Abstract
Treatment of real textile wastewater by electro-Fenton method was investigated using Ti/RuO2 electrodes. The performance of the treatment process was evaluated in terms of %chemical oxidation demand removal; %color removal; and energy consumed, at three electro-Fenton process parameters: current, electrolysis time and ferrous sulfate concentration. To determine the optimum operating conditions, multiple responses optimization based on Box–Behnken design with desirability function was used. The optimum value of parameters were found to be current= 0.32 A, time = 90 min and ferrous sulfate concentration = 0.53 mM. Box–Behnken design suggested the %chemical oxidation demand removal; %color removal; and energy consumed were 100%, 90.30% and 1.27 Wh, respectively at optimized process parameters. The predicted performance parameters agree well with the experimental data. Second-order kinetic model was fitted to the experimental data, at optimum conditions. GC–MS analysis confirmed that dye components were totally eliminated after electro-Fenton treatment of textile effluent. To determined the toxicity of the treated textile effluent bioassay analysis was performed. It was identified that the generated by-products were non-toxic in nature. The total cost to treat the 1 m3 of real textile wastewater by electro-Fenton at optimum conditions was $ 3.13.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global industrialization and urbanization has attained high pace leading to enormous generation of wastewater. Textile industries require large amount of freshwater for textile processing and generate huge amount of wastewater (Gregory 1986; Phalakornkule et al. 2010). To improve the performance of finished textile products, textile industries use dyes, which are recalcitrant in nature and varieties of chemicals (bleaching agents, salts, acids, alkalis, etc.), which are lost/remaining in processing water during the processing and goes with discharged wastewater. Therefore, the high-strength wastewater is produced having burly color, high suspended particles, high pH and chemical oxygen demand (COD) (Olmez et al. 2007; Vlyssides et al. 1999). Furthermore, the composition of textile effluent is altered depending on different types of dyestuff and auxiliary chemicals used. This wastewater if discharged without treatment may pose problem to the environment. Moreover, environmental regulatory agencies have set the discharge standards for release of the wastewater in to the environment.
Treatment of real textile wastewater is tedious task to meet the discharge standards. Various physical and chemical treatment methods like chemical coagulation, adsorption processes and membrane filtration are not preferred for the treatment of such type of effluents because of their unsuitability due to expensive chemical coagulants, adsorbents, membranes and membrane cleaning and production of large volume of secondary pollutants (Georgiou et al. 2002; Cañizares et al. 2006). The conventional treatment technology used in the textile industries employs activated sludge process (ASP) as a secondary treatment facility. ASP is prone to bulking of sludge and is very sensitive toward variation in COD and BOD loadings, as happens in case of textile industries wastewater. ASP also requires large area for its installation and high level of controlling. Except these, non-biodegradability of dyes limits the use of biological methods for the treatment of textile wastewater (Chen and Liu 2012; Bansal et al. 2013). Anaerobic processes are also not suitable for wastewater containing dyes, because the dyes degradation under anaerobic conditions leads to the formation of toxic organic compounds (Meric et al. 2004).
The inefficiency of the traditional treatment methods has motivated researchers to explore other techniques like electrochemical advanced oxidation processes (EAOPs). These methods are based on the in situ generation of ·OH radicals and the degradation of pollutants. Among the different EAOPs, several researches have demonstrated that the electro-Fenton (EF) process is a promising technology to be more efficient and environmental friendly to remove organic matter (Brillas et al. 2009; Sandhwar and Prasad 2017).
In classical Fenton process, H2O2 is added with Fenton reagent to generate ·OH radicals. EF process allows better control of the process and avoids the storing and transport of the highly reactive H2O2. Moreover, EF process does not produce secondary pollutants. In the EF process, organics are eliminated by the action of both Fenton’s reaction (Eq. 2) in the bulk and anodic oxidation at the anode surface (Eq. 4). The oxidation of pollutants is achieved by anodic oxidation by using high-oxygen-overvoltage anodes (such as dimensionally stable anode (DSA), Pt and BDD (boron-doped diamond) anode (Lin et al. 2014; Nidheesh and Gandhimathi 2014). H2O2 is continuously generated in the solution during electrolysis in EF process due to two-electron oxygen reduction at cathode (Brillas et al. 2009; Sirés and Brillas 2012) in an acidic medium (Eq. 1), and ferrous ion added into the system analogously generates the ·OH radicals in the classical Fenton’s reaction (Eq. 2). Due to high standard redox potential (E° (OH/H2O) = 2.80 V/SHE) of ·OH radicals, organics present in the effluent are oxidized to CO2, water and inorganic ions. At the same time, in this process, the ferrous ion is regenerated at the cathode (Eq. 3), reducing its further addition.
