Abstract
Wastewater treatment with bioelectrical generation is an attractive feature with microbial fuel cells. The chitosan/biodegradable copolymer proton exchange membrane was used to assess its performance with brewery wastewater in a dual chambered microbial fuel cell. The biodegradable copolymer was made by thermal condensation of malic acid and citric acid in 3:1 ratio and then blended with chitosan to form a membrane via solution casting and solvent evaporation techniques. The performance of the chitosan/biodegradable copolymer membrane was evaluated in bioelectricity production with brewery effluent as an anolyte in a carbon electrode microbial fuel cell. Additionally, the competence of the prepared blend proton exchange membrane is compared with the commercial Nafion 117 membrane and Agar salt bridge in separate microbial fuel cell units with the same effluent and electrodes. At neutral pH, the effect of adding metabolites such as glucose and acetate to the anolyte was also investigated. The maximum current density and power density generated with chitosan/biodegradable copolymer membrane was 111.94 mA m−2 and 3022.39 mW m−2, respectively, whereas the Nafion 117 membrane had a maximum current density of 120.23 mA m−2 and power density of 3486.73 mW m−2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Conventional wastewater treatment methods such as aerobic digestion and activated sludge treatment are energy intensive and sometimes ineffective (Perlman 2014). Therefore, various investigations have been carried out to minimize energy expenditure in wastewater treatment (Heidrich et al. 2011; Lefebvre et al. 2011). One of these investigations introduces microbial fuel cell (MFC) technology that generates electricity along with the treatment of wastewater. The dual function of MFC emerges due to the exploitation of metabolic cycles of respiring bacteria in addition to mitigating harmful greenhouse gas emissions, especially carbon dioxide (Logan and Regan 2006).
In the renewable energy sector, bioelectricity generation from waste has garnered significant interest in recent years. Researchers like Allen and Benetto (1993), Kim et al. (2004), Oh and Logan (2005), Ahn and Logan (2013) among many others made significant progress in bioelectricity production through MFC technology throughout the years. Studies are further extended to microbial optimization, MFC design and operation and characterization (Lefebvre et al. 2011). The majority of these studies are focused on bioenergy recovery from liquid waste streams (Feng et al. 2008; Zhang et al. 2013); however, limited research has been conducted on the development of efficient proton exchange membranes in MFC (Du et al. 2007).
Proton exchange media (PEM) is integral in the performance of MFC as it keeps charge separation, minimizes the effect of recombination and supports prototrophic transport (Logan 2008). MFC’s treatment level is dependant upon microbial activity and the amount of ions transported across the membrane. In polymer electrolyte membrane fuel cells, the membrane is the most expensive component due to its high manufacture cost, particularly the Nafion 117 membrane; as a result researchers started to develop alternative materials based on natural resources (Winfield et al. 2013). Moreover, utilization of synthetic polymers generates secondary pollution to the environment and this led towards the development of a new proton exchange membrane by using organic waste resources. Chitosan (CT) is the deacylated derivative of Chitin, the second most abundant biopolymer after cellulose as it is present in the shells of sea creatures and insects. Chitosan bears excellent properties such as hydrophilicity, biocompatibility, non-toxicity and chemical and thermal stability (Rao et al. 2007; Dash et al. 2011; Suginta et al. 2015). Chitosan is receiving great attention as an alternative membrane electrolyte material in various types of fuel cells due to its low cost that possibly lead to the development of economical fuel cells (Ma and Sahai 2013; Vaghari et al. 2013). Furthermore, chitosan can be modified through blending, sulphonation, phosphorylation, quaternization, and crosslinking that makes it as an efficient electrolyte membrane with high ion conductivity (Mat and Liong 2009). Due to the presence of free amino and hydroxyl groups in the structure of chitosan, it can be modified easily to enhance its properties for the desired applications. As CT is hydrophilic, it undergoes excessive swelling in wastewater which results in the permeation of oxygen across the membrane. To employ CT as a proton exchange membrane in MFC and reduce swelling behaviour, the cationic CT biopolymer is blended and crosslinked ionically with anionic copolymer synthesized from malic acid and citric acid in 3:1 ratio (Popuri et al. 2014), which contains multiple carboxylic groups. Citric acid and malic acid are found in produce and are presently used as crosslinking agents or modifiers in polymer synthesis (Werpy et al. 2004; Isiklan and Sanli 2005; Reddy and Yang 2010). The presence of carboxylic and oxide groups makes the copolymer poly(malic acid-citric acid) (PMC) hydrophilic, ionically active and easily modified with other polymers. Therefore, with the theoretical assumptions of introducing multiple charged groups for enhanced prototrophic transportation and energy production from brewery wastewater, this novel attempt is approached by blending the hydrophilic and biodegradable polymers with opposite charges to prepare a proton exchange membrane for MFC. In addition, it also seeks to evaluate the performance of the synthesized CT/PMC membrane by comparing it with other proton exchange media such as Nafion 117 membrane and Agar in bioelectrical generation. Then, the synthesized CT/PMC membrane with 1:1 ratio is characterized by Fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD), thermal gravimetric analysis (TGA) and scanning electron microscopy-energy dispersion spectra (SEM–EDS) to determine its physicochemical properties. The present work was carried out in Department of Biological and Chemical Sciences, The University of the West Indies, Barbados during 2013–2014 academic years and the characterization of the membranes is done at Southern Taiwan University of Science and Technology and Da-Yeh University during January to December, 2014.
