment of dairy effluents by electrocoagulation using aluminium electrodes. Sci Total Environ 408:947–952
Turan M (2004) Influence of filtration conditions on the performance of nanofiltration and reverse osmosis membranes in dairy wastewater treatment. Desalination 170(1):83–90
Vourch M, Balannec B, Chaufer B, Dorange G (2008) Treatment of dairy industry wastewater by reverse osmosis for water reuse. Desalination 219(1–3):190–202
Weast RC (1969) Handbook of chemistry and physics. Am J Med Sci 257:423
Wheatley A (1990) Anaerobic digestion: a waste treatment technology. Elsevier Applied Science, London and New York
Yavuz Y, Öcal E, Koparal AS, Öğütveren ÜB (2011) Treatment of dairy industry wastewater by EC and EF processes using hybrid Fe-Al plate electrodes. J Chem Technol Biotechnol 86:964–969
Zhang H, Zhang D, Zhou J (2006) Removal of COD from landfill leachate by electro-Fenton method. J Hazard Mater 135:106–111
Zhang H, Fei C, Zhang D, Tang F (2007) Degradation of 4-nitrophenol in aqueous medium by electro-Fenton method. J Hazard Mater 145:227–232
Acknowledgements
The authors are grateful Atesoglu Dairy Factory for their collaboration. The authors also would like to thank the anonymous reviewers for their valuable comments and suggestions to improve the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: M. Abbaspour.
Rights and permissions
About this article
Cite this article
Akkaya, G.K., Erkan, H.S., Sekman, E. et al. Modeling and optimizing Fenton and electro-Fenton processes for dairy wastewater treatment using response surface methodology. Int. J. Environ. Sci. Technol. 16, 2343–2358 (2019). https://doi.org/10.1007/s13762-018-1846-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-018-1846-0