Rate of Fenton’s reaction in EF process is predominantly controlled by the H2O2 production rate, which depends on factors such as O2 solubility, temperature, pH, cathode type and current density. Current controls not only the H2O2 generation rate (Eq. 1), but also regeneration rate of Fe2+ (Eq. 3). At the same time, high current density supports other side reactions leading to reduction in the H2O2 production (Eqs. 5–7) and production of H2 at cathode (Eq. 8).
Nature and concentration of catalyst used significantly affect the process efficiency. Usually, Fe2+ has been reported the best catalyst in the EF process due to its efficient activity even at lower concentrations (Sirés et al. 2014).
In the literature, various authors reported the EF treatment performance for textile wastewater using different types of electrodes (Brillas and Casado 2002; Nasr et al. 2005; Wang et al. 2005; Zhou and He 2007; Martinez-Huitle and Brillas 2009; Salazar et al. 2012; Oturan et al. 2012; Pajootan et al. 2014; Ghanbari and Moradi 2015; Asghar et al. 2015; Garcia-Segura and Brillas 2016). In the reported literature, investigators discussed about the treatment of simulated/dye textile wastewater only, by using EF process (Lin et al. 2014, Nidheesh and Gandhimathi 2014). Some investigators perform EF with the combination of other processes for the treatment of real textile wastewater (Santos et al. 2010). The treatment performance of EF for real textile wastewater is not investigated in the reported literature. Real textile wastewater contains number of components, which can promote or hinder the treatment process like chloride content, TS, composition of different types of dyes. Therefore, performance investigation and applicability of EF process require more study on real industrial textile wastewater.
Real textile wastewater contains high chloride; however, dimensionally stable active electrodes may be suitable for the textile wastewater treatment. Textile wastewater electro-oxidation has been reported by various dimensionally stable electrodes like Pt, BDD and metal oxide-coated anodes such as Ti/IrO2, Ti/PbO2 and Ti/RuO2 (Kaur et al. 2015, 2017). Ti/RuO2 has been reported dimensionally stable active electrode with high electrode potential (Santos et al. 2010). Ti/RuO2 anodes generate various chlorine products such as Cl2, HOCl and ClO− in solutions containing high chloride. These chlorine products additionally oxidize the pollutants by mediated oxidation method in association with direct oxidation by ·OH radicals. But, in the presence of Fenton reagent the generation of ·OH radicals increases which leads to the ·OH radicals-mediated organics oxidation of pollutants. Therefore, during the textile wastewater treatment, Ti/RuO2 anode is capable of delivering high degradation of pollutants by both electro-oxidation and Fenton ·OH radicals-mediated organics oxidation. However, degradation mediated by chlorine products yields suspicion of carcinogenic/toxic chlorinated organic compounds generation. Therefore, there is a need to explore the true efficiency of EF process with Ti/RuO2 electrodes and its applicability for the treatment of real textile wastewater.
The purpose of the present study is to investigate EF treatment of actual textile wastewater using Ti/RuO2 electrodes. Effects of various EF process parameters are as follows: current (i), time (t) and concentration of ferrous sulfate (CFe) (Fenton reagent) on %chemical oxidation demand (COD) removal (M1), %color removal (M2) and energy consumed (M3). GC–MS at optimum conditions was performed for safe disposal point of view. Moreover, COD removal and color removal kinetics were investigated. To determine the economic feasibility of EF process to treat the textile wastewater, operating cost exploration was executed.
This study was performed in Industrial Pollution Abatement Laboratory, Department of Chemical Engineering, Thapar Institute of Engineering & Technology, Patiala, Punjab, India (April 2015–February 2016).