Materials and methods
Materials
Citric acid (CA), DL-malic acid (MA), l-cysteine hydrogen chloride monohydrate and chitosan were purchased from Sigma Aldrich Co., USA. Sodium dihydrogen orthophosphate, potassium dichromate, potassium ferrous cyanide, mercuric sulphate, silver sulphate, potassium dihydrogen orthophosphate were obtained from BDH Chemicals. Glucose and sodium acetate and commercial Nafion 117 membrane were also procured from Sigma Aldrich Co., USA. Agar was procured from Thomas Scientific, USA, and the 800-mL polypropylene containers along with the ½ inch connecting poly (vinyl chloride) piping were obtained from a local hardware store. The effluent being used was collected from a local brewery, and the carbon brush electrodes were supplied by a local mechanical service company. Deionized and distilled water was used throughout this study.
Preparation of CT/PMC membrane
CT/PMC blend membranes used in this study were synthesized by solution casting and solvent evaporation technique. 2% chitosan solution was prepared by dissolving 4 g of chitosan in a 2% acetic acid solution overnight at 55 °C and combined with 2% of PMC copolymer solution in equal proportions (1:1 ratio). This blend solution was mixed by stirring until the resulting solution appeared homogenous. The bubble free solution was casted on a clean glass plate, and the solvent was allowed to evaporate slowly at room temperature for 48 h. The resulted CT/PMC blend membrane was peeled off from the glass plate, dried and kept in a desiccator.
The biodegradable copolymer PMC was synthesized through thermal condensation process by mixing the monomers malic acid and citric acid in 3:1 ratio. Monomers were subjected to melting followed by condensation polymerization using programmable thermal furnace. The detailed description of synthesis of PMC reported elsewhere (Popuri et al. 2014).
Microbial fuel cell setup
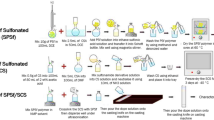
Dual chambered MFC setups with different proton exchange media were constructed in the laboratory as presented in Scheme 1. The laboratory MFC setup contained 800-mL volume polypropylene containers joined by a PVC hollow tube with a detachable coupling. The anolyte brewery effluent (pH 6) and the catholyte 50 mM potassium ferricyanide (pH 13.5) solutions with a volume of 650 mL were transferred into respective chambers. Carbon brushes (2.4 × 3 cm; 9 mm thickness; surface area 24.12 cm2) were used as electrodes and were submerged into the catholyte and anolyte. The chamber caps and perforations used for electrodes were sealed with Teflon tape to ensure anaerobic environment.
A total of three MFC units were fabricated in the laboratory for studying the effect of various proton exchange media in bioelectricity production. The Agar salt bridge was prepared by dissolving 0.5 g of potassium chloride and 100 g of Agar in 1000 mL of deionized water. The molten agar solution was casted into the tube and allowed to cool at room temperature. Nafion 117 and synthesized CT/PMC membranes were arch punched, sandwiched between nonwoven fabrics and fixed in between the O-ring washers of PVC coupling (see inset in Scheme 1).
These MFC setups were continuously stirred while in operation and voltage measured daily at regular intervals, with 100 Ω resistance to obtain the current and power density profiles. The current and power densities were calculated by the following Eqs. (1) and (2):
where J is current density, P is power density, V is voltage, A is electrode area, R is resistance and I is current.
The pH of the anolyte was maintained neutral using 1 M sodium hydroxide solution, and the performance of each MFC setup was also evaluated by adding two metabolites such as glucose and sodium acetate. Care was taken to keep out oxygen from the anodic chamber by adding a small amount of l-cysteine monohydrate to the solution to maintain anaerobic environment.
