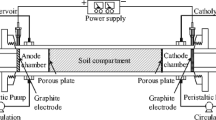
Abstract
A two-stage system for octachlorinated dibenzo-p-dioxin (OCDD)-contaminated soil remediation was developed. Soil washing using emulsified oil (EO) was applied in the first stage for OCDD extraction followed by the second stage of bioremediation using P. mendocina NSYSU for remaining OCDD biodegradation. The major tasks included (1) determination of optimal soil washing conditions for OCDD extraction by EO, (2) evaluation of feasibility of OCDD biodegradation by P. mendocina NSYSU under aerobic cometabolic conditions using EO as the primary substrate, and (3) assessment of the effectiveness of OCDD removal using the two-stage system. During the soil washing stage, EO with two different oil-to-water ratios (1:50 and 1:200) and pore volumes were tested with initial soil OCDD concentration of 21,000 µg/kg. Results indicate that EO could effectively improve the solubility and desorption of OCDD in soils. Up to 74% of OCDD removal could be obtained after washing with 60 PVs of EO and dilution factor of 50. After the soil washing process, enriched P. mendocina NSYSU solution was added into the reactor to enhance the aerobic biodegradation of remaining OCDD in soils. P. mendocina NSYSU could use adsorbed EO globules as substrates and caused significant OCDD degradation via the aerobic cometabolic mechanism. Approximately 82% of the remaining OCDD could be removed after 50 days of operation, and P. mendocina NSYSU played important roles in OCDD biodegradation. Up to 87% of OCDD was removed through the EO washing and biodegradation process. The two-stage system is a potential technology to remediate dioxin-contaminated soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dioxins, which are persistent and toxic chemicals, include 75 polychlorinated dibenzo-p-dioxins (PCDDs) (Warenik-Bany et al. 2016). Researchers have reported that many dioxin isomers [including the highly chlorinated octachlorinated dibenzo-p-dioxin (OCDD)] have been released to the environments due to various natural and man-made chemical and combustion processes, which result in the contamination of the ecosystems and environmental media (e.g., sediments and soils) (Shin et al. 2016; Warenik-Bany et al. 2016).
Although physicochemical treatment technologies have been used for dioxin-contaminated sites remediation (Hung et al. 2016), they are costly to cleanup significant amounts of polluted media (e.g., soils and sediments). Bioremediation can be a more cost-effective technology for dioxin removal. However, it might not be applicable for dioxin sites with high dioxin concentrations due to the bacterial inhibition effect (Piskorska-Pliszczynska et al. 2016). Thus, a combined system containing physicochemical and biological techniques could be more feasible to remediate highly dioxin-contaminated sites.
Dioxins can be biotransformed to less-chlorinated by-products through the anaerobic reductive dechlorinating mechanisms (Liu et al. 2014). There are two different processes of reductive dehalogenation: hydrogenolysis and dichloroelimination. Reductive dechlorination can be carried out under cometabolic conditions, which proceeds with enzymes that catalyze other reactions (Hiraishi 2008). Some aerobic bacteria also possess unique catabolic pathways, which enable them to degrade less-biodegradable and persistent chlorinated compounds. These bacteria could oxidize and open the aromatic ring structures via the dioxygenase processes (Jeon et al. 2016). Compared to anaerobic biodegradation, aerobic biodegradation can be used to enhance and accelerate the biodegradation rates (Nam et al. 2014). However, aerobic transformation of chlorinated organic compounds is usually occurred under cometabolic conditions, indicating that addition of primary substrates is required. Less-chlorinated dioxins are susceptible to partial degradation via aerobic cometabolism, and only monochlorinated congeners can serve as the primary substrates in some cases (Bastviken 2013).
Soil washing is a more efficient soil remedial technique compared with bioremediation. Surfactant is a commonly used soil washing reagent used for contaminated soil cleanup (Mousset et al. 2016; Trellu et al. 2016). Soil washing is based on the desorption of contaminants from soils via the washing reagents (e.g., water and surfactant). After soil washing, the water phase containing contaminants is disposed or further treated by other methods. The efficiency of soil washing process is dependent on the desorption and solubility rates of contaminants (Ishiguro and Koopal 2016). Some researchers indicate that applying specific washing solutions (e.g., surfactant solution and solvents) could enhance contaminant removal and reduce the soil cleanup time (Im et al. 2015; Mousset et al. 2016; Trellu et al. 2016).
When surfactant solution is mixed with soils, surfactant molecules would adsorb onto the surface of soil particles and interactions with the hydrophobic contaminants would occur (Im et al. 2015; Mao et al. 2015). Surfactants could result in the increase in the contaminant solubility, and thus, the bioavailability and contaminant biodegradation rate could also increase (Mousset et al. 2016; Trellu et al. 2016).
