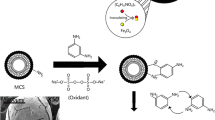
Abstract
In this paper, we modified chitosan beads in order to simultaneously detect and adsorb Co2+ from aqueous solution. Firstly, 4-(5-chloro-2-pyridylazo)-1,3-phenylenediamine (5-Cl-PADAB) was used as selective probe for Co2+ with color changing from yellow to pink, and the UV–Vis spectra showed that the λ max changed from 439 to 504 nm. Then a novel biomaterial was synthesized with 5-Cl-PADAB (metal indicator), chitosan (biosorbent) and EDTA anhydride (cross-linker and chelating agent). The analysis of Fourier transform infrared and energy-dispersive X-ray spectra proved that 5-Cl-PADAB and EDTA were successfully connected to chitosan. The modified chitosan bead was selective probe for Co2+ with a remarkable color change from white to pink, and the UV–Vis spectra showed that the λ max changed from 441 to 459 nm. The adsorption of cobalt ions onto the modified chitosan beads followed pseudo-second-order (R 2 = 0.99) kinetics and the Langmuir isotherm model (R 2 = 0.80). Comparing with chitosan beads, the q e of the modified one increased from 2.00 to 7.97 mg/g. The modified chitosan beads are promising biomaterial for simultaneous detection and removal of Co2+ from aqueous solution.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among various pollutants, radioisotopes, such as 90Sr, 137Cs, and 60Co, are very dangerous if not taken good care of. For the safe of whole nature, the concentration of radioisotopes should be diminished to certain acceptable level before discharge. To meet the strict environmental regulations, cobalt ions in solution are removed by various methods, such as adsorption (Chen et al. 2007; Missana and Garcia-Gutierrez 2007; Zhu et al. 2014; Xing et al. 2016; Xing and Wang 2016), flocculation precipitation (Wang et al. 2007), biosorption (Suhasini et al. 1999; Wang and Chen 2006, 2009, 2014; Jo et al. 2015), membrane separation (Cojocaru et al. 2009; Uzal et al. 2011; Zakrzewska-Trznadel 2013) and the like. Among those techniques, biosorption is considered to be a good option because it is cheap, easy to use, and environmentally friendly.
Chitosan is an N-deacetylated derivative of chitin, which is the second most abundant natural organic resource in the earth. It is rich in arthropods, fungi, and yeast. With abundant amino groups and hydroxyl groups, this natural polymer can act as sorbent and be modified for certain purpose (Minamisawa et al. 1999; Mostafa et al. 2005; Zhu et al. 2012; Kongkaoroptham et al. 2015). Besides, it has a variety of current and potential applications in many fields, such as cosmetics (Tombs and Harding 1998), drug-delivery materials (Casimiro et al. 2005; Pérez-Calixto et al. 2016), agriculture (El-sawy et al. 2010) and biotechnological materials (Sinch and Ray 1994; Li et al. 2013), etc.
The chromogenic agent, 4-(5-chloro-2-pyridylazo)-1, 3-phenylenediamine (5-Cl-PADAB), has been widely used in spectrophotometric determination since Shibata’s study on it (Shibata et al. 1973; 1974). As a typical azo-compound, it can undergo a color change when combing to some certain metal ions and some color changes can even be seen by naked eyes (Huang et al. 1997; Wu et al. 2013). For years, the spectrophotometric determination of cobalt ions with 5-Cl-PADAB has been well established. However, it is still not convenient to detect the cobalt ions on the spot for it needs heavy and expensive machine and time and energy to deal with the solution.
EDTA is known as chelating agent for most metal ions. Its anhydride product is called EDTA anhydride. With two anhydride groups, it can react with other two molecules, which have amino groups or hydroxyl groups, at the same time (Capretta et al. 1995; Roosen and Binnemans 2014). Thus, it can be used to modify other material for the purpose of creating amphoteric compound or increasing the absorption capability (Shen et al. 2007; Tan et al. 2015; Fujita and Sakairi 2016).
The objective of this work was to synthesize a novel biomaterial by the reaction of 5-Cl-PADAB (metal indicator), chitosan (biosorbent) and EDTA anhydride (cross-linker and chelating agent) in order to simultaneously detect and adsorb radioisotope Co2+. After adsorption of Co2+, the color of modified chitosan beads changed from white to pink, which could be observed by naked eyes. This work was conducted in 2015–2016, in the Laboratory of Environmental Technology, Tsinghua University, Beijing, China.
