Abstract
There has been an increasing interest among occupational hygienists and public health officials in indoor air quality assessment in industrial workplace environments. Exposure to bioaerosols may pose health risks to workers operating in the processing of recycled waste paper. This study was conducted in two waste paper recycling factories. Air sampling of the factory was performed on weekly basis over a period of 3 months employing passive air sampling technique (the settle plates) with standard Petri dishes in which appropriate culture media are used for counting bacteria and fungi in air samples. In addition, metagenome analyses were conducted to obtain more details about the diversity and abundance of bacteria in the investigated factory. Results showed abundant bacterial and fungal counts in the waste paper factory in all the manufacturing stages. In particular, the cutting/shredding unit exhibited high microbiological contamination; thus, corrective measures should be taken in order to achieve better indoor air quality. The most dominant genus detected according to both DGGE sequencing and metagenome analysis was Thermicanus. The dominance of this thermophilic, microaerophilic fermentative species especially during summer season reflects the condition within the factory as well as the source of the recycled paper and the way it has been handled and stored. An air flow ventilation system that prevents contamination from areas where raw waste paper is handled is recommended.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recycling of waste paper is progressively increasing in urban areas worldwide. This has been accompanied with a steady growth in waste paper processing and manufacturing plants. In such facilities, bioaerosols are emitted which are airborne particles of biological origin. They are composed of organic suspended materials such as live or dead bacteria, fungi, viruses, endotoxins, antigens, toxins and mycotoxins, as well as various allergens (Del Cimmuto et al. 2010). Exposure to bioaerosols may pose environmental health risks to workers in the processing of recycled waste paper. It may cause a number of adverse effects such as respiratory, gastrointestinal, dermatologic and allergy problems (Hamada and Fujita 2002; Krajewska-Kulak et al. 2011; Gaskin et al. 2012). Endotoxin (a component of the outer membrane of Gram-negative bacteria) is recognized as a health hazard in various occupations and has been associated with asthma (Parker et al. 2001).
Evaluation of bioaerosols includes the use of variety of methods for sampling depending on the concentration of microorganism expected (Srikanth et al. 2008). There have been problems in developing standard sampling methods and in establishing threshold limit values for exposures due to the complexity of composition of bioaerosols, variations in human response to their exposure and difficulties in recovering microorganisms (Srikanth et al. 2008). In organic waste, the main microbial populations are bacteria and fungi (Miller and Clesceri 2002). Studies in the field of occupational medicine showed that occupational exposure to bioaerosols containing high concentrations of bacteria and fungi, e.g. at workplaces of agriculture, composting and waste management, can lead to respiratory diseases including allergies and infections (Walser et al. 2015). Some studies have investigated exposure levels to bioaerosols in the organic waste management field. However, most studies focused on workers’ exposure, particularly in composting facilities (Schlosser et al. 2016). Despite advances in waste recycling and recovery technologies, studies on emissions and dispersal of bioaerosols from waste processes are limited and focused on commercial composting facilities (O’Connor et al. 2015) and landfill sites (Schlosser et al. 2016; Fraczek et al. 2017). Facing the lack of data on bioaerosols from waste processes, this study was conducted to assess the microbiological air quality within a waste paper recycling/processing factory in the state of Kuwait by determining airborne culturable bacteria and fungi in the processing plant.
Materials and methods
Description of the manufacturing plant
The studied factory belongs to the United Paper Industries Company in Kuwait which is located in the Shuaiba industrial area. It processes 120 ton/day of waste paper as well as 50 ton/day of virgin paper pulp and employs 24 workers on 8-h shifts per day. The factory encompasses three manufacturing steps within a single ventilated, closed building as well as an air-conditioned cube of offices for personnel. The raw, waste paper bales are received and stored in a reception station before being transferred to the manufacturing plant. The plant encompasses three consecutive manufacturing steps which are fully automated and include: (1) a waste paper cutting/shredding step, (2) a paper pulping/processing step and (3) a paper finishing step. In the first step, the “baled” waste paper is shredded, screened (through coarse screens, fine screens, and ash removal). In the second step, the shredded paper is pulped (after adding water and chemicals) and the pulped waste paper is mixed with virgin pulp, dried and converted into wrapping paper sheets through machine belts. In the third (paper finishing, rolling) step, the wrap paper rolls are prepared and packed in plastic bags. The finished product is transferred outside the plant to a product storage facility to be ready for sale.