Food-grade edible oils have been applied as the environmentally friendly solvents for hydrophobic chemical sorption (Gong et al. 2006; López-Blanco et al. 2016). The edible oils can also serve as the long-lasting substrates to enhance the biodegradation processes. However, edible oils might cause the clogging of soil pores because of the large diameters of the oil globules. Thus, edible oils need to be emulsified to reduce the globule diameters (Sheu et al. 2015; López-Blanco et al. 2016). The emulsion process could create higher capacity of hydrophobic contaminant solubilization than the normal micellar system, and thus, the contaminant removal rate could be facilitated (Sheu et al. 2015).
In our previous study, emulsified oil (EO) has been produced to provide biodegradable substrates to enhance the reductive dechlorination process (Liang et al. 2013). The produced EO contained edible oil, cane molasses, and surfactants [soya lecithin (SL) and Simple Green™ (SG)]. The EO (with a droplet size of 0.2–0.9 μm) could supply primary substrates continuously for the enhancement of trichloroethylene (TCE) biodegradation in groundwater and also remove TCE via the sorption mechanism. Results from our previous study show that EO could improve contaminant solubilizing activity and bioavailability (Liang et al. 2013; Tsai et al. 2014).
The Pseudomonas mendocina NSYSU (P. mendocina NSYSU) was isolated and enriched from soils collected from a PCP spill site (Kao et al. 2005). The site was also polluted by OCDD with concentrations up to 20 mg/kg (NSC 2012). Results from NSC (2012) show that the site soils and groundwater were under aerobic conditions and that higher cell density was observed for P. mendocina NSYSU when it was incubated under aerobic conditions. Thus, it is possible that P. mendocina NSYSU has the potential to biodegrade dioxins under aerobic biodegradation processes.
Because dioxins have high potential toxicological impact, the development of cost-effective processes with high pollutant removal efficiency is a major challenge for researchers and soil remediation companies. In this study, OCDD was treated as the target compound, and its biodegradability by P. mendocina NSYSU was evaluated. Batch soil washing and biodegradation studies were performed to assess the effectiveness of the developed two-stage system (soil washing using EO followed by biodegradation with inoculation of P. mendocina NSYSU) on OCDD removal. The goals of this study were to (1) assess the feasibility of biodegrading OCDD under aerobic conditions with the addition of P. mendocina NSYSU as the inocula, (2) evaluate the feasibility of using EO as the OCDD desorption and solubilization reagent to cleanup OCDD-contaminated soils and determine the optimal operational conditions of the soil washing process, (3) evaluate feasibility of biodegradation of the remaining OCDD in soils using P. mendocina NSYSU as the inocula after the soil washing process, (4) evaluate the changes of bacterial diversity and dominant bacteria in aerobic biodegradation mechanisms.
Materials and methods
Preparation of EO and growth of P. mendocina NSYSU
EO was prepared following the procedures described in Liang et al. (2013). Preliminary study was performed to assess an appropriate component of the EO mixture to generate stable O/W emulsions with small globules with a uniform size.
In our previous study, a PCP-degrading bacterium (P. mendocina NSYSU) was isolated from the PCP-contaminated site (Kao et al. 2005). The bacterial strain was kept in a freezer at −80 °C before cultivation. In this study, P. mendocina NSYSU culture was cultivated in the 10-mL nutrient broth (NB, Difco 003-01) under aerobic conditions. NB was applied for substrate (carbon and energy sources) supplement to grow P. mendocina NSYSU. Spectrophotometry method was applied for cell density and growth conditions of the P. mendocina NSYSU evaluation (APHA 2005). All chemicals (reagent grade) for bacterial incubation were obtained from Sigma-Aldrich Co. (USA).
OCDD biodegradability study
This experiment was performed to evaluate the effectiveness of EO addition on OCDD biodegradation enhancement. Collected OCDD-contaminated soils from low contamination zone (OCDD concentration was approximately 3.5 mg/kg) were used in this study. Soils were air-dried, and a 2-mm mesh sieve was used for preliminary separation.
In the aerobic microcosms, EO was supplied as the substrate. Each microcosm contained 35 mL of mineral solution, 20 g of OCDD-polluted soils, 5 mL of P. mendocina NSYSU solution, and 1 mL of EO in a 100-mL serum bottle sealed with Teflon-lined rubber septa. In the control batch group, same amount of mineral solution was used to replace the P. mendocina NSYSU and EO solution. The following components were added in the media (units are in mg per liter of water): CaCl2·2H2O, 44.1; Mg2SO4·7H2O, 98.6; NH4Cl, 10.7; KH2PO4, 326.4; Na2HPO4, 1263.8; and 3.35 mg of trace elements [MnSO4·4H2O, 1; FeSO4·7H2O, 1; Na2B4O7·10H2O, 0.25; (NH4)6Mo7O24·4H2O, 0.25; CoCl2·6H2O, 0.25; CuCl2·2H2O, 0.25; NH4VO3, 0.1; ZnCl2, 0.25]. The preparation of the mineral solution was described in Tu et al. (2014).