Chemical oxygen demand determination
The chemical oxygen demand (COD) was determined to find the level of organic substance removal by the bacteria in according to the American Public Health Association standard methods of water and wastewater analysis (AHPA 2005).
Characterization of the membranes
Morphology studies were carried out to determine the characteristics of the synthesized CT/PMC and Nafion 117 membranes before and after MFC operation. Surface texture of the membranes was captured by a multi-function (Joel JSM-7401F) scanning electron microscope (SEM) at 1000× and 3000× magnifications. Energy dispersion spectrum (EDS) was generated by a potential of 15 kV, and an image was captured by PentaFETx3-75 EDS simultaneously. Fourier transfer infrared spectroscopy (FTIR) was conducted to the CT/PMC and Nafion 117 membranes using Shimadzu IR affinity-1 Spectrophotometer over the range of 4000–500 cm−1 with a resolution of 50 cm−1. X-ray diffraction (XRD) was conducted using a Shimadzu XRD-6000 powder X-ray diffractometer to further analyse the solid-state morphology of the membranes. X-rays were generated using Cu-Kα radiation in the range of 10°–110° with a rate of 3° per minute, with an accelerating potential of 30 kV and electron probe current of 30 mA. Additionally, thermogravimetric analysis was studied in the range 29–900 °C in 2 °C intervals at scan rate of 10 °C min−1 for the membranes before and after MFC operation to check its thermal properties. The membrane samples were cut into small pieces according to the sample holder size and analysed by using a Da-Yeh University-self-designed thermo-destructive analyzer.
Sorption studies were carried out with the synthesized CT/PMC blend and Nafion 117 membranes to study the swelling behaviour of the membranes during MFC operation. Small pieces of membranes were weighed then placed in solutions of pure water and brewery effluent. The films were taken out after 24- and 48-h soaking periods and weighed after carefully wiping out excess liquid to estimate the amount absorbed.
The percentage sorption and degree of swelling were calculated from the following Eqs. 3 and 4:
where M d is the weight of dry membrane and M s is the weight of the swollen membrane.
Results and discussion
Biodegradable polymers are environmentally attuned and can be tailored in various ways. The biodegradability, non-toxic behaviour, hydrophilicity and easy chemical modification properties of these polymers allow them to be used in various applications and replace of many synthetic polymers utilized presently. In the present investigation, the synthesized copolymer PMC possesses reactive carboxylic and hydroxyl functional groups and the biopolymer chitosan has amino and hydroxyl functional groups. Due to the anionic nature of PMC copolymer and cationic nature of chitosan, it is expected that both polymers may interact ionically during the blending step of the CT/PMC membrane manufacture. The free carboxylic groups of the PMC chains are mostly present as carboxylate, and the amino groups of chitosan are protonated, resulting in the formation of new salt bonds (Cardenas et al. 2003) through electrostatic interactions. The ionic complexation reaction or poly-ion complex formation between cationic group (–NH3 +) of chitosan polymer and anionic group (–COO–) of copolymer is presented in Scheme 2. Since both the chitosan and PMC copolymer possesses hydrophilic attributes, it is projected that the resulting blend polymer CT/PMC membrane absorb water preferentially owing to the extensive interaction arising from intra- and intermolecular hydrogen bonding and dipole–dipole interactions between water and the functional groups of CT/PMC membrane, such as amine, acetal and hydroxyl besides unreacted amine moieties (Scheme 2).
Characterization of the membranes
The physicochemical properties of the synthesized CT/PMC membrane are needed to understand the underlying microstructures that may possibly affect electrical performance. As aforementioned, measurements to determine these properties were taken and described in detail.