Bacterial and fungal counts
Air sampling of the factory was performed on weekly basis over a period of 3 months using passive air sampling technique (the settle plates). Monitoring of bacteria and fungi was conducted through the settle-plates standard Petri dishes technique in which culture media that are opened and exposed for a given time are used (Yassin and Almouqatea 2010). This is an inexpensive and easy to use technique requiring no special equipment. It was conducted by exposing four Petri plates at each of four selected points located at the corners within each of the three manufacturing steps as well as from the office cubical at the exit of the plant (which provided background data for the study). Microbiological analysis included plate counts of bacteria and fungi using specific culture media NA (nutrient agar) and PDA (potato dextrose agar), respectively. Each of the NA and PDA Petri dishes was subjected to the indoor air for 20 min placed at a fixed height and then closed and transferred to the incubator operated at 37 ± 1 °C for 48 h in case of bacteria “NA” plates and for 5 days in case of fungi “PDA” plates. Statistical analysis was conducted to determine median values of CFU (colony-forming unit) for each sampling location. The index of microbial air contamination was based on the count of the microbial fallout onto Petri dishes left open to the air inside the factory. The sampling was conducted at a height of 1.5 m from the factory floor (near workers’ breathing zone).
Samples were assessed in the laboratory for enumeration of bacteria and fungi. Bacterial and fungal results were reported as the number of colony-forming units (CFUs) of each per plate and further adjusted by the volume of air sampled to obtain concentrations as CFU/m3. This was calculated by (Gaskin et al. 2012):
Metagenomic analyses
The metagenome analyses were conducted to obtain more details about the diversity and abundance of bacteria in the investigated factory. In these analyses, another but similar waste paper factory in Sabhan industrial area was also considered for comparison and included both summer and winter testing. Pooled DNA samples from all indoor air DNA of the two factories sampled at the same season were used for metagenomic analysis. Both 16SV4 and 18SV9 metagenome libraries were prepared to be sequenced with Miseq (Source BioScience, UK) for gene determination.
Results and discussion
Microbial counts
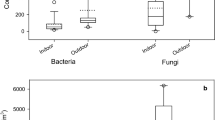
Table 1 presents bacterial and fungal occurrence in the indoor air samples collected from four sites, namely (1) waste paper cutting/shredding stage, (2) paper pulping/processing stage, (3) paper finishing stage and (4) personnel offices (used as background). Four locations, namely a, b, c and d, distributed around the machine(s) in sites 1, 2 and 3 or the offices in site 4, were chosen to collect four different samples in each site in order to obtain the mean value. Similarly, Table 2 shows statistical analysis of both bacterial and fungal data at same locations where the data obtained (as CFU) in Table 1 were further used to calculate the CFU/m3. The results showed some variability in bacterial and fungal counts so that data should focus on the median of CFU/m3 (either bacterial or fungal) for each sampling point. Bacterial counts were always higher than fungal counts after 2 days of incubation. It is to be noted that fungi incubation may need 5 days, but the plates were covered with mycelia that made fungal counting impossible after 2 days. The results obtained for both fungal and bacterial counts in the studied factory were higher compared to those reported in the literature for either indoor bacterial or fungal counts in factories or office buildings (Jasmine et al. 2002; Tsai et al. 2002; Hamada and Fujita 2002; Krajewska-Kulak et al. 2011; Hsu et al. 2012; Gaskin et al. 2012).
Given the type of activity performed in the factory, different levels of contamination were observed. Total bacterial counts as high as 575 CFU/m3 and fungal counts of 302 CFU/m3 were observed at the waste paper shredding location within the factory which, from the occupational exposure standards, would be considered as “heavy” microbiological contamination source within the factory with a potential health risk (Lavoie and Guertin 2001). Other locations, such as the paper processing and finishing areas, are less contaminated as the flow of air along the factory, provided through ventilation, disperses and dilutes the microbes, and air contamination may only be “mild”. This study confirms that a correct management of air flows within an industrial building where sources of bioaerosols are present can result in a good control of air quality in a waste paper processing facility. However, some areas require a higher level of attention in terms of workers protection.