Table 1 lists the components of four groups of aerobic microcosms. Group A was live control microcosms without P. mendocina NSYSU and EO addition. Groups B to C were used to evaluate the effectiveness of P. mendocina NSYSU and EO supplement on OCDD biodegradation, respectively. Group D was used to evaluate the efficiency of OCDD removal with the addition of both P. mendocina NSYSU and EO. P. mendocina NSYSU strain was added into Groups B and D, and EO was only added into Groups C and D microcosms. P. mendocina NSYSU was incubated in NB under aerobic conditions for 36 h until a cell density of 1.7 × 109 cells/mL was obtained. Microcosms were inoculated in the dark at room temperature (20 °C). The quantification of the dioxins in all samples from the experiments was performed by high-resolution gas chromatography–high-resolution mass spectrometry (HRGC/HRMS) analysis. The QA/QC criteria followed US EPA Method-1613B (EPA 1994). The procedures for OCDD analytical methods were described in Tu et al. (2014).
Stage 1—soil washing experiment
Collected OCDD-contaminated soils from dioxin-contaminated site were analyzed to determine the initial OCDD concentration. Soils were collected from highly contaminated area with OCDD concentrations of approximately 21,000 µg/kg of soil. Table 2 presents the characteristics of tested soils. A semi-continuous batch experiment was performed to assess the feasibility of using the proposed two-stage scheme on OCDD-polluted soil remediation. During the soil washing stage, EO with two different oil-to-water ratios (1:50 and 1:200) and five pore volumes (PVs) (5, 10, 20, 30, and 60) (1 PV = 64 mL) was applied for their effectiveness on OCDD removal. In each batch experiment, 200 g of OCDD-polluted soils was added into the reactor (500-mL glass bottle), and then, 1 PV of EO was added each time for soil washing. The reactors were shaken in a shaker at 200 strokes per min for 5 min before analysis. After washing with a certain amount of PVs of EO solution, the second stage of remedial process was performed to bioremediate the residual OCDD in soils. In the control experiment, deionized (DI) water was used as the soil washing reagent for OCDD washing. Thus, no EO was applied in the control group. Duplicate samples were analyzed at each monitoring time point.
Stage 2—biodegradation experiment
After the EO washing process, the biodegradation process was applied to further cleanup the remained OCDD in soils. The biodegradation experiment was performed to evaluate the feasibility of OCDD biodegradation after the soil washing process. P. mendocina NSYSU strain was used as the inocula in the batch bottles. The optimal soil washing conditions were applied to prepare the EO washed soils for the second-stage experiment. After soil washing, EO solution was removed and replaced with P. mendocina NSYSU culture solution.
After the soil washing stage, 1 PV (64 mL) of P. mendocina NSYSU culture solution (or 1 PV of mineral solution) and 1 PV of mineral solution (or 1 PV of DI water or 0.5 PV of mineral solution +0.5 PV of NB solution) were added into each bottle for the biodegradation of residual OCDD adsorbed on the soils (the second stage). In the control bottle, no P. mendocina NSYSU culture solution was supplied. Table 3 describes the characteristics of the second-stage biodegradation batch experiments.
In the aerobic experiments, the remaining EO was used as the primary substrate. Each 500-mL batch bottle contained 64 mL of mineral medium (or NB or DI water), 200 g of contaminated soils after EO washing, and 64 mL of P. mendocina NSYSU solution as inocula (or 64 mL of mineral solution in the control bottles) sealed with Teflon-lined rubber septa.
Group A was live control group without P. mendocina NSYSU strain addition. Group B was the group with P. mendocina NSYSU strain addition but no mineral or NB addition. In Groups C and D batch bottles, mineral nutrient solution and NB solution were added to enhance the OCDD biodegradation efficiency, respectively. Live control group was used to assess the effectiveness of indigenous soil bacteria on OCDD removal after the soil washing by EO. Duplicate samples from different bottles were collected and analyzed for OCDD concentrations at each time point. Molecular biological techniques were used to evaluate the changes in bacterial diversity of the collected soil samples.
The OCDD degradation efficiency was determined as a percentage of the initial OCDD concentration. The characteristics of the four groups of batch experiments are presented in Table 3. At each sampling event, the water sample in each bottle was also analyzed for other parameters including dissolved oxygen, pH, and oxidation–reduction potential (ORP). The batch bottles were shaken in a shaker at 100 rpm and incubated in the dark at room temperature for 50 days. DO meter (Oxi 330) was used for DO measurement, and a MP120 pH/Eh meter (Mettler Toledo) was used for pH and ORP analyses. The total plate counts were conducted using plate count agar (Difco) to assess the approximate size of the total heterotrophic bacteria in samples using the spread plate method (APHA 2005).
Statistical analysis was applied in this study to evaluate the performance of each treatment process on OCDD removal as directed. The results in all tables and figures are presented as the mean ± standard deviation. The one-way analysis of variance (ANOVA) was used to evaluate differences between two different tests of experiments, and a level of 0.05 was used as the lower bound to determine the significance of the variation. The stochastic package for social science (SPSS software, version 17) (SPSS 2007) was applied to perform the statistical analyses.