Materials and methods
Wastewater sampling, characterization and experimental setup
Real textile wastewater was provided by a mink blanket industry (Ludhiana, Punjab, India). Textile wastewater contains most of the basic dyes that are used in mink blanket industry. The physicochemical characterization of textile wastewater for water quality parameters is given in Table 1. A cubical shaped EF batch reactor made from plexi glass sheet of 1.5 L working volume was used (Fig. 1). Ti/RuO2 electrodes of dimensions 100 mm × 85 mm × 1.5 mm were supplied by Titanium Tantalum Products Limited, Chennai, India, and were used as anode, whereas cathode was of aluminum (Al) plate of same dimensions. A digital power supply provided by DIGITECH, Roorkee, India (Model: 4818A10; 0–20 V, 0–5 A), was used to supply the DC during experiments. A pump with air sparger was used for continuous supply of air to the real textile wastewater sample in the reactor. To adjust the pH of textile wastewater for a particular run with 0.1 N HCl solution was used. Magnetic stirrer was used to agitate the real textile wastewater sample in the reactor.
Experimental design, mathematical modeling and optimization
The EF process was modeled and analyzed using Box–Behnken design (BBD) under response surface methodology (RSM). RSM facilitates in designing experiments, modeling, process parameter effects assessment and identifying optimum conditions for responses (Sangal et al. 2013). BBD under RSM allows the identification and quantification of interactive effects of various parameters. Equation 9 shows the relationship between the response and the process parameters as the surface of the x1, x2, …, xk coordinated in the graphical sense.
where M is the response, x1, x2, …, xk are quantitative factors, f is the response function and δr is the statistical error
In the present study, predicted response (M) refers to the %COD removal, %color removal and energy consumed. A second-order polynomial equation (Eq. 10) was used to determine the relevant model terms where ω0 is the model constant; ωi represents the linear coefficient; ωii and ωij are the interaction coefficients; k is the number of the factors or variables; and δr is the statistical error. The interaction between the process variables and the responses was analyzed from an analysis of variance (ANOVA).
The statistical Design-Expert software version 6.06 (STAT-EASE Inc., Minneapolis, USA) was used for designing the experiments by BBD. The process parameters: current (0.25–3 A), time (10–90 min) and ferrous sulfate concentration (0.20–1.0 mM), were selected from preliminary experiments. Multi-response optimization with desirability function was applied for maximizing the %COD removal and %color removal and minimizing the energy consumption simultaneously. The electro-Fenton responses, i.e., %COD removal, %color removal, energy consumption of textile effluent, were measured according to the design matrix (Table 2) to optimize the EF process.
Operational details of experimental system and data analysis
Experiments were conducted, as per the design matrix shown in Table 2. The pH of textile wastewater collected was found to be 9.84. It has been reported that the best working pH for EF processes is pH = 3 (Sun and Pignatello 1993). Therefore, before conducting the EF experiments, the pH of textile wastewater was altered to pH = 3. The EF treatment time (t) measured from the moment of power supply button was switched on. During the EF treatment, the supplied current intensity (i) was kept constant. In EF process, oxygen is required for generation of H2O2 (Eq. 1), and hence, ·OH radicals are generated (Eq. 2). In the present study, oxygen was supplied in the form of air with the help of air diffuser at the bottom (Fig. 1). Further, the supplied air/oxygen should be distributed evenly in the reactor and its concentration and mass transfer rate should be high in order to generate high concentration of oxidants (H2O2 and ·OH). To ensure this, magnetic stirrer was used to stir the content of the reactor, and high flow rate of air was maintained. After the desired treatment time (t), the power supply was switched off, and samples were collected from the reactor for further analysis.
The samples were then analyzed for color and COD with double-beam UV visible spectrophotometer (HACH, DR 5000, USA) and COD digestion unit (Spectra lab, 2015D, India). Energy consumed (E) during the EF process was assessed by Eq. 11:
where i = current (A); V = voltage (volt); t = treatment time (h); Z = sample volume (L); CODr = COD removed (mg/L).
GC–MS of treated textile wastewater (at optimum conditions) and untreated textile wastewater was carried out to identify various transformation products formed during EF degradation and for disposal study. The sample for GC–MS (Perkin-Elmer Clarus 500 MS) analysis was prepared by solvent extraction method. The Fused silica capillary columns coated with a 5% diphenyl/95% dimethyl polysiloxane of 25 m length and 0.20 µm internal diameter were used at injector temperature of 300 °C. The helium (carrier gas) with a flow rate of 1 ml/min was used. The temperatures of the inlet line and ion source were set at 250 and 280 °C, respectively. The run time for the samples was 26 min.