Figure 1 presents the mean, minimum and maximum counts, while Fig. 2 shows the range and standard deviation reported for bacteria. Similarly, Figs. 3 and 4 display the mean, minimum and maximum counts as well as the range and standard deviation reported for fungi, respectively. These figures illustrate a gradient in the counts moving from site 1 to site 3, presumably due to air passage through ventilation leading to microbial dilution, while site 4 represents a blank reference. Progressive air flow control should be implemented at the cutting/shredding area, where personnel are present throughout the entire work shift, in order to prevent the intake of bioaerosols from sources of heavy contamination. The cutting/shredding unit, as described above, exhibits high microbiological contamination; thus, corrective measures should be taken in order to achieve better air quality. Specifically, the implementation of an air flow system that prevents contamination from areas where raw waste paper is handled should be undertaken.
Further microscopic examination showed that the highest concentration of Gram-negative bacteria was found in the delivery pit, machine shop, waste dropping gate and the waste paper cutting/shredding unit with medians of 688, 284, 316 and 233 CFU/m3, respectively. These values do not exceed the suggested occupational exposure limit of 1000 CFU/m3 (Lavoie and Guertin 2001). However, this limit is largely exceeded at single time points in the waste delivery areas. Again, the lowest contamination levels for Gram-negative bacteria were found in the personnel offices (medians < 1 CFU/m3). Factory workers and employees are exposed to high bacterial and fungal contamination. Dirt, in addition to temperature and humidity, may be factor of the bacterial and fungal contamination. Adequate ventilation as well as the use of fungicides could be employed to control microbiological contamination in the factory.
Molecular analyses
These included two factories as mentioned earlier. The 16S library gave good amplification for the DNA, while the 18S gave very poor quality and quantity for the DNA (Fig. 5). Moreover, the two pie charts (Fig. 6) show the dominant bacterial phyla in the air collected during summer (Fig. 6a) and winter (Fig. 6b) from the two factories studied. Members that belong to Firmicutes and Proteobacteria dominated the factories indoor air during the summer season, while bacteria that belong to Proteobacteria, Firmicutes, Actinobacteria and Bacteroidetes dominated the factories indoor air during the winter season. The results showed that the air samples collected from the two factories during summer season were dominated with unclassified bacteria (40%) while the most dominant known genus was Thermicanus (26.3%) followed by Burkholderia (21.1%), Vibrio (2.04%), Thermosinus (1.3%) and Thermoanaerobacterium (1.02%). In addition, there were 412 different genera in the same air samples with occurrences percentage less than 1% for each. On the other hand, the samples collected from the two factories during winter were dominated with both Thermicanus (21.8%) and unclassified genera (21.4%). The Burkholderia (19.8%), Prevotella (11.5%), Bifidobacterium (10.03%), Corynebacterium (7.74%) and Acinetobacter (2.3%) all showed > 1% presence in the air samples, while another 392 different genera were also detected in the same samples with abundance percentage less than 1%.
The most dominant genus in the current study according to both DGGE sequencing and metagenome analysis was Thermicanus. The dominance of this thermophilic, microaerophilic fermentative species especially during the summer season may reflect the condition within the factory as well as the source of the recycled paper and the way it has been handled and stored. This soil bacterium can contaminate the recycled paper during storing and before being processed and hence dominates the inner air. It is a fact that in a paper recycling factory there is tendency to close all water loops or reduce the consumption of water to minimum in order to save water. The reduction in water consumption is reported by Öqvist et al. (2008) to increase the process temperatures and to increase the colloidal and dissolved material in the process circulation. The increase in the nutrient level in the water circuits may favour microbial growth and fouling that is dominated by thermophilic bacteria. The resulted aerosols that form due to the activity in the paper factory and machine activities help in launching of these thermophilic bacteria in the air. In addition, because the indoor bacteria as mentioned above are originated mainly from indoor sources, this explains the dominance of the Thermi-members of the phylum Firmicutes in the two factories indoor air during summer.
The Thermicanus proved to be more successful in this system because it grows at a broad range of substrates, including oligosaccharides such as stachyose, raffinose, maltose, sucrose, cellobiose, lactose, galactose, glucose, fructose, mannose, acetate, formate, succinate and xylose (Gobner et al. 1999). As an organism that resides in an environment prone to fluctuations in O2 and organic carbon levels, these factors likely contribute to the competitiveness of this genus under in situ conditions (Gobner et al. 1999). Thermicanus despite being Gram-positive does not contain peptidoglycan, but instead it has S-layer which makes it very resistant to harsh conditions. The optimal growth of this bacterium is reported at 55–60 °C and at pH 6.5–7. No record is available about the effect of this genus on human health, and more work is needed to be done to investigate its ability in forming biofilms.