Materials and methods
Chemicals
Chitosan (molecular weight: 130 kDa, degree of deacetylation: 90%), methyl alcohol, cobalt chloride and acetic acid were purchased from Sinopharm Chemical Reagent Co., Ltd. 4-(5-chloro-2-pyridylazo)-1,3-phenylenediamine (5-Cl-PADAB) was purchased from Aladdin. EDTA anhydride and dialysis bag (MW: 3500) were purchased from Aladdin. All chemicals were used as received without further purification.
Modification of chitosan with 5-Cl-PADAB and EDTA
Chitosan (0.175 g) was dissolved in 5 mL aqueous acetic acid solution (10%, v/v), and methyl alcohol (30 mL) was added to the solution above and stirred for 20 min. Then, 5-Cl-PADAB (20 mg) and EDTA anhydride (20 mg) were dissolved in the solution above in order. The reaction mixture was stirred for 48 h at room temperature. Later the reaction mixture was dialyzed for 2 d and the distilled water was changed every 2 h. The solution in the dialysis bag was put into the laboratory oven at 50 °C to concentrate (to about 10 mL). Then the pure solution of modified chitosan was achieved.
Preparation of chitosan beads
Chitosan (0.15 g) was dissolved in 5 mL aqueous acetic acid solution (4%, v/v) and stirred for 3 h. When the bubbles in the solution disappeared, the mixed solution was injected into NaOH solution (15%, w/v). The chitosan beads were then washed with distilled water to eliminate any remaining sodium hydroxide. Finally, the chitosan beads were kept in distilled water for later use.
Preparation of modified chitosan beads
Chitosan (0.3 g) and acetic acid solution (0.2 mL) were added to the pure solution of modified chitosan and stirred for 3 h. When the bubbles in the solution disappeared, the mixed solution was injected into NaOH solution (15%, w/v). The modified chitosan beads were then washed with distilled water to eliminate any remaining sodium hydroxide. Finally, the modified chitosan beads (about 95% moisture) were kept in distilled water for later use.
Adsorption experiments
Co2+-containing stock solution was prepared using CoCl2 to achieve the final concentration of 200 mg/L. Then it was diluted to various concentrations before use without pH adjustment. Biosorbents were added to the solutions with different concentrations of cobalt ions at room temperature (about 26 °C). Then, the samples were shaken at 150 rpm. The concentration of cobalt ions was measured at different time until equilibrium was reached.
The adsorption capacity (q t ) and equilibrium adsorption capacity (q e) can be calculated by the following equation:
where C 0, C e and C t are the initial concentration, equilibrium concentration and at time t’s concentration of cobalt ions, respectively; V is the volume of the solution; and m is the mass of the dry adsorbents (calculated).
Analytical methods
IR spectra were obtained on a VERTEX 70 FT-IR (Bruker). The data were received from 400 to 4000 cm−1 with resolution of 2 cm−1 and 16 scans. UV–Vis absorption spectra were obtained on a Lambda 25 spectrometer (PerkinElmer, USA). The concentrations of cobalt ions were determined using a ZA3000 Polarized Zeeman Atomic Absorption Spectrophotometer (HITACHI, Japan). Energy-dispersive X-ray (EDX) data were obtained on scanning electron microscopy QUANTA 200 FEG (EDAX, USA).
Results and discussion
Color change of 5-Cl-PADAB and variation of UV–Vis spectra
Figure 1 shows the color variation of Co2+-containing solution and 5-Cl-PADAB solution. 5-Cl-PADAB (0.01 g) was dissolved in anhydrous alcohol (100 mL) and then diluted with pure water for analysis. Figure 1a shows the color of 5-Cl-PADAB solution, CoCl2 solution and distilled water, respectively (from left to right); Fig. 1b shows the color of 5-Cl-PADAB solution, 5-Cl-PADAB solution + Co2+ and distilled water + 5-Cl-PADAB, respectively (from left to right); Fig. 1c shows the reaction of 5-Cl-PADAB with Co2+.