Molecular biological analyses
Soil DNA extraction and the PCR amplification process were conducted using procedures in Shrestha et al. (2010). The amplified PCR was conducted with denaturing gradient gel electrophoresis (DGGE) to evaluate the bacterial species and dominant bacteria (Shrestha et al. 2010). Microcosm soils were applied for the PCR analyses to determine the bacteria responsible for the aerobic biodegradation of OCDD. Soil DNA extraction and the PCR amplification process were conducted using procedures in Shrestha et al. (2010). The PCR-amplified products were electro-eluted from gel and sequenced. The sequences were assessed using the alignment search tool to determine the closest relatives in the GenBank (http://www.ncbi.nlm.nih.gov).
Results and discussion
OCDD biodegradability study
In the biodegradability study, low level of OCDD-polluted soils (approximate OCDD concentration = 3500 µg/kg) was collected from a dioxin site located in Tainan City, Taiwan. Figure 1 presents the variations in OCDD concentrations in batch bottles during the 50-day operational period. In Group A microcosms (live control without P. mendocina NSYSU and EO addition), no significant OCDD concentration drop (3% of OCDD removal) was detected. This demonstrates that indigenous soil bacteria could not effectively biodegrade OCDD without appropriate bacteria and substrate supplement.
In Group B microcosms (with P. mendocina NSYSU addition only), approximately 6% of OCDD was removed (OCDD concentration dropped from 3702 to 3489 µg/kg) after 50 days of operation. This indicates that OCDD biodegradation could not be enhanced without primary substrate supplement although P. mendocina NSYSU was added. The slight OCDD removal could be due to the adsorption and intrinsic biodegradation mechanisms using the natural organic carbon as the primary substrate. Approximately 12% of OCDD could be removed (OCDD concentration dropped from 3761 to 3302 µg/kg) in Group C microcosms after 50 days of operation. The OCDD removal could be due to sorption and the occurrence of OCDD biodegradation by indigenous OCDD-degrading bacteria using the EO as the primary substrate.
In Group D microcosms (with P. mendocina NSYSU and EO addition), approximately 53% of OCDD was removed (OCDD concentration dropped from 3730 to 1769 µg/kg) after 50 days of operation. This indicates that OCDD could be biodegraded with P. mendocina NSYSU and substrate addition. Results also imply that P. mendocina NSYSU can only biodegrade OCDD under cometabolic conditions, and thus, primary substrate supplement is required to accelerate the OCDD biodegradation. Moreover, results demonstrate that EO can serve as the carbon source for the growth of P. mendocina NSYSU during OCDD biodegradation.
In Fig. 1, the significance of variance for OCDD decay curves between microcosm groups of A (live control) and D (NSYSU strain and EO addition), B (NSYSU strain addition only) and D, and C (EO addition only) and D (NSYSU strain and EO addition) was performed using the ANOVA analyses. Results from the ANOVA analysis show that significant variations were observed (at p < 0.05) for CODD decay curves of A and D, B, and D, and C and D microcosms. This indicates that NSYSU strain and EO addition could effectively enhance the OCDD removal in microcosms. However, results from the ANOVA analyses show that no significant variation was observed (at p > 0.05) for the OCDD decay curves of A to C microcosms. This indicates that addition of NSYSU strain or EO only could not significantly enhance the OCDD removal under aerobic conditions.
Soil washing experiment (Stage 1)
Figure 2 presents the OCDD concentrations in soils versus the PVs using DI water and EO as the washing reagents. Results show that less than 6% of OCDD removal was observed after 60 PVs of DI water washing. This indicates that OCDD could not be significantly removed via the water extraction solely. Because OCDD had high octanol–water partition coefficient (logKow = 8.2), it would be sorbed onto the soil particles tightly with low mobility (McKay 2002). Thus, surfactant application is required when soil washing is applied to remediate OCDD-contaminated soils.
Results also show that approximately 74 and 67% of OCDD removal were obtained after washing with 60 PVs of EO solution with EO-to-water ratio of 1:50 and 1:200, respectively. Results demonstrate that using EO solution as soil washing reagent obtained much higher OCDD removal efficiency compared with the tests applying DI water along. Thus, EO addition could enhance OCDD mobility and solubility in the soil washing system. Higher OCDD removal efficiency was obtained in batch system with higher EO-to-water ratio (1:50). However, the trend of OCDD removal leveled off after approximately 20–30 PVs of washing (Fig. 2). This implies that the system approached equilibrium conditions after 20–30 PVs of EO washing.
In Fig. 2, the significance of variance for OCDD extraction curves (extracted with EO-to-water ratio of 1:50 and 1:200) was performed using the ANOVA analyses. Results from the ANOVA analysis show that a significant variation was observed (at p < 0.05) for CODD decay curves of EO-to-water ratio of 1:50 and 1:200. This indicates that using the extraction solution of EO-to-water ratio of 1:50 could obtain a higher OCDD extraction efficiency.