Results and discussion
Model fitting and statistical analysis
The responses %COD removal, M1; %color removal, M2; and energy consumed, M3, for the EF were measured after conducting experiments as per design matrix listed in Table 2. Quadratic model was found to be best fitted to the experimental responses. Quadratic model also supported by ANOVA with F-values of 77.47, 72.1 and 1.1 × 104 for the responses M1, M2 and M3, respectively, shown in Table 3. Adequate precision ratio above 4, in the present study, shows that model is efficient in navigating the design space. The statistical regression coefficient values R2, adjusted R2 and predicted R2 for responses M1, M2 and M3 found to be 0.99, 0.98 and 0.84; 0.99, 0.98 and 0.83; and 0.99, 0.99 and 0.99, respectively, which shows a good relation between predicted and experimentally observed values. The values of “Prob > F” smaller than 0.05 show that model terms are significant with 95% confidence level. ANOVA indicated that i, t, i2, C 2Fe are highly significant model terms for M1; t, i2, t2, CFe are highly significant model terms for M2, while i, t, CFe, i2, t2, C 2Fe , i* CFe are highly significant for M3 (Table 3). The quadratic equations for M1, M2 and M3 in terms of coded variables after the elimination of insignificant terms are given below:
Normal probability, outlier-t-residual plots and studentized residuals explained that the quadratic model will satisfy the ANOVA.
Parametric study, optimization and disposal study
To explore the effects of EF parameters and their interactions, 3D response surface graph was drawn. Figure 2a and b shows the interaction of CFe and i on the M1 and M2 with time, t, respectively. The 3D plot showed that M1 decreases at higher and lower CFe at all the i values. However, M1 increases with the increase in i from 0.25 to ≈ 0.81 A, after it starts decreasing. M1 also increases with the increase in CFe from 0.20 to ≈ 0.53 Mm. This trend was also observed for M1. On the other side, with increasing CFe along with the increase in i value from 0.30 to ≈ 0.81 A, increases M2. 100% M2 was observed, when CFe was up to 0.53 mM at i = 0.32 A.
Figure 2c, d shows that M1 and M2 increases with the increase in t for all i value. However, increase in t at particular i between 0.50 and ≈ 0.81 A showed 100% M1 and M2. M3 value increases with the increase in CFe, t and i.
It is asserted that electro-oxidation (EO) and Fenton ·OH radicals-mediated organics oxidation are responsible for the removal of COD and color from the real textile wastewater. It was well known that Fenton’s reaction can be applied in acidic pH (Sun and Pignatello 1993) to efficiently produce ·OH radicals, but EO, at acidic pH, generates oxidants such as Cl2, HOCl and ·OH. It has been reported that chloro-oxidants have lower oxidation potential and are toxic/carcinogenic in nature (Deborde and Von Gunten 2008). However, oxidation of pollutant particles occurs at the surface of Ti/RuO2 electrodes due to chloro-oxidants and ·OH radicals. Due to lower oxidation potential, chloro-oxidants transform pollutants into chloro-compounds. Furthermore, these chloro-compounds are mineralizing by Fenton ·OH radicals-mediated oxidation. Therefore, M1 and M2 are increased with the increase in CFe at all i values. But, it has been also seen that after CFe = 0.53 mM, there is a sharp decrease in M1 and M2 along with electrolysis time (t) at particular i value. Due to the high generation of ·OH radicals at the higher concentration of Fenton reagent, a competitive reaction occurs between the Fe2+ and hydroxyl radicals. It could reduce the concentration of hydroxyl radicals to the pollutant particles (Bouafia-Chergui et al. 2010). Therefore, above CFe = 0.53 mM, there is a sharp decrease in M1 and M2.
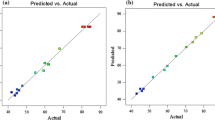
Optimum operating conditions were derived for simultaneous optimization of the multiple responses. For this purpose, the process parameters (i, t and CFe) were selected within whole range of study and responses (M1 and M2) maintained as maximum; however, response (M3) maintained as minimum. Using minimum and maximum acceptability of the responses, individual and simultaneous desirability was calculated. The one-sided desirability for all the responses was 1.0, and overall desirability was 0.92. The optimum values of parameters were found to be i = 0.32 A, t = 90 min and CFe = 0.53 mM with corresponding desirability value 0.920 for simultaneous optimization. At optimum conditions, the M1, M2 and M3 suggested by BBD were found to be 90.30, 100% and 1.27 Wh, respectively. At optimum values of parameters, experiments were performed in duplicate and average values of responses were found to be 89.75, 99.49 and 1.067 Wh, for M1, M2 and M3, respectively. This validation explains good correlation between the values of experimental and predicted responses and the reliability of modeling by RSM.