It was proved that 5-Cl-PADAB had high selectivity for Co2+ and could indicate the presence of Co2+ with color change, which could be observed by naked eyes. The color of the low concentration of 5-Cl-PADAB dissolved in water was slight yellow (Fig. 1b, right). It changed into pink color when Co2+ was added (Fig. 1b, middle). Moreover, it kept almost the same color in the presence of different metal ions (e.g., Sn2+, Ag+ Pb2+, Sr2+, Al3+, Ce3+, Cs2+, Fe3+, and Mg2+). Besides, it is obvious that Co2+ is slightly pink (Fig. 1a, middle). When Co2+ combined with 5-Cl-PADAB, the color changed from slight pink to pink (Fig. 1b, middle), and the color of pink was strengthened, indicating that the colorization reaction between Co2+ and 5-Cl-PADAB was taken place, as shown in Fig. 1c.
Figure 2 shows a variation in the UV–Vis spectra of 5-Cl-PADAB before and after the addition of Co2+. Because 5-Cl-PADAB is very slightly soluble in water (without pH adjustment and other materials), the supernatant was diluted for the experiment. After adding Co2+, the previous pure 5-Cl-PADAB solution showed a universal redshift with its maximum absorption wavelength changing from 439 to 504 nm, so the color was changed from white to pink, which can be obviously observed by the naked eye.
Modification of chitosan with 5-Cl-PADAB and EDTA anhydride
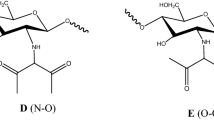
As shown in Scheme 1, three main reactions were conducted during the procedure of preparing modified chitosan. From the reaction mechanism, it could be seen that EDTA anhydride had several important roles in the preparation process. Firstly, EDTA anhydride could react with the amino groups of chitosan and connect different chitosan through amide bonds; secondly, EDTA anhydride could modify the amino groups of chitosan with EDTA chelating groups, which may enhance adsorbing capacity; thirdly, EDTA anhydride could connect 5-Cl-PADAB and chitosan via amide bonds. Other side reactions may occur at the same time, while the side products were taken out of the system during the dialyzing.
The evidence of the existence of modified chitosan could be further achieved by Fourier transform infrared (FT-IR) analysis. From the FT-IR spectrum of the chitosan, we can see the characteristic bands at 3365, 2877 and 1598 cm−1, which are assigned to be O–H stretching vibration, polymer backbone’s C–H stretching vibration and N–H bending vibration, respectively. As shown in Fig. 3, modified chitosan has other special characteristic bands added at 1737, 1634 and 827 cm−1 besides chitosan’s characteristic bands. The band at 1737 cm−1 is assigned to be the C=O stretch vibration of –COOH groups. The band at 1634 cm−1 is assigned to be C=O stretching vibrations of –CONH- generated from the reaction of –NH2 with EDTA anhydride. In addition, the band at 827 cm−1 is assigned to be out of plane bending vibration of 1,2,4-substituted phenyl ring’s adjacent C–H, which indicates that 5-Cl-PADAB is successfully grafted into the chitosan. At least, the third reaction of Scheme 1 is well proved by FT-IR analysis. Besides, after dialyzing for 2 days, the small molecules, such as 5-Cl-PADAB, were removed from the solution, while the yellow color of the modified chitosan solution can easily be seen by naked eyes. This can add evidence to this conclusion to some degree.
Detection of Co2+ with modified chitosan beads
Considering that the adsorbent can concentrate metal ions, small portion of indicator in chitosan beads is able to result in great color change. Besides, the indicator is much more expensive than chitosan. Thus, more chitosan was added to form the modified chitosan beads.
With diameter ranging from 3 mm to 4 mm, the modified chitosan beads were added to the Co2+-containing solution. As repeated every time in the laboratory, the color of the beads changed from white to pink very quickly and the diameter changed little, which can be seen in Fig. 4.
Considering that cobalt (II) has pale pink color, there would be a coloration of cobalt (II)-chitosan complex (Guan and Cheng 2004; Shaabani et al. 2015). However, the coloration is rather slow and after a long contact time in the same Co2+-containing solution, the pure chitosan beads can turn into pink color, suggesting that it was the complex formation with cobalt (II) and 5-Cl-PADAB that contributes to the color change at the beginning, while the coloration of cobalt (II)-chitosan complex strengthen the pink color as time goes by.