On the other hand, other bacteria found dominating the metagenome libraries during summer and winter such as Burkholderia are very important in paper recycling factories. A previous study done by Väisänen et al. (1998) showed that Burkholderia, Bacillus coagulans and Acinetobacter which were also detected in the current study are able to form biofilms on the machines, especially in the press section where both types of bacteria have high affinity to form biofilms on stainless steel. Not only that but also both Burkholderia and Bacillus coagulans are able to degrade paper-making chemicals. Meanwhile, Tiirola et al. (2009) proved that Cloacibacterium was among the primary colonizers of biofilms forming on machines in paper mills and so it contributes intensively to the functioning of the machine where it gets degraded, hence affecting the operating and maintenance (O&M) costs by increasing the budget required for cleaning and maintaining the machines. Many microorganisms degrade paper-making materials such as cellulosic fibre, starch, casein and resin sizers or may cause deterioration in product quality by producing odour, taste, undesirable colouring and contamination (Väisänen et al. 1998). So it is important to deeply investigate the ability of the identified bacteria in Shuaiba and Sabhan factories and assess their role. For instance, many scientists find it essential to prevent attachment of the primary colonizing bacteria to the machines than to control the growth of secondary communities, which are sheltered by exopolysaccharide slime layers because the latter action is more difficult and costly. It should be pointed out that Burkholderia, the most dominant proteobacteria identified in the current study, were reported by many scientists to have extreme adhesiveness to stainless steel, indicating that they will be difficult to remove from any industrial system in which they have colonized. Biofilm bacteria have poor sensitivity to any biocides (Brown and Gilbert 1993), and adhesion frustrates mechanical cleaning (Väisänen et al. 1998).
The metagenomic results also showed seasonal variations in indoor air microbes in the two factories where summer samples were dominated with unclassified bacteria and Firmicutes, while winter samples were dominated by Proteobacteria and Firmicutes. The temperature and relative humidity may be the major factors that determine the survival of the bacteria in the air and hence the recorded variation in the bacterial diversity between the two seasons (Frankel et al. 2012).
Health impact
Exposure to bioaerosols may pose health risks to workers operating in the processing of recycled paper. Gram-negative bacteria may exert adverse effects on exposed workers, as evidenced by high concentrations of airborne endotoxin and the presence of numerous potentially pathogenic species. Thus, these microorganisms pose a potential risk of respiratory diseases for the workers in the factories (Madsen et al. 2016).
Exposure levels in the organic waste chain vary widely between various waste management sites. Highest exposure levels are found in those jobs in which waste is intensively disturbed and/or handled indoors. In the highest exposure categories, mean values exceeded Dutch occupational exposure limits, suggesting that at all sites workers are at risk of developing adverse health effects (Wouters et al. 2015). Exposure levels at the factory sites showed large variability, with exposure levels varying more over time within workers than between workers. This implies that in the paper recycling factories, more and repeated measurements are needed to assess exposure precisely.
Depending on particle size, bioaerosols may penetrate deep into the lungs and become embedded in alveoli. Principal types of health effects of bioaerosols identified in published studies (Pearson et al. 2015) on humans include, but are not limited to:
-
1.
Allergic asthma, rhinitis, hypersensitivity pneumonitis (HP)/extrinsic allergic alveolitis, allergic bronchopulmonary aspergillosis (ABPA), eye and skin irritations.
-
2.
Toxic non-allergic asthma, rhinitis, mucous membrane irritations (MMI), chronic bronchitis, chronic airway obstruction such as chronic obstructive pulmonary disease (COPD), organic dust toxic syndrome (ODTS), toxic pneumonitis.
-
3.
Infectious aspergillosis and zygomycosis.
Biological hazard to man arises from exposure to high concentrations or unfamiliar forms of bioaerosols, and three major groups of diseases associated with bioaerosol exposure are infectious diseases, respiratory diseases and cancer, but current knowledge is unclear regarding risk to cancer. Moreover, various bacterial diseases such as legionellosis (caused by legionella pneumophila) and tuberculosis (transmitted by tubercle bacilli) are linked to cause significant public health concern due to their low infectious dose. Fungal diseases due to respiratory infections and allergic reactions include Penicillium, Aspergillus, Acremonium, Paecilomyces Mucor and Cladosporium (Srikanth et al. 2008).