At optimum conditions, both EO and Fenton ·OH radicals-mediated oxidation are participating in the oxidation of pollutant species of actual textile wastewater, which can be confirmed by GC–MS analysis of both the untreated and treated real textile wastewater (at optimum conditions). The intermediate/transformation products from GC–MS analysis of untreated and treated real textile wastewater are shown in Tables 4 and 5, respectively. There is no chlorinated compound identified, so it shows that chloro-compounds are mineralizing by Fenton ·OH radicals-mediated oxidation. GC–MS analysis also shows that dye components are totally eliminated after treatment by EF. After treatment, there was no component observed that was previously present in real textile wastewater. Butyl phthalate was found in treated sample with highest matching % as shown in Table 5. It is non-toxic and biodegradable component. It is observe from GCMS analysis that there is no toxic component present in treated real textile wastewater. To confirm this, the bioassay analysis was performed. The toxicity analysis of untreated and treated wastewater was carried out using E coli DH-α strain with standard Kirby–Bauer method. The result of bioassay analysis shows a zone of inhibition with less number of bacterial colonies in case of untreated, and there is no zone of inhibition in case of treated wastewater. There is a heavy count of bacterial colonies around the puncher well for treated wastewater (Fig. 3).
Reaction kinetics
At optimum parameters of the EF process, kinetics of COD and color removal was studied. Second-order model was fitted to the experimental data at optimum conditions (i = 0.32 and CFe = 0.53 mM) for COD removal and color removal according to the rate equation.
On integrating between known limits and rearranging, the above model becomes
where C0 is initial color intensity or initial COD concentration, Cs is final color intensity or final COD concentration, t is degradation time (min) and k is second-order rate constant (L/mg min). Figure 3a and b shows the fitting of second-order reaction kinetics to the %color removal and %COD removal for the treatment of textile effluent by EF process. The kinetic raw data for the textile wastewater treatment by EF method has been shown in Table 6. The values of rate constant of second-order reaction kinetics are 0.001 and 2.756 L/mg min along with the R2 value 0.983 and 0.886 for M1 and M2, respectively (Fig. 4).
Cost analysis for EF process for the treatment of textile wastewater
The electrical energy and electrode cost required for the treatment of 1 m3 and 1 kg of COD removal of textile wastewater by EF process were calculated at optimum conditions.
-
Electricity price in India = $ 0.069 kWh
-
Electrical energy consumed = 0.789 kWh/m3 of real textile wastewater or 1.306 kWh/kg of COD removal of real textile wastewater
-
Electrical energy consumption cost (CE) = $ 0.054/m3 of real textile wastewater
-
Electrical energy consumption cost (CE (per Kg COD removed)) = $ 0.10/kg of COD removed
-
Ti/RuO2 electrodes price (CEL) = $ 3.08/m3 of textile effluent
-
Ti/RuO2 electrodes price in India (CEL (per Kg COD removed)) = $ 5.66/kg of COD removed
-
Total cost (CT (per Kg COD removed)) = CE (per Kg COD removed) + CEL (per Kg COD removed) = $ 5.76/kg of COD removal
-
Total cost (CT) to treat one m3 of textile wastewater = CE + CEL = $ 3.13
The total treatment cost of real textile wastewater by EF is $ 5.76/kg of COD removal and $ 3.13/m3. Cost of the treatment process can be reduced by avoiding the deterioration of catalytic electrodes. In electrochemical system, the major reason for the deterioration of catalytic electrode is generation of electric double layer, which affects the mechanism and kinetics of electrochemical reaction. In case of cathode, fermi level increases, electrons jump to unoccupied level to reduce the pollutant particles, but in case of anode fermi level goes on decreasing; then, electrons from bulk jump to the electrode and participate in oxidation on the surface of anode (Newns 1969). Most of the catalyst atoms have d orbital. Bonding and antibonding levels affect the chemisorption bonds; however, filling of antibonding orbital will strengthen the chemisorption and weaken the intermolecular bond of adsorbed molecule. Filling of antibonding orbital also affects the catalyst bonding to the surface of a metal and leads to slowly deterioration of catalytic electrode (Anderson 1961).