Figure 5 shows the EDX analysis results of modified chitosan beads before and after adsorption Co2+. Figure 5a shows the presence of chlorine (0.52 wt % or 0.2 at %), indicating the successful connection of 5-Cl-PADAB into chitosan. As discussed above, the proportion of 5-Cl-PADAB was not high. Besides, the presence of cobalt in Fig. 5b shows the adsorption of cobalt ions (9.51 wt % or 2.47 at %) into modified chitosan beads. Because the Co2+-containing solution was prepared from CoCl2, there is an increase in Cl content after adsorption, as shown in Fig. 5b. Although EDTA, 5-Cl-PADAB and chitosan are all composed of the elements carbon, hydrogen, oxygen and nitrogen with different elemental proportion, there are still some difference among them. The accurate elemental proportion of EDTA, 5-Cl-PADAB can be obtained. However, chitosan is a polymer which has not accurate elemental proportion (Varma et al. 2004). Besides, comparing to chitosan, there are only small amount of EDTA and 5-Cl-PADAB in the modified chitosan beads. Thus, the change of the elemental proportion is not obvious and the data could not be used to calculate the grafting degree of EDTA and 5-Cl-PADAB in the modified chitosan beads. Even so, according to their dosage added in the preparation process, it can be calculated that the maximum substitution degree of EDTA group on the modified chitosan beads was less than 4% (w/w), i.e., 20 mg (EDTA)/515 mg(chitosan beads).
In addition, Fig. 6 shows that after adding Co2+ (0.002 mol/L), the modified chitosan showed a universal redshift with its maximum absorption wavelength changing from 441 to 459 nm. Considering the pale pink color of Co2+, there would be a coloration of cobalt (II)-chitosan complex (Guan and Cheng 2004; Shaabani et al. 2015). However, according to Fig. 6, the maximum absorption wavelength of Co2+ is 513 nm, which is quite different from that of the modified chitosan after adsorption of Co2+. In addition, the coloration reaction of cobalt (II)-chitosan complex is rather slow. After a long exposure time in the same Co2+-containing solution, the pure chitosan beads can turn into pink, indicating that the complex formation with cobalt (II) and 5-Cl-PADAB contributed to the color change at the beginning, while the coloration of cobalt (II)-chitosan complex strengthen the pink color as time goes by.
As expected, after addition of other metal ions (e.g., Sn2+, Ag+, Pb2+, Sr2+, Al3+, Ce3+, Cs2+, Fe3+ and Mg2+), the color of modified chitosan beads kept almost unchanged, indicating that modified chitosan beads had high selectivity for Co2+ and could probe Co2+ in aqueous solution by naked eyes quickly.
Adsorption capacity
The adsorption experiments were carried out by mixing modified chitosan beads and chitosan beads with 50 mL of 100 mg/L Co2+-containing solution, respectively. The mixture was agitated at 150 rpm in a temperature-controlled shaker to reach the equilibrium.
Figure 7 gives the typical results for the adsorption of Co2+ onto modified chitosan beads and chitosan beads, respectively. Both for the modified chitosan beads and chitosan beads, the plots were characterized by a monotonous increasing trend with a gradual rise, then reaching equilibrium at 90 min (modified chitosan beads) or 20 min (chitosan beads).
Pseudo-second-order kinetic model was used to simulate the equilibrium adsorption capacity (q e) by chitosan beads and the modified chitosan beads. It is derived assuming second-order dependence of the sorption rate on available sites and described by following equation:
Its linearized form is given as:
where k 2 is the kinetic rate constant for pseudo-second-order and t is time.
As listed in Table 1, the experimental data fitted well with the pseudo-second-order model with R 2 up to 0.99 (the modified chitosan beads) or 0.97 (chitosan beads). It was obvious that q e of the modified chitosan beads (7.97 mg/g) was much higher than that of chitosan beads (2.00 mg/g). The increasing equilibrium adsorption capacity of Co2+ by the modified chitosan beads might contribute to the introduction of EDTA in the modified beads, which is chelating agent for most metal ions (Fujita and Sakairi 2016; Tan et al. 2015; Roosen and Binnemans 2014). It was calculated that the maximum substitution degree of EDTA group on modified chitosan beads was less than 4% (w/w).