Control measures/effect of ventilation
Certain control measures and technologies can be followed to reduce bioaerosol loads in indoor environments (Luengas et al. 2015). These include proper elimination of the microbial source, maintenance of equipment, humidity control, natural ventilation, the use of filters in ventilation and the use of disinfectants and biocides for air cleaning.
To reduce exposure inside the factory, focus should be on interventions related to the unloading of waste and on good hygiene in the processing unit. Bioaerosol exposure indoors is an important factor influencing human health. Thus, understanding the dynamic behaviour of biological particles inside the factory is essential in the design of effective engineering controls to reduce microbial concentrations and to limit exposures. Building air exchange is an important removal mechanism for indoor bioaerosols and a means by which outdoor bioaerosol particles are brought indoors. Building air exchange is the replacement of indoor air with outdoor air. Air exchange is used to limit the accumulation of bioaerosols. When outdoor air is uncontaminated, then increasing the air exchange rate consistently improves indoor air quality (Nazaroff 2014). The use of mechanical ventilation is recommended in the waste paper recycling factories. In this regard, fans could be used to induce mechanical ventilation.
The transport and ultimate settling of a bioaerosol are affected by its physical properties such as size, density and shape of particles and by environmental factors which include magnitude of air currents, relative humidity and temperature, which determine the capacity to be airborne (Srikanth et al. 2008). Increased humidity of the outdoor–indoor air in the summer season as well as dust storms may generate bioaerosols as dust or powders that partially rehydrate and may also lead to the presence of moulds which would aggravate the conditions inside the waste paper recycling factories.
Conclusion
The results indicate the presence of considerable microbial levels of bacteria and fungi in the waste paper factory in all the manufacturing stages as compared to the office cubical. In particular, the total bacterial counts and fungal counts observed at the waste paper cutting/shredding location within the factory were high, but the mean values did not exceed the occupational exposure limits. The observed levels would be considered as “heavy” microbial contamination source within the factory. Other locations, such as the paper processing and finishing areas, are less contaminated as the flow of air along the factory, which was provided through ventilation, would disperse and dilute the microbes; thus, air contamination may only be “mild”. The most dominant genus detected according to both DGGE sequencing and metagenome analysis was Thermicanus. The dominance of this thermophilic, microaerophilic fermentative species especially during summer season reflects the condition within the factory as well as the source of the recycled paper and the way it has been handled and stored. An air flow system that prevents contamination from areas where raw waste paper is handled should be implemented. This study confirms that correct management of air flows within an industrial building where sources of bioaerosols are present can result in a good control of air quality in the waste paper recycling facility. However, some areas in the factory such as the receiving and the waste paper cutting/shredding facilities require a higher level of attention in terms of workers protection. In general, workers and employees of the waste paper recycling factory should use respiratory protection equipment as a precautionary health measure.
References
Brown MRW, Gilbert P (1993) Sensitivity of biofilms to antimicrobial agents. J Appl Microbiol 74:875–975
Del Cimmuto A, D’Acunzo F, Marinelli L, De Giusti M, Boccia A (2010) Microbiological air quality in an urban solid waste selection plant. Italian J Public Health 7(1):20–27
Fraczek K, Kozdroj J, Gorny RL, Cyprowski M, Golofit-Szymczak M (2017) Fungal air contamination in distinct sites within a municipal landfill area. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-017-1344-9
Frankel M, Bekö G, Timm M, Gustavsen S, Hansen EW, Madsen AM (2012) Seasonal variations of indoor microbial exposures and their relation to temperature, relative humidity, and air exchange rate. Appl Environ Microbiol 78(23):8289–8297