FTIR analysis
Fourier transform infrared spectroscopy (FTIR) was done to analyse the structure of the synthesized CT/PMC membrane and Nafion 117 membrane before and after MFC operation as shown in Fig. 1. A 2% chitosan membrane was also analysed as a term of reference to determine comparable characteristic trends within the CT/PMC membrane. The chitosan membrane in Fig. 1 showed the characteristic –OH and –NH stretching peaks, –CH peak at 3296 and 2939 cm−1, respectively, along with a prominent –CO stretch at 1041 cm−1. A peak at 1632 cm−1 indicates the amide group of chitosan. The FTIR spectra of copolymer PMC showed peaks at around 3450, 1700–1740, and 2900–3100 cm−1 which represents –OH stretching, –COO− stretching and –CH stretching, respectively (Popuri et al. 2014). The band at 1240 cm−1 was due to –CO stretching of ether groups formed after condensation of the monomers (Popuri et al. 2014). These peaks were also observed in the CT/PMC membrane; however, the peaks have decreased in its intensity in comparison with the CT and PMC membrane. The peak at 1668 cm−1 can be attributed to the NH3 + deformation, while a peak at 1282 cm−1 corresponds to –CO stretching and –OH deformation that occurs on polymer blending (Beppu et al. 2007). The columbic interactions between amino groups in chitosan and carboxylate ions lead to an increase in the affinity of CT/PMC membrane towards water by the increase in charge density. The intensity of the characteristic peaks decreased and presence of additional peaks was observed in the FTIR analysis post MFC operation (Fig. 1c), indicates the presence of foulants on the membrane (Her et al. 2000; Xu et al. 2012). The Nafion 117 membrane spectra before MFC operation in Fig. 1 possessed characteristic peaks at 2987 cm−1 and a doublet peak at 1034 cm−1 which indicates the presence of –CH, –CF and –SO3 − groups, respectively. The slight shift to the right of the spectra of Nafion 117 membrane after MFC operation indicates the presence of a foulant which could have been from bacterial accumulation to the membrane (Her et al. 2000).
Surface morphology of PEMs: SEM/EDS studies
SEM studies were conducted to determine the surface morphologies of the synthesized CT/PMC and Nafion 117 membranes, along with EDS which was used to determine the surface chemistry of the membranes prior and subsequent to their operation in the MFC units. Similarly, plain CT membrane was used to compare the degree of contribution of the PMC copolymer in the CT/PMC membrane matrix.
The SEM images of the CT, CT/PMC and Nafion 117 membranes used before and after MFC operation are shown in Fig. 2. From Fig. 2a, b, the surface of the CT/PMC membrane before MFC operation generally appeared clean and uniform with no major cavities or protrusions. However, after MFC operation as shown in Fig. 2d, e, the surface morphology was discernibly contrasting, as many contaminants accumulated on the surface of the membrane, thus resulting in the dramatic reduction of membrane coverage that could be observed by the SEM. It was speculated that the entire membrane surface of the CT/PMC after MFC operation was covered with contaminants, both biotic and abiotic entities, thus the resulting SEM image should be attributed solely to the dehydrated form of the suspended solids in the anolyte (Oh et al. 2010; Jang et al. 2013). However, it is worthy to note that due to agitation of the liquor in the dual chamber MFC unit, and the high biological constituents in the anolyte, possibly biological self-layering occurred and it is reasonable to assume that this bacterial self-assembly surface layering could be biofilm formation on the anodic chamber PEM surface (Park et al. 2011; Lee et al. 2014). Although microbial analysis was not performed, aggregated microbial communities were observed with film-layer morphology, with colonies aggregated to form arbitrarily grouped shapes with community sizes of about 4–6 μm (Ping et al. 2014). In addition, due to MFC time evolution and with the required nutrients available, there was a probability that biofilm formation was supported (Erable et al. 2012).
SEM images of CT/PMC membrane before MFC operation at ×1000 (a), ×3000 (b) and after MFC operation at ×1000 (d), ×3000 (e); Nafion 117 membrane before MFC operation at ×1000 (g), ×3000 (h) and Nafion 117 after MFC operation at ×1000 (j), ×3000 (k) chitosan at ×1000 (m), ×3000 (n). EDS images of CT/PMC membrane before (c) and after MFC usage (f); Nafion 117 membrane before (i) and after (l) MFC usage and chitosan membrane (o)
The Nafion 117 membrane surface morphology prior to use in the MFC was quite comparable to that of the CT/PMC membrane, as was shown in the SEM image, represented by Fig. 2g, h. In addition, the surface of the Nafion 117 membrane had minor but distinct grain marks on its surface, which was deemed to be negligible as it was expected that these impressions were attributed to the impact of the packaging material and packaging process, on the surface of the purchased Nafion 117 membrane (Hamel and Fréchette 2011). The observed Nafion 117 membrane in Fig. 2j, k after MFC operation also appeared to exhibit a similar trend of accumulation of biotic and abiotic substrates on the anolyte surface of the PEM. This phenomenon has been previously ascribed as accumulation or membrane fouling (Xu et al. 2012).
EDS analysis of the membranes (Fig. 2c, f, i, l, o along with Table 1) revealed that the surface chemistries varied significantly as a result of MFC operation. In the CT/PMC membrane, the major constituents used for synthesis were biopolymers chitosan and PMC, where PMC possess a carbon backbone with oxygen moieties to complete their chemical composition (Popuri et al. 2014). The initial atomic carbon content of the CT/PMC was 60.7%, and increased to 71% after MFC operation, conversely a decrease in atomic oxygen content was observed in the spectra shifting from 39.2 to 20%. These variances have been attributed to the accumulation of foulants on the PEM surface. In addition, the non-metal sulphur, an essential component in bacteriological cells also appeared on the surface of the membrane as a minor with 7.68% (Wu and Fane 2012; Ping et al. 2014).