Figure 3 presents the increased organic carbon content in soils after the soil washing process using EO as the washing reagent. Results indicate that the organic carbon content increased from 0.1% before the EO washing to 2.1 and 2.6% after 60 PV of EO washing using 1:200 and 1:50 EO-to-water ratio, respectively. Results also show that the organic carbon content approached equilibrium conditions after 50 PV of EO washing (EO-to-water ratio = 1:50). This indicates that the equilibrium conditions could be reached shortly with higher EO-to-water ratio. Because the EO contained substantial amounts of vegetable oil (around 35%) with small globule diameter (around 200–900 nm) (Liang et al. 2013), part of the EO globules would be adsorbed onto soil particles, resulting in increased soil organic content, which could be used as the primary substrate for OCDD biodegradation.
In Fig. 3, the significance of variance for two soil organic carbon content curves (extracted with EO-to-water ratio of 1:50 and 1:200) was performed using the ANOVA analyses. Results from the ANOVA analysis show that a significant variation was observed (at p < 0.05) for two soil organic carbon content curves. This indicates that using the extraction solution of EO-to-water ratio of 1:50 could result in the increase in the soil organic content significantly.
Although the percentages of OCDD removal went up from 67 to 74% when the EO-to-water ratios increased from 1:200 to 1:50, the increment was not significant. To minimize the possible soil pore clogging problem and reduce the cost of EO, further increase in the EO-to-water ratio would not be a necessity.
Because EO contained surfactant component, the phospholipids structures of the surfactant molecules could form micelles, bilayer sheets, or lamellar structures, which makes EO a special amphipathic chemical with smaller diameter (Liang et al. 2013). Moreover, OCDD is the hydrophobic compound with relatively higher oil affinity; the OCDD originally existed within the soil pores or adsorbed onto the surface of soil particles would dissolve in the EO phase. Thus, linear desorption mechanism (physical adsorption mechanism) was observed in the initial soil washing process (0–10 PVs EO washing). Because most of the removable OCDD was desorbed during the early phase of soil washing process, slower desorption mechanism was observed after the occurrence of equilibrium during the latter part of the EO washing (Mao et al. 2015). Results also show that up to 25–33% of the OCDD could not be desorbed during the EO washing process. This could be due to the following three phenomena: (1) Some of the OCDD was adsorbed inside the micropores of the soil particles, and the diameter of EO globule was smaller than the diameter of soil particle. Thus, the EO could not effectively contact and react with the OCDD molecular retained within the microsites in soils during the soil washing process. Although the molecular diffusion mechanisms could remove the OCDD in microsites after solubilization mechanisms, the solubilization time might not be appropriate from the engineering and remediation point of view (Ishiguro and Koopal 2016). (2) Because the EO globules had negative electric charges (Tsai et al. 2014), they would be adsorbed onto some of the soil particles with positive electric charges (chemical adsorption mechanism). This rapid chemical reaction between the electrons with opposite electric charges would limit the OCDD removal from the EO globules. (3) Aggregation of EO globules resulted in the increase in the diameter of EO globules, which limited the EO globule transport and OCDD removal due to the sorption of aggregates onto the soil particles (Lowry et al. 2012). Thus, other feasible remedial techniques needed to be applied to remove the remained OCDD in soil micropores (Li and Chen 2009). OCDD-degrading microorganism, which could transport into the EO globules, was able to degrade OCDD via the biological mechanisms.
Biodegradation study (Stage 2)
In the biodegradation study (Stage 2), OCDD-polluted soils (OCDD concentration = 21,000 µg/kg) were collected and pretreated with EO solution (EO-to-water ratio = 1:50) for OCDD removal. After 60 PVs of EO washing, the pretreated soils were used for the Stage 2 biodegradation process to bioremediate the remaining OCDD.
Figure 4 presents the remained OCDD in microcosms along the 50-day operational period. In Group A batch bottles (no P. mendocina NSYSU addition), only slight OCDD removal was obtained (8% of OCDD removal) after 50 days of operation. However, up to 32% of OCDD was removed from Group B bottles (with P. mendocina NSYSU addition). This implies that the indigenous soil bacteria were not able to biodegrade OCDD significantly. This was due to the fact that the dioxin-biodegrading bacterial strains were not the dominant bacteria in site soil environment. Therefore, dioxin-degrading strain inoculation is necessary to improve OCDD biodegradation efficiencies. Results demonstrate that the P. mendocina NSYSU was able to use the remaining EO and enhance the OCDD biodegradation under aerobic cometabolic conditions.
Approximately 39 and 50% of OCDD was removed in Groups C and D batch bottles, respectively. This demonstrates that the mineral nutrient media and NB broth (with carbon source) could enhance the aerobic biodegradation. Because the soil contained low levels of total nitrogen and phosphorus (Table 2), the low N and P concentrations might limit bacterial growth, and thus, nutrient addition could improve the bacterial growth and subsequent OCDD removal. Results from Group D batch experiment indicate that the carbon source in NB (beef extract) was more biodegradable than vegetable oil in EO. Thus, more efficient OCDD biodegradation rate was observed in Group D bottles due to the addition of more applicable substitute primary substrate. Results show that substrate supplement (e.g., EO and NB) is required to enhance the OCDD biodegradation under aerobic cometabolic conditions.