Gaskin S, Taylor M, Bentham R, Pisaniello D (2012) Understanding and managing risks associated with fungal contamination in indoor environments. Final Report, The University of Adelaide
Gobner AS, Devereux R, Ohnemuller N, Acker G, Stackebrandt E, Drake HL (1999) Thermicanus aegyptius gen. nov., sp. nov., isolated from oxic Soil, a fermentative microaerophile that grows commensally with thermophilic acetogen moorellathermoacetica. Appl Environ Micro 65(11):5124–5133
Hamada N, Fujita T (2002) Effect of air-conditioner on fungal contamination. Atmos Environ 36:5443–5448
Hsu YC, Kung PY, Wu TN, Shen YH (2012) Characterization of indoor air bioaerosols in Southern Taiwan. Aerosol Air Qual Res 12:651–661
Jasmine CH, Schwartz J, Milton DK, Harriet AB (2002) Populations and determinants of airborne fungi in large office buildings. Environ Health Perspect 110(8):777–782
Krajewska-Kulak E, Lukaszuk C, Chadzopulu A, Bousmoukilia S, Terovitou C, Theodosopoulou E, Amanatidou A, Danilidis D (2011) Indoor air studies of fungi contamination at the tobacco factory in Kavala, Greece. Prog Health Sci 1(1):21–26
Lavoie J, Guertin S (2001) Evaluation of health and safety risks in municipal solid waste recycling plants. J Air Waste Manag 51(3):352–360
Luengas A, Barona A, Hort C, Gallastegui G, Platel V, Elias A (2015) A review of indoor air treatment technologies. Rev Environ Sci Biotechnol 14(3):27
Madsen AM, Alwan T, Orberg A, Uhrbrand K, Jorgensen MB (2016) Waste workers’ exposure to airborne fungal and bacterial species in the truck cab and during waste collection. Ann Occup Hyg 60(6):651–668
Miller PA, Clesceri NL (2002) Waste sites as biological reactors; characterization and modeling. CRC Press, Boca Raton
Nazaroff WW (2014) Indoor bioaersol dynamics. Wiley, New York
O’Connor DJ, Daly SM, Sodeau JR (2015) On-line monitoring of airborne bioaerosols released from a composting/green waste site. Waste Manag 42:23–30
Öqvist CK, Kurola J, Pakarinen J, Ekman J, Ikävalko S, Simell J, Salkinoja-Salonen M (2008) Prokaryotic microbiota of recycled paper mills with low or zero effluent. J Ind Microbiol Biotechnol 35(10):1165–1173
Parker JH, Spiegelman DL, Gold DR (2001) Predictors of airborne endotoxin in the home. Environ Health Perspect 109:859–864
Pearson C, Littlewood E, Douglas P, Robertson S, Gant TW, Hansel AL (2015) Exposures and health outcomes in relation to bioaerosol emissions from composting facilities: a systematic review of occupational and community studies. J Toxicol Environ Health B Crit Rev 18(1):43–69
Schlosser O, Robert S, Debeaupuis C (2016) Aspergillus fumigatus and mesophilic moulds in air in the surrounding environment downwind of non-hazardous waste landfill sites. Int J Hyg Environ Health 219:239–251
Srikanth P, Sudharsanam S, Steinberg R (2008) Bioaerosols in indoor environment: composition, health effects and analysis. Indian J. Med Microbiol 26:302–312
Tiirola M, Lahtinen T, Vuento M, Oker-Blom C (2009) Early succession of bacterial biofilms in paper machines. J Ind Microbiol Biotechnol 36(7):929–937
Tsai FC, Macher JM, Hung Y-Y (2002) Concentrations of airborne bacteria in 100 US office buildings. In: Proceedings of Indoor Air Quality, pp 353–358
Väisänen OM, Weber A, Bennasar A, Rainey FA, Busse H-J, Salkinoja-Salonen MS (1998) Microbial communities of printing paper machines. J Appl Microbiol 84:1069–1084
Walser SM et al (2015) Evaluation of exposure–response relationships for health effects of microbial bioaerosols—a systematic review. Int J Hyg Environ Health 218:577–589
Wouters IM, Span S, Douwes J, Doekes G, Heederik D (2015) Overview of personal occupational exposure levels to inhalable dust, endotoxin, β(1 → 3)-Glucan and fungal extracellular polysaccharides in the waste management chain. Ann Occup Hyg 50:39–53
Yassin MF, Almouqatea S (2010) Assessment of airborne bacteria and fungi in an indoor and outdoor environment. Int J Environ Sci Technol 7(3):535–544
Acknowledgements
This study was financially supported by a research grant (Project RW 02/12) from the Research Administration, Kuwait University. The authors would like to thank Mr. Ammar Al-Ibrahim for his assistance in air sampling and analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Mohamed Fathy Yassin.
Rights and permissions
About this article
Cite this article
Hamoda, M.F., Mahmoud, H. Microbiological characteristics of indoor air bioaerosols in a waste paper recycling factory. Int. J. Environ. Sci. Technol. 16, 2601–2610 (2019). https://doi.org/10.1007/s13762-018-1694-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-018-1694-y