To investigate isotherm models for modified chitosan beads, a series of adsorption experiments were performed. The most common equilibrium adsorption isotherm models, i.e., the Langmuir and the Freundlich models, were used to fit the experimental data. The Freundlich isotherm model is an experimental model and it is linearized form as follows:
where K F is the Freundlich constant describing the sorption capacity; and n is the Freundlich constant, which is indicator of heterogeneity of the sorbent surface;
The Langmuir isotherm model is a theoretical model for monolayer adsorption, whose linearized form is given as follows:
where q m (mg/g) and K L (L/mg) are the maximum monolayer capacity of the gel beads and the affinity constant, respectively.
The modeling results of cobalt ions adsorption by modified chitosan beads are listed in Table 2. It was found that the data fitted the Langmuir model (R 2 = 0.80) better than Freundlich’s (R 2 = 0.56), indicating that monolayer adsorption can better explain this adsorption procedure. From Langmuir model’s adsorption constants, the q m was about 7.61 mg/g, which was much closer to the pseudo-second-order model’s result (q e = 7.97 mg/g).
Minamisawa et al. (1999) investigated the effect of pH on the adsorption of cobalt(II) onto chitosan. They found that cobalt(II) was quantitatively adsorbed onto chitosan over a wide pH range from 5.0 to 10.0. The adsorption of cobalt(II) decreased with a decrease of pH in the lower pH range because the adsorption of cobalt(II) was incomplete due to the dissolution of chitosan in the lower pH range. In this study, we performed the adsorption of cobalt(II) onto the modified chitosan beads in the neutral region (without pH adjustment).
The adsorption of cobalt ions has been studied by means of diverse adsorbent materials. To evaluate different adsorbent material’s sorption performance, Langmuir q m values were used for the comparison. As shown in Table 3, the CSIS possesses highest q m (53.51 mg/g) and shortest equilibrium time (70 min) for cobalt ions owing to isatin’s modification for chitosan and the resin type. However, comparing with other modified chitosan beads (XMCS and MCS) and particles (CTS-MMT), this article’s modified chitosan beads have shortest equilibrium time (90 min) and a higher q m (7.61 mg/g) than MCS’s (2.98 mg/g) and CTS-MMT’s q m (0.5098 mg/g) while lower than XMCS’s q m (18.5 mg/g). Above all, in terms of maximum adsorption capacity, the modified chitosan beads synthesized is a potential adsorbent for Co2+-containing wastewater treatment.
Conclusion
The novel biosorbent was synthesized and analyzed by FT-IR spectra and EDX. The analysis of FT-IR spectra proved that EDTA anhydride and 5-Cl-PADAB were successfully connected with chitosan. The EDX analysis results showed the presence of chlorine, indicating the successful connection of 5-Cl-PADAB to the modified chitosan. When combing with Co2+, the color of the modified chitosan beads was remarkably changed from white to pink, which can be observed by naked eyes, and the maximum absorption wavelength changed from 441 to 459 nm. The adsorption of Co2+ by the modified chitosan beads fitted well with pseudo-second-order kinetic model (R 2 = 0.99). After modification, the adsorption capacity of modified chitosan beads increased from 2.00 to 7.97 mg/g. The adsorption data fitted better with Langmuir isotherm model (R 2 = 0.80) than Freundlich isotherm model (R 2 = 0.56). The maximum adsorption capacity was 7.61 mg/g from Langmuir isotherm model for Co2+. The modified chitosan beads are promising biomaterial for simultaneous detection and removal of Co2+ from aqueous solution.