In Fig. 4, the significance of variance for OCDD removal curves between four microcosm groups (A to D) was performed using the ANOVA analyses. Results from the ANOVA analysis show that significant variations were observed (at p < 0.05) for CODD removal curves between Groups A (no NSYSU strain addition) and B, A and C, and A and D microcosms. This indicates that NSYSU strain addition could effectively enhance the further OCDD biodegradation after EO washing. However, results from the ANOVA analyses show that no significant variation was observed (at p > 0.05) for the OCDD decay curves between Groups B and D, C, and D, and B and C microcosms. The results could be due to the fact that the initial OCDD concentrations in Groups C to D microcosms were similar, which resulted in insignificant differences between these three degradation curves. Result also reveal that NB and mineral solution addition had similar weights on the improvement of OCDD removal compared to NSYSU strain addition only.
Results from the ANOVA analyses also show that a significant variation was observed (at p < 0.05) for the OCDD decay curves between B and D, C, and D, and B and C microcosms only during the operational period from Day 30 to Day 50. Thus, higher OCDD biodegradation rate was detected in Groups C and D microcosms with EO and NB supplement in the latter part of the operation. This also implies that carbon substrates could enhance the OCDD removal when a loner operational period was reached.
Tables 4, 5, 6 present the averages of analytical results during the operation of biodegradation experiment (Stage 2). Results demonstrate that decreased pH, DO, and ORP measurements were observed after the biodegradation process. The total bacterial population in Groups B to D increased (>109 CFU/mL) during the early operational period but dropped to below 106 CFU/mL during the latter operational period. Because the P. mendocina NSYSU culture was cultivated and added into the Groups B, C, and D, compared to the Group A without P. mendocina NSYSU addition, the increased total bacterial population could be due to the increased population of P. mendocina NSYSU. This indicates that the EO, nutrient, and NB supplement enhanced the bacterial growth at the beginning of the experiment. However, the EO biodegradation also resulted in the consumption of DO and drop of ORP. The aerobic biodegradation of EO caused the fatty acid production, which resulted in decreased total bacterial population. The phenomena might be the causes of decreased OCDD removal efficiency after 30 days of operation.
Table 7 presents the removal efficiencies of four groups of batch systems after the two-stage treatment. Results show that the removal efficiencies of the EO washing stage ranged from 73 to 75%. The total OCDD removal efficiencies in Groups 1–4 system reached 75, 82, 83, and 87%, respectively. Results indicate that the two-stage system (EO washing and biodegradation) could remove OCDD in soils significantly. Although EO washing could remove abundant OCDD within a short period of time, the high OCDD soil/water partition coefficient limits its complete desorption. Thus, biodegradation could be applied to replace the soil washing step. Due to its biodegradable and slow-release characteristics, EO could be used as a continuous substrate supplement to enhance the cometabolism of OCDD. The decreased pH and DO values might cause the declined OCDD removal efficiencies after 30 days of operation (Table 4, 5, 6). In the practical field-scale study, pH and DO monitoring and control would be a necessary task to maintain an acceptable OCDD removal efficiency.
To accelerate the site cleanup efficiency, application of soil excavation and on-site or ex situ two-stage batch reactor system can be applied for the OCDD-contaminated soil treatment for the future practical and field application to cleanup OCDD-contaminated soils. In the batch reactor system, controlled operational conditions (including EO-to-water ratio, number of PV, P. mendocina NSYSU inoculation) can be applied to achieve the optimal OCDD removal efficiency during the two-stage operational processes.
Microbial diversity analyses
Figure 5 presents the DGGE trends of the PCR-amplified 16S rDNA for soils from four groups of experiments on Days 5 and 40 during the operational period. DGGE results demonstrate that soil sample from Group A (no P. mendocina NSYSU addition) had lower bacterial diversity. This could be due to the fact that the soil samples were highly contaminated with OCDD, which inhibited bacterial growth. Because the soils did not contain appropriate and significant OCDD-degrading bacteria, only slight OCDD removal was observed (Fig. 4). After the addition of P. mendocina NSYSU in Group B, P. mendocina NSYSU and mineral nutrient in Group C, and P. mendocina NSYSU and NB in Group D, higher bacterial diversities were observed in these three groups of microcosms. P. mendocina NSYSU was the dominant bacterium in these three groups of microcosms during the operational period. Thus, results imply that the inoculated P. mendocina NSYSU played a significant role in OCDD biodegradation.
DGGE results also show that the decay of P. mendocina NSYSU was observed in Groups B, C, and D microcosms on Day 40. This could be due to the changes of the environmental conditions (e.g., decreased substrates, pH, total bacteria, DO) after 40 days of incubation, which resulted in the decreased P. mendocina NSYSU population. The results also corresponded with the decreased OCDD degradation rates in these three microcosms (Fig. 4). To maintain a higher OCDD removal rate, frequent P. mendocina NSYSU inoculation might be required.