Analogously, there was a similar trend observed for the changes in chemical composition on the surface layer of the Nafion 117 membrane. The new surface morphology was previously speculated to be bacterial self-assembled surface layering or biofilm formation, however, due to the high atomic ratios of the metals, it can also be deduced that the dissolved metals chelated on the putative metal-binding sites on the protein surfaces of the biofilm (Paquete et al. 2014). Thus, these metals such as potassium (0.82%), sodium (0.6%), iron (0.5%) and platinum (0.11%) were subsequently present on the surface of the dried Nafion 117 membrane.
XRD analysis
X-ray diffraction studies can provide information about the morphological properties of the crystalline structures present in materials. Thus, X-ray diffraction analysis of the CT/PMC and Nafion 117 membranes prior and subsequent to MFC operation, as well as a pure chitosan membrane, was performed, and the results are shown in Fig. 3.
The X-ray diffractogram of Nafion 117 membrane exhibited the characteristic 2θ peaks at ~16.5° and ~18.8°; however, the convulsion of these peaks increased after MFC operation. These low 2θ diffraction angles have been previously attributed to hexagonal structure of Nafion 117, in which the amorphous and crystalline regions overlap due to the presence of perfluorocarbon chains. In addition, after MFC operation, these peaks shifted slightly to the left being observed at lower 2θ diffraction angles and exhibited peak contraction, thus indicative of a reduction of the most probable maximum grain size. The broad band observed in both Nafion 117 membranes at about 2θ = 40° is associated with the characteristic polyfluorocarbon chains. It is seen that the characteristic peaks of the CT/PMC membrane in Fig. 3 appeared at 2θ = 38°, 44°, 64° and 77° (Popuri et al. 2014); however, these peaks shifted slightly to the left after MFC operation, which is indicative of oxidation of organic material on the membranes surface, but no significant change in their 2θ values indicate that the average intermolecular distance is same in blended polymer membrane chains. The level of noise was increased in the X-ray diffractogram of CT/PMC membrane after MFC operation indicates the accumulation of impurities on the membrane surface. The shift in the CT/PMC membrane after MFC operation is very small, and this shows that there was little or no change in crystalline structure which is indicative of anti-fouling properties (Her et al. 2000).
Thermal degradation behaviour of CT/PMC membrane
The measurement of the change in mass of the synthesized CT/PMC membrane due to thermal degradation was acquired via thermal gravimetric analysis (TGA). In this study, an inert nitrogen atmosphere was used to acquire these weight measurements with respect to temperature and presented in Fig. 4. The membrane under study had a major weight loss at 500 °C with the final decomposition at 550 °C although weight loss started at 450 °C. The PMC copolymer was detached and degraded from 281 °C onwards (Popuri et al. 2014) followed by chitosan degradation at 450 °C and undergoes depolymerization (Dhawade and Jagtap 2012). Thermal stability of the membrane was up to 400 °C; this indicates the extent of ionic crosslinking and the resilience of the membrane to be used in MFC or other fuel cell applications (Reddy and Yang 2010).
Sorption and COD studies
Sorption studies were conducted for the Nafion 117 and CT/PMC membrane in pure water and brewery effluent to evaluate the swelling behaviour of the membranes and its suitability for MFC experiments. The effect of equilibrium sorption percentage of CT/PMC blend membrane and Nafion 117 membrane in brewery effluent and water are shown in Table 2. From the table, it was demonstrated that the sorption percentage of CT/PMC membranes was same for 24 and 48 h of swelling for both water and effluent; however, Nafion 117 membrane sorption percentage decreased from 24 to 48 h in pure water, but remained the same in brewery effluent. The synthesized CT/PMC membrane showed significant increases in the degree of swelling and sorption percentage from brewery effluent to pure water. The maximum sorption % of CT/PMC and Nafion 117 membranes was 275 and 14.29 for brewery effluent and 350 and 12.50 for pure water, respectively. This was mainly due to the hydrophilicity of the CT/PMC membrane and thus indicates that CT/PMC blend membrane had more interaction towards water than Nafion 117 membrane (Riva et al. 2011). The Nafion 117 membranes showed little water uptake as its surface was not as available for the absorption of water molecules (Moilanen et al. 2008). Additionally, the proton conductive channels are not as connected in Nafion 117 membrane as it is with the synthesized CT/PMC membrane, where the latter has a greater degree of connectivity due to design and structure (Wu et al. 2013) (Table 2).