Conclusion
Electro-Fenton method was successfully performed in batch mode for real textile wastewater treatment. Statistical analysis advocated good correlation between the observed and predicted values. 3D response surface graph concluded that very high concentration of the Fenton reagent hinders the degradation process during electro-Fenton. The optimization of process parameters was successfully performed. EO and Fenton ·OH radicals-mediated oxidation were responsible for %COD removal and %color removal in association with chloro-oxidant-mediated oxidation. Furthermore, chloro-compounds were mineralized by Fenton ·OH radicals-mediated oxidation. It was clear by GCMS analysis that there was no generation of chloro-compounds, and bioassay analysis confirmed that treated real textile wastewater was non-toxic. Therefore, textile wastewater after treatment by electro-Fenton method is safe to dispose in the environment. Therefore, no further treatment is required to dispose of the treated textile wastewater and it is found to be cost-effective than other electrochemical treatment methods.
References
Anderson PW (1961) Localized magnetic states in metals. Phys Rev 124(1):41–53
Asghar A, Raman AAA, Daud WMAW (2015) Advanced oxidation processes for in situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: a review. J Clean Prod 87:826–838
Bansal S, Kushwaha JP, Sangal VK (2013) Electrochemical treatment of reactive black 5 textile wastewater: optimization, kinetics, and disposal study. Water Environ Res 85(12):2294–2306
Bouafia-Chergui S, Oturan N, Khalaf H, Oturan MA (2010) Parametric study on the effect of the ratios [H2O2]/[Fe3+] and [H2O2]/[substrate] on the photo-Fenton degradation of cationic azo dye basic blue 41. J Environ Sci Health A 45(5):622–629
Brillas E, Casado J (2002) Aniline degradation by Electro-Fenton and peroxi-coagulation processes using a flow reactor for wastewater treatment. Chemosphere 47(3):241–248
Brillas E, Sirés I, Oturan MA (2009) Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem Rev 109(12):6570–6631
Cañizares P, Martínez F, Jiménez C, Lobato J, Rodrigo MA (2006) Coagulation and electro coagulation of wastes polluted with dyes. Environ Sci Technol 40(20):6418–6424
Chen W, Liu J (2012) The possibility and applicability of coagulation-MBR hybrid system in reclamation of dairy wastewater. Desalination 285:226–231
Deborde M, Von Gunten URS (2008) Reactions of chlorine with inorganic and organic compounds during water treatment—kinetics and mechanisms: a critical review. Water Res 42(1):13–51
Garcia-Segura S, Brillas E (2016) Combustion of textile monoazo, diazo and triazo dyes by solar photoelectro-Fenton: decolorization, kinetics and degradation routes. Appl Catal B Environ 181:681–691
Georgiou D, Melidis P, Aivasidis A (2002) Use of a microbial sensor: inhibition effect of azo reactive dyes on activated sludge. Bioprocess Biosyst Eng 25(2):79–83
Ghanbari F, Moradi M (2015) A comparative study of electrocoagulation, electrochemical Fenton, electro-Fenton and peroxi-coagulation for decolorization of real textile wastewater: electrical energy consumption and biodegradability improvement. J Environ Chem Eng 3:499–506
Gregory P (1986) Azo dyes: structure-carcinogenicity relationships. Dyes Pig 7(1):45–56
Kaur P, Kushwaha JP, Sangal VK (2015) Modeling and evaluation of electro-oxidation of dye wastewater using artificial neural networks. RSC Adv 5:34663–34671
Kaur P, Kushwaha JP, Sangal VK (2017) Evaluation and disposability study of actual textile wastewater treatment by electro-oxidation method using Ti/RuO2 anode. Proc Saf Environ Prot 111:13–22
Lin H, Zhang H, Wang X, Wang L, Wu J (2014) Electro-Fenton removal of Orange II in a divided cell: reaction mechanism, degradation pathway and toxicity evolution. Sep Purif Technol 122:533–540
Martinez-Huitle CA, Brillas E (2009) Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: a general review. Appl Catal B Environ 87(3):105–145