DGGE results show that there were three other apparent bands on the DGGE gels in Groups B, C, and D microcosms. To determine the representatives for bacteria, the three specific bands (labeled as a, b, and c) were amplified and sequenced for the nucleotide sequences. The nucleotide sequences of 16S rDNA of three dominant bacteria were compared with the database from GenBank. Up to 99% of identities of specific bacteria of the nucleotide sequences of three bands were observed as compared to database of GenBank. Based on the results from GenBank, Acidobacteria bacterium, Brevundimonas sp., and Pseudaminobacter sp. were the most possible bacterial species for bands a, b, and c, respectively. Researchers reported that Acidobacterium capsulatum could biodegrade polychlorinated biphenyls (PCB) (Nogales et al. 1999). Brevundimonas sp. (MCM B-427) and Brevundimonas sp. (X08) were able to degrade dimethoate and phenanthrene, respectively (Xiao et al. 2010). Pseudaminobacter sp. (C147 or C195) could biodegrade atrazine (Zhang et al. 2011). Because these chemicals had structures similar to dioxins, the specific bacteria might be able to biodegrade dioxins under the aerobic cometabolic conditions.
Conclusion
In this study, a two-stage treatment system for OCDD-contaminated soil remediation was developed. Results indicate that the addition of EO caused the increase in OCDD dissolution and solubility and resulted in increased OCDD removal in the soil washing stage. Results show that up to 74% of OCDD removal could be obtained after washing with 60 PVs of EO solution (EO-to-water ratio = 1:50). Because the EO contained substantial amounts of vegetable oil with small globule diameter, it would adsorb onto soil particles causing increased soil organic carbon content, which could be used as the primary substrate for OCDD biodegradation.
The OCDD-degrading bacterium, P. mendocina NSYSU, was able to use EO as its carbon and energy sources and caused significant degradation of OCDD via the aerobic cometabolic mechanism (53% of OCDD removal within 50 days in the biodegradability study). After the soil washing process, enriched P. mendocina NSYSU solution was added into the batch bottles to enhance the aerobic biodegradation of the remaining OCDD in soils. More than 87% of OCDD removal could be obtained through the EO washing (first stage) followed by the biodegradation process (second stage). Results indicate that the on-site two-stage (washing and bioremediation) remedial system has the potential to be developed into a practical technology to remediate dioxin-polluted site.
References
APHA (American Public Health Association) (2005) American water works association and water environment federation, standard methods for the examination of water and wastewater, (22st ed.). Washington
Bastviken D (2013) Rapid and extensive natural chlorination & dechlorination of soil organic matter. Euro Chlor 17:1–4
EPA (1994) US Environmental Protection Agency, Tetra-through octa-chlorinated dioxins and furans by isotope dilution HRGC/HRMS. Method 1613, Washington DC, USA
Gong Z, Wilke BM, Alef K, Li P, Zhou Q (2006) Removal of polycyclic aromatic hydrocarbons from manufactured gas plant-contaminated soils using sunflower oil: laboratory column experiments. Chemosphere 62:780–787
Hiraishi A (2008) Biodiversity of dehalorespiring bacteria with special emphasis on polychlorinated biphenyl/dioxin dechlorinators. Microbes Environ 23:1–12
Hung PC, Chang SH, Ou Yang CC, Chang MB (2016) Simultaneous removal of PCDD/Fs, pentachlorophenol and mercury from contaminated soil. Chemosphere 144:50–58
Im J, Yang K, Jho EH, Nam K (2015) Effect of different soil washing solutions on bioavailability of residual arsenic in soils and soil properties. Chemosphere 138:253–258
Ishiguro M, Koopal LK (2016) Surfactant adsorption to soil components and soils. Adv Colloid Interface Sci 231:59–102
Jeon JR, Murugesan K, Baldrian P, Schmidt S, Chang YS (2016) Aerobic bacterial catabolism of persistent organic pollutants: potential impact of biotic and abiotic interaction. Curr Opin Biotechnol 38:71–78
Kao CM, Liu JK, Chen YL, Chai CT, Chen SC (2005) Factors affecting the biodegradation of PCP by Pseudomonas mendocina NSYSU. J Hazard Mater 124:68–73
Li JL, Chen BH (2009) Surfactant-mediated biodegradation of polycyclic aromatic hydrocarbons. Materials 2:76–94
Liang SH, Kuo YC, Chen SH, Chen CY, Kao CM (2013) Development of a slow polycolloid-releasing substrate (SPRS) biobarrier to remediate TCE-contaminated aquifers. J Hazard Mater 254–255:107–115
Liu H, Park JW, Häggblom MM (2014) Enriching for microbial reductive dechlorination of polychlorinated dibenzo-p-dioxins and dibenzofurans. Environ Pollut 184:222–230