The COD studies (see Table 3) revealed that the highest rate of organic removal from the brewery wastewater came from the Nafion 117 membrane MFC unit, followed by the CT/PMC membrane and the Agar MFC units. The COD removal rate and in turn the columbic efficiency of the MFC setups of different proton exchange media are dependent on the removal of electrons and protons, where Nafion 117 membrane is most efficient, although CT/PMC membrane is a promising alternative.
Bioelectricity generation in MFCs
The MFCs with different proton exchange media (CT/PMC membrane, Nafion 117 membrane and Agar) were operated for 86 days with continuous monitoring, and readings were recorded twice in a day with an average of one reading for every 12 h. Different types of substrate such as glucose and sodium acetate were added to the three MFCs when the potential (voltage) values fell to minimum and remained stable. The power density and current density values of the MFCs before and after the addition of substrate with different proton exchange media are presented in Figs. 5 and 6.
Effect of proton exchange media on energy production
Figure 5 indicates the generation of power density and current density of pristine brewery effluent in MFCs with different proton exchange media. The results indicate that the power density of CT/PMC membrane and Nafion 117 membrane showed a general trend of increase from commencement. The initial power density of MFC unit containing CT/PMC membrane (1226 mW m−2) was higher than the Nafion 117 membrane (159 mW m−2) on day 1 and the trend continues up to day 7 where the CT/PMC membrane produced 3882 mW m−2. Current density fluctuated within the first 4 days of operation and this is probably due to the acclimation of the microbes to the environment. The exponential increase in potential can also suggest that there is a greater amount of exoelectrogenic bacteria present in the substrate, which will directly contribute to electrical generation (Wang et al. 2008). The power generation of CT/PMC membrane from day 7 onwards decreased exponentially with time and finally reach to minimum of 272 mW m−2 by day 13; however, the power generation of Nafion 117 membrane maintained the increasing trend with time up to day 14 and generated a maximum of power density 4245 mW m−2, followed by a sharp decrease trend to 824 mW m−2 by day 17. On contrary of CT/PMC and Nafion 117 membranes, the Agar media generated a maximum power density of 637 mW m−2 by day 13 and reduced to a minimum of 374 mW m−2 within a day. In the Agar salt bridge, the protons have to travel farther in the media than with the PEM membranes CT/PMC and Nafion 117. Additionally, the maximum electrical generation time needed to extract the maximum amount of power in Nafion 117 membrane; CT/PMC membrane and Agar MFCs are 14, 7 and 13 days, respectively. The synthesized CT/PMC membrane MFC converts the substrate’s chemical energy into electricity in half the time of Nafion 117 MFC unit and also generates higher energy in the initial days; however, the maximum energy generation was marginally less than the Nafion 117 membrane and relatively unsustainable. The CT/PMC membrane showed a greater bioelectricity generation due to the hydrophilic nature of chitosan and the proton conductive channels that are present in the CT/PMC membrane, that eventually allow the positively charged ions to move faster through the membrane barrier via proton conductive mechanisms: vehicle and structural diffusion (Wu et al. 2013). Vehicular conductive mechanism is prototrophic movement in bulk water and as the membrane reaches a high hydrated state, it allows the protons to be carried across the membrane at an increased rate via a mechanism called structural diffusion or Grotthuss diffusion (Moilanen et al. 2008; Wu et al. 2013). Agmon (1995) describes this diffusion as protons “hopping” from one aqueous cationic structure to another via the forming and cleavage of covalent and/or hydrogen bonds. The increase in prototrophic movement (Grotthuss diffusion) would relate to an increase in current density, as shown in Fig. 5. Moreover, the key functional groups (for example carboxylic groups of PMC copolymer and amino group of chitosan) possess the ability to absorb bulk water and swell faster than Nafion 117 membrane (see Table 1) (Asano et al. 2006; Wu et al. 2013). These functional groups may also attract and hold the positive ions with lesser binding forces than the highly electronegative functional groups of Nafion 117 at low hydration levels, and can deliver the protons to the cathodic chamber. The Nafion 117 membrane would be at a low hydration level within the first 5 days, and at this point the electronegative groups would make it difficult for protons to pass through (Moilanen et al. 2008).
Effect of glucose on MFC performance
Glucose 160 mg L−1 glucose was added to the brewery wastewater in anodic chamber of each MFC and operated for 23 days with the measurement of both voltage and current. The performances of three MFC units were evaluated by measuring voltage and current output. Glucose is a molecule that can be used for microbial respiration without prior modification (Logan and Rabaey 2012).