References
Capretta A, Maharajh RB, Bell RA (1995) Synthesis and characterization of cyclomaltoheptaose-based metal chelants as probes for intestinal permeability. Carbohydr Res 267:49–63
Casimiro MH, Botelho ML, Leal JP, Gil MH (2005) Study on chemical, UV and gamma radiation-induced grafting of 2-hydroxyethyl methacrylate onto chitosan. Radiat Phys Chem 72:731–735
Chen YW, Wang JL (2012) The characteristics and mechanism of Co(II) removal from aqueous solution by a novel xanthate-modified magnetic chitosan. Nucl Eng Des 242:452–457
Chen CL, Xu D, Tan XL, Wang XK (2007) Sorption behavior of Co(II) on γ-Al2O3 in the presence of humic acid. J Radioanal Nucl Chem 273:227–233
Cojocaru C, Zakrzewska-Trznadel G, Miskiewicz A (2009) Removal of cobalt ions from aqueous solutions by polymer assisted ultrafiltration using experimental design approach Part 2: optimization of hydrodynamic conditions for a crossflow ultrafiltration module with rotating part. J Hazard Mater 169:610–620
El-sawy NM, El-rehim HAA, Elbarbary AM, Hegazy EA (2010) Radiation-induced degradation of chitosan for possible use as a growth promoter in agricultural purposes. Carbohydr Polym 79:555–562
Fujita S, Sakairi N (2016) Water soluble EDTA-linked chitosan as a zwitterionic flocculant for pH sensitive removal of Cu(II) ion. RSC Adv 6:10385–10392
Ghassabzadeh H, Torab-mostaedi M, Mohaddespour A, Ghannadi M, Javad S, Zaheri P (2010) Characterizations of Co(II) and Pb(II) removal process from aqueous solutions using expanded perlite. Desalination 261:73–79
Guan HM, Cheng XS (2004) Study of cobalt(II)-chitosan coordination polymer and its catalytic activity and selectivity for vinyl monomer polymerization. Polym Adv Technol 15:89–92
Huang CZ, Li K, Tong SY (1997) Determination of nanograms of nucleic acids by their enhancement effect on the resonance light scattering of the cobalt(II)/4-[(5-chloro-2-pyridyl)azo]-1,3-diaminobenzene complex. Anal Chem 69:514–520
Jo A, Jang G, Namgung H, Kim C, King D, King Y, King J, Lee TS (2015) Simultaneous detection and removal of radioisotopes with modified alginate beads containing an azo-based probe using RGB coordinates. J Hazard Mater 300:227–234
Kongkaoroptham P, Piroonpan T, Hemvichian K, Suwanmala P, Rattanasakulthong W, Pasanphan W (2015) Poly (ethylene glycol) methyl ether methacrylate-graft-chitosan nanoparticles as a biobased nanofiller for a poly (lactic acid) blend: radiation-induced grafting and performance studies. J Appl Polym Sci 132:42522
Li R, Wang HD, Wang WF, Ye Y (2013) Simultaneous radiation induced graft polymerization of N-vinyl-2-pyrrolidone onto polypropylene non-woven fabric for improvement of blood compatibility. Radiat Phys Chem 88:65–69
Minamisawa H, Iwanami H, Arai N, Okutani T (1999) Adsorption behavior of cobalt(II) on chitosan and its determination by tungsten metal furnace atomic absorption spectrometry. Anal Chim Acta 378:279–285
Missana T, Garcia-Gutierrez M (2007) Adsorption of bivalent ions (Ca(II), Sr(II) and Co(II)) onto FEBEX bentonite. Phys Chem Earth 32:559–567
Monier M, Ayad DM, Wei Y, Sarhan AA (2010) Adsorption of Cu(II), Co(II), and Ni(II) ions by modified magnetic chitosan chelating resin. J Hazard Mater 177:962–970
Mostafa TB, Naguib HF, Sabaa MW, Mokhtar SM (2005) Graft copolymerization of itaconic acid onto chitin and its properties. Polym Int 54:221–225
Pérez-calixto MP, Ortega A, Garcia-uriostegui L, Burillo G (2016) Synthesis and characterization of N-vinylcaprolactam/N,N-dimethylacrylamide grafted onto chitosan networks by gamma radiation. Radiat Phys Chem 119:228–235
Roosen J, Binnemans K (2014) Adsorption and chromatographic separation of rare earths with EDTA- and DTPA-functionalized chitosan biopolymers. J Mater Chem A 2:1530–1540
Shaabani A, Boroujeni MB, Sangachin MH (2015) Cobalt-chitosan: magnetic and biodegradable heterogeneous catalyst for selective aerobic oxidation of alkyl arenes and alcohols. J Chem Sci 127:1927–1935
Shen XC, Zhang LY, Jiang XQ, Hu Y, Guo J (2007) Reversible surface switching of nanogel triggered by externa stimuli. Angew Chem Int Ed 46:7104–7107