López-Blanco R, Gilbert-López B, Rojas-Jiménez R, Robles-Molina J, Ramos-Martos N, García-Reyes JF, Molina-Díaz A (2016) Evaluation of processing factors for selected organic contaminants during virgin olive oil production: distribution of BTEXS during olives processing. Food Chem 199:273–279
Lowry GV, Gregory KB, Apte SC, Lead JR (2012) Transformations of nanomaterials in the environment. Environ Sci Technol 46:6893–6899
Mao X, Jiang R, Xiao W, Yu J (2015) Use of surfactants for the remediation of contaminated soils: a review. J Hazard Mater 285:419–435
McKay G (2002) Dioxin characterisation, formation and minimisation during municipal solid waste (MSW) incineration: review. Chem Eng J 86:343–368
Mousset E, Huguenot D, van Hullebusch ED, Oturan N, Guibaud G, Esposito G, Oturan MA (2016) Impact of electrochemical treatment of soil washing solution on PAH degradation efficiency and soil respirometry. Environ Pollut 211:354–362
Nam IH, Hong HB, Schmidt S (2014) Is the biotransformation of chlorinated dibenzo-p-dioxins by Sphingomonas wittichii RW1 governed by thermodynamic factors? J Microbiol 52:801–804
Nogales B, Moore ERB, Abraham WR, Timmis KN (1999) Identification of the metabolically active members of a bacterial community in a polychlorinated biphenyl-polluted moorland soil. Environ Microbiol 1:199–212
NSC (2012) Development of treatment technologies to remediate toxic chemical contaminated sites. National Science Council, Taipei, Taiwan Report No. 101-2622-E-006-001-C-C1
Piskorska-Pliszczynska J, Strucinski P, Mikolajczyk S, Maszewski S, Rachubik J, Pajurek M (2016) Pentachlorophenol from an old henhouse as a dioxin source in eggs and related human exposure. Environ Pollut 208(Part B):404–412
Sheu YT, Chen SC, Chien CC, Chen CC, Kao CM (2015) Application of a long-lasting colloidal substrate with pH and hydrogen sulfide control capabilities to remediate TCE-contaminated groundwater. J Hazard Mater 284:222–232
Shin ES, Kim JC, Choi SD, Kang YW, Chang YS (2016) Estimated dietary intake and risk assessment of polychlorinated dibenzo-p-dioxins and dibenzofurans and dioxin-like polychlorinated biphenyls from fish consumption in the Korean general population. Chemosphere 146:419–425
Shrestha HK, Hwu KK, Chang MC (2010) Advances in detection of genetically engineered crops by multiplex polymerase chain reaction methods. Trends Food Sci Technol 21:442–454
SPSS, Stochastic Package for Social Science (2007) Statistics Base 17.0 User’s Guide. ISBN-13-978-1-56827-400-3, https://www.jou.ufl.edu/assets/researchlab/SPSS-Statistcs-Base-Users-Guide-17.0.pdf
Trellu C, Mousset E, Pechaud Y, Huguenot D, van Hullebusch ED, Esposito G, Oturan MA (2016) Removal of hydrophobic organic pollutants from soil washing/flushing solutions: a critical review. J Hazard Mater 306:149–174
Tsai TT, Liu JK, Chang YM, Chen KF, Kao CM (2014) Application of polycolloid-releasing substrate to remediate trichloroethylene-contaminated groundwater: a pilot-scale study. J Hazard Mater 268:92–101
Tu YT, Liu JK, Lin WC, Lin JL, Kao CM (2014) Enhanced anaerobic biodegradation of OCDD-contaminated soils by Pseudomonas mendocina NSYSU: microcosm, pilot-scale, and gene studies. J Hazard Mater 278:433–443
Warenik-Bany M, Strucinski P, Piskorska-Pliszczynska J (2016) Dioxins and PCBs in game animals: interspecies comparison and related consumer exposure. Environ Int 89–90:21–29
Xiao J, Guo L, Wang S, Lu Y (2010) Comparative impact of cadmium on two phenanthrene-degrading bacteria isolated from cadmium and phenanthrene co-contaminated soil in China. J Hazard Mater 174:818–823
Zhang S, Wan R, Wang Q, Xie S (2011) Identification of anthracene degraders in leachate–contaminated aquifer using stable isotope probing. Int Biodeterior Biodegrad 65:1224–1228
Acknowledgements
This project was funded in part by Ministry of Science and Technology, Taiwan. The authors would like to thank the personnel at Ministry of Science and Technology and researchers at the Department of Biological Science, National Sun Yat-Sen University, Taiwan, for their assistance and support throughout this project.
Funding was provided by Ministry of Science and Technology, Taiwan (Grant No. 103-2622-E-006-018-CC2).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: J. Aravind.
Rights and permissions
About this article
Cite this article
Lin, J.L., Dong, C.D., Chen, C.W. et al. Development of a two-stage washing and biodegradation system to remediate octachlorinated dibenzo-p-dioxin-contaminated soils. Int. J. Environ. Sci. Technol. 14, 1919–1930 (2017). https://doi.org/10.1007/s13762-017-1286-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-017-1286-2