Experimental data presented in Fig. 6a revealed that the power density after the addition of glucose increases significantly within a 1 day time period for each MFC setup due to the ease of which glucose can be metabolized. The synthesized CT/PMC membrane MFC unit showed a trifold increase in bioelectricity generated from initial with a maximum power and current densities of 2349 mW m−2 and 100 mA m−2. The current density at its peak almost doubly increased from its initial and then decreases to 10 mA m−2 while power density steadily decreased for the next 3 days from its maximum value to 238 mW m−2. Nafion 117 membrane MFC unit produced the highest bioelectricity generation after the addition of glucose among three MFC units used in this study with a fourfold increase from its initial to a maximum power density of 2468 mW m−2 with a corresponding current density 96 mA m−2. The power density showed a decline from maximum value in the period of 1.5 days to 1021 mW m−2 and remained constant for 5 days. Agar salt bridge had the lowest degree of bioelectrical production, reaching a maximum power density of 1184 mW m−2 with a corresponding current density of 65 mA m−2, which relates to a onefold increase from initial.
After the addition of glucose to the anodic chamber, the microbes in wastewater would have consumed the glucose rapidly and produce an increased number of protons which would be carried across the membrane at increased levels and correlates to the sharp increases of bioelectricity production in the MFC units. The exhaustion of the additive left the microbes with little to utilize to generate electricity resulting in the power and current densities were decreasing to its minimum then remained constant. Glucose has sustained bioelectricity generation for 4 days for CT/PMC MFC unit and 3 days for both Nafion 117 and Agar MFC units. The bioelectricity sustainability of these MFCs with glucose metabolite was significantly smaller than the generation times quoted prior with original brewery wastewater (see Fig. 5), and this is due to the unintentional reactions (fermentation acetogenesis and methanogenesis) that glucose undergo (Logan and Rabaey 2012; Chae et al. 2009; Pant et al. 2010).
Effect of sodium acetate on MFC performance
Sodium acetate is a non-fermentable substrate that readily dissociates in aqueous solutions to provide microbes with acetate, which can be metabolized to create protons and electrons (Logan and Rabaey 2012). As sodium acetate does not undergo unwanted side reactions like methanogenesis, it is more widely used as a substrate in MFC (Pant et al. 2010).
Therefore, 160 mg L−1 of sodium acetate was introduced to each MFC unit; it is revealed in Fig. 6b that the power generated was sustained over a longer time period compared to glucose at the same concentration. The synthesized CT/PMC membrane MFC unit generated a 20-fold increase in maximum bioelectricity generation with power density of 1434 mW m−2 with a current density of 60 mA m−2 in comparison with the initial electrical energy production. The maximum power generation of this MFC was obtained 3 days after initiation and tapered slowly for 4 days until minimum at day 48. The Nafion 117 membrane MFC unit had the highest power production of 1675 mW m−2, with a current density of 78 mA m−2 which was reached in 4 days and is possibly due to the increase in proton conductivity (Wu et al. 2013). This electrical generation dropped within 1.5 days to 647 mW m−2 at day 47 and gently decreased to a minimum of 203 mW m−2 on day 53. Agar salt bridge MFC unit outperformed the synthesized CT/PMC MFC unit by generating a maximum power density of 1388 mW m−2 at a current density of 70 mA m−2, 4 days after initiation. The Agar MFC unit may have at this time been hydrated enough to allow a mass movement of protons across chambers, despite the distance needed to travel. Power generation sharply decreased within 1.5 days from maximum to 313 mW m−2 on day 49 where the power generation subsequent gently decreased and reached its minimum at 107 mW m−2 on day 53.
When the microbes exhaust the substrate, the electricity generated goes to a constant minimum where the microbes are in a dormant state (Lappin-Scott and Costerton 1990) and continues in this state to ensure survival. When substrates are introduced to the microbial system, the microbes would increase the rate of metabolism and thus an increased rate of bacterial respiration. This high metabolism rate will create electrons and protons which can result in sharp increases of bio electricity generation for each MFC setup (Prakash et al. 2010). The Nafion 117 MFC setup began to produce significant amounts of electrical energy just after 1 day of operation, due to its state of hydration. The Nafion 117 membrane possesses a slower hydration rate than that of the synthesized CT/PMC membrane and reached a state of high levels of hydration at day 41 which allows more protons to be are carried across the membrane via the aforementioned Grotthuss diffusion (Wu et al. 2013). The current density graph for the Nafion 117 membrane MFC unit showed that the highest current generation was between day 42 and day 44, which relates to a significant increase in bioelectricity generation. The sharp increase in power generation in the CT/PMC MFC unit is also attributed to the rapid metabolism of the introduced substrate, but as the membrane is highly hydrophilic, it would have been fully hydrated at day 41. This maximum hydration indicates that Grotthuss diffusion is at a maximum for prototrophic mobility and plays a minimal role in this investigation. The Agar salt bridge showed a slower response time to the addition of the additive, as the prototrophic distance is increased in this MFC unit but still generated a significant amount of energy.