Shentu B, Zhu Q, Liu Q, Weng Z (2007) Kinetics and equilibrium of cobalt ion adsorption on cross-linked polyethylenimine membrane. J Appl Polym Sci 105:1964–1967
Shibata S, Ishiguro Y, Nakashima R (1973) The spectrophotometric determination of palladium with 4-[(5-chloro-2-pyridyl)azo]-1,3-diaminobenzene. Anal Chim Acta 64:305–309
Shibata S, Furukawa M, Goto K (1974) Spectrophotometric studies on the reaction of cobalt with 4-(2-pyridylazo)-1,3-diaminobenzene and its halogen derivatives. Anal Chim Acta 71:85–96
Sinch DK, Ray AR (1994) Graft copolymerization of 2-hydroxyethylmethacrylate onto chitosan films and their blood compatibility. J Appl Polym Sci 53:1115–1121
Suhasini IP, Sriram G, Asolekar SR, Sureshkumar GK (1999) Biosorptive removal and recovery of cobalt from aqueous systems. Process Biochem 34:239–247
Tan ZY, Peng H, Liu HF, Wang LL, Chen J, Lu XH (2015) Facile preparation of EDTA-functionalized chitosan magnetic adsorbent for removal of Pb(II). J Appl Polym 132:42384
Tombs M, Harding SE (1998) An introduction to polysaccharide biotechnology. Taylor & Francis, London, p 144
Uzal N, Jaworska A, Miskiewicz A, Zakrzewska-Trznadel G, Cojocaru C (2011) Optimization of Co2+ ions removal from water solutions via polymer enhanced ultrafiltration with application of PVA and sulfonated PVA as complexing agents. J Colloid Interface Sci 362:615–624
van Hullebusch ED, Peerbolte A, Zandvoort MH, Lens PNL (2005) Sorption of cobalt and nickel on anaerobic granular sludges: isotherms and sequential extraction. Chemosphere 58:493–505
Varma AJ, Deshpande SV, Kennedy JF (2004) Metal complexation by chitosan and its derivatives: a review. Carbohydr Polym 55:77–93
Wang JL, Chen C (2006) Biosorption of heavy metals by Saccharomyces cerevisiae: a review. Biotechnol Adv 24:427–451
Wang JL, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27:195–226
Wang JL, Chen C (2014) Chitosan-based biosorbents: modification and application for biosorption of heavy metals and radionuclides. Bioresour Technol 160:129–141
Wang JP, Chen YZ, Ge XW, Yu HQ (2007) Gamma radiation-induced grafting of a cationic monomer onto chitosan as a flocculant. Chemosphere 66:1752–1757
Wang HL, Tang HQ, Liu ZT, Zhang X, Hao ZP, Liu ZW (2014) Removal of cobalt(II) ion from aqueous solution by chitosan—montmorillonite. J Environ Sci 26:1879–1884
Wu LP, Huang CZ, Yang X, He J, Li LL, Liu H (2013) Determination of heparin based on the reaction with Co(II)/5-Cl-PADAB complex using the resonance Rayleigh scattering technology. Anal Methods 5:2511
Xing M, Wang JL (2016) Nanoscaled zero valent iron/graphene composite as an efficient adsorbent for Co(II) removal from aqueous solution. J Colloid Interface Sci 474:119–128
Xing M, Xu LJ, Wang JL (2016) Mechanism of Co(II) adsorption by zero valent iron/graphene nanocomposite. J Hazard Mater 301:286–296
Yavuz O, Altunkaynak Y, Guzel F (2003) Removal of copper, nickel, cobalt and manganese from aqueous solution by kaolinite. Water Res 37:948–952
Zakrzewska-Trznadel G (2013) Advances in membrane technologies for the treatment of liquid radioactive waste. Desalination 321:119–130
Zhu YH, Hu J, Wang JL (2012) Competitive adsorption of Pb(II), Cu(II) and Zn(II) onto xanthate-modified magnetic chitosan. J Hazard Mater 221:155–161
Zhu YH, Hu J, Wang JL (2014) Removal of Co2+ from radioactive wastewater by polyvinyl alcohol (PVA)/chitosan magnetic composite. Prog Nucl Energy 71:172–178
Acknowledgments
The research was supported by the National Natural Science Foundation of China (51578307), the National Key Research and Development Program (2016YFC1402507), the Program for Changjiang Scholars and Innovative Research Team in University (IRT-13026) and the National S&T Major Project (2013ZX06002001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Binbin Huang.
Rights and permissions
About this article
Cite this article
Zhuang, S.T., Yin, Y.N. & Wang, J.L. Simultaneous detection and removal of cobalt ions from aqueous solution by modified chitosan beads. Int. J. Environ. Sci. Technol. 15, 385–394 (2018). https://doi.org/10.1007/s13762-017-1388-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-017-1388-x