Meric S, Kaptan D, Olmez T (2004) Color and COD removal from wastewater containing reactive black 5 using Fenton’s oxidation process. Chemosphere 54(3):435–441
Nasr B, Abdelatif G, Cañizares P, Saez C, Lobato J, Rodrigo MA (2005) Electrochemical oxidation of hydroquinone, resorcinol, and catechol on boron-doped diamond anodes. Environ Sci Technol 39(18):7234–7239
Newns DM (1969) Self-consistent model of hydrogen chemisorption. Phys Rev 178(3):1123–1135
Nidheesh PV, Gandhimathi R (2014) Removal of Rhodamine B from aqueous solution using graphite–graphite electro-Fenton system. Des Water Treat 52(10–12):1872–1877
Olmez T, Kabdasli I, Tunay O (2007) The effect of the textile industry dye bath additive EDTMPA on colour removal characteristics by ozone oxidation. Water Sci Technol 55(10):145–153
Oturan N, Brillas E, Oturan MA (2012) Unprecedented total mineralization of atrazine and cyanuric acid by anodic oxidation and electro-Fenton with a boron-doped diamond anode. Environ Chem Lett 10(2):165–170
Pajootan E, Arami M, Rahimdokht M (2014) Discoloration of wastewater in a continuous electro-Fenton process using modified graphite electrode with multi-walled carbon nanotubes/surfactant. Sep Purif Technol 130:34–44
Phalakornkule C, Polgumhang S, Tongdaung W, Karakat B, Nuyut T (2010) Electrocoagulation of blue reactive, red disperse and mixed dyes, and application in treating textile effluent. J Environ Manag 91(4):918–926
Salazar R, Brillas E, Sirés I (2012) Finding the best Fe2+/Cu2+ combination for the solar photoelectro-Fenton treatment of simulated wastewater containing the industrial textile dye disperse blue 3. Appl Catal B Environ 115:107–116
Sandhwar VK, Prasad B (2017) Terephthalic acid removal from aqueous solution by electrocoagulation and electro-Fenton methods: process optimization through response surface methodology. Proc Saf Environ Prot 107:269–280
Sangal VK, Kumar V, Mishra MI (2013) Optimization of a divided wall column for the separation of C4–C6 normal paraffin mixture using Box–Behnken design. Comput Chem Eng 19(1):107–119
Santos ID, Afonso JC, Dutra AJB (2010) Behavior of a Ti/RuO2 anode in concentrated chloride medium for phenol and their chlorinated intermediates electrooxidation. Sep Purif Technol 76(2):151–157
Sirés I, Brillas E (2012) Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: a review. Environ Int 40:212–229
Sirés I, Brillas E, Oturan MA, Rodrigo MA, Panizza M (2014) Electrochemical advanced oxidation processes: today and tomorrow (A review). Environ Sci Pollut Res 21(14):8336–8367
Sun Y, Pignatello JJ (1993) Photochemical reactions involved in the total mineralization of 2,4-D by iron (3+)/hydrogen peroxide/UV. Environ Sci Technol 27(2):304–310
Vlyssides AG, Loizidou M, Karlis PK, Zorpas AA, Papaioannou D (1999) Electrochemical oxidation of a textile dye wastewater using a Pt/Ti electrode. J Hazard Mater B70(1):41–52
Wang A, Qu J, Ru J, Liu H, Ge J (2005) Mineralization of an azo dye acid red 14 by electro-Fenton’s reagent using an activated carbon fiber cathode. Dyes Pig 65(3):227–233
Zhou M, He J (2007) Degradation of azo dye by three clean advanced oxidation processes: wet oxidation, electrochemical oxidation and wet electrochemical oxidation—a comparative study. Electrochim Acta 53(4):1902–1910
Acknowledgements
Authors are thankful to the University Grant Commission (UGC), India, for providing MANF fellowship (MANF-2015-17-PUN-49188) to first author of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: Josef Trögl.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaur, P., Sangal, V.K. & Kushwaha, J.P. Parametric study of electro-Fenton treatment for real textile wastewater, disposal study and its cost analysis. Int. J. Environ. Sci. Technol. 16, 801–810 (2019). https://doi.org/10.1007/s13762-018-1696-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-018-1696-9