The electricity generation times for all of the MFC units were approximately 6 days, which when compared to the glucose investigation is an increase. This is because sodium acetate is metabolized at a slower rate in microbes and can thus sustain the electrical generation for longer periods. Additionally, there is not as much microbes available to metabolize acetates, so this effect can also be a factor to the enlargement of these times (Pant et al. 2010).
Effect of metabolite concentration
Figure 6c shows the effect of adding an increasing concentration of a metabolite; sodium acetate on different proton exchange media in MFC and the sections indicate the region by concentration. Upon the increase in concentration of the metabolite, the trend is a general increase in electrical production (Cheng and Logan 2011) and was seen in the Nafion 117 membrane MFC unit (see Fig. 6c) power density graph. The CT/PMC membrane setup showed an attenuation of maximum power generation, with the increase in sodium acetate concentration from 1434 mW m−2 at 160 mg L−1 to 91 mW m−2 at 1000 mg L−1. The Agar MFC setup also follows the trend of decrease, even though the maximum power generation increases slightly when the concentration 1000 mg L−1. It should be noted that the Agar MFC unit generally outperformed the CT/PMC MFC unit as the agar was now fully solvated and could carry a greater number of protons via vehicular mechanisms. However, the Nafion 117 membrane MFC setup has shown a significant power output at the 1000 mg L−1 mark which can be attributed to Grotthuss and vehicular diffusion. There is a large drop in power when the concentration of acetate was increased to 500 mg L−1 which can be attributed to the loss of bacteria in the anodic chamber due to the harsh change in environmental conditions. In addition, the increase in concentration may have had an inhibitory effect on the CT/PMC and Agar salt bridge MFC setups as the excess acetate could not have been utilized at a rate to limit microbial death and this could have led to fouling of the proton exchange media which is solely responsible for prototrophic transport which is seen in the CT/PMC FTIR spectra after MFC operation (Her et al. 2000; Ghoreyshi et al. 2011).
Conclusion
This comparative study revealed that the use of CT/PMC membrane in a microbial fuel cell using brewery wastewater can yield a maximum power density of 3882 mW m−2, which is competitive to the bioelectricity output from Nafion 117 membrane MFC, with a power density of 4245 mW m−2. The CT/PMC membrane also has shown competiveness in its MFC unit bioelectricity start-up times, which allowed it to generate electrical energy earlier than the comparative proton exchange media Nafion 117 membrane and Agar salt bridge MFC unit. The decrease in maximum hydration times is an interesting property of the synthesized CT/PMC membrane, as it can improve prototrophic movement; hence improve retention times for MFC electrical generation. Sodium acetate metabolite showed a greater bioelectricity output over time, with the longest duration of generation. The increase in concentration of the metabolite only resulted in a nominal increase in the Nafion 117 membrane unit; conversely an attenuation of generation with the synthesized CT/PMC membrane was seen. The efficiency of the CT/PMC membrane as PEM is high in bioelectricity generation using brewery effluent along with fermentable and non-fermentable additives. The usage of unwanted materials to create a PEM for MFC has a twofold advantage as it would minimize the impact of liquid and solid waste in the environment, along with electrical generation. Further research is needed to investigate the anti-fouling properties of the synthesized CT/PMC membrane in MFC as it is promising for long-term usage.
References
Agmon N (1995) The Grotthuss mechanism. Chem Phys Lett 244:462–465
Ahn Y, Logan B (2013) Saline catholytes as alternatives to phosphate buffers in microbial fuel cells. Bioresour Technol 132:436–439
AHPA (2005) Standard methods for the examination of water and waste water. American Public Health Association, Washington
Allen RM, Bennetto PH (1993) Microbial fuel-cells. Appl Biochem Biotechnol 39:27–40
Asano N, Aoki M, Suzuki S, Miyatake K, Uchida H, Watanabe M (2006) Aliphatic/aromatic polyimide ionomers as a proton conductive membrane for fuel cell applications. J Am Chem Soc 128:1762–1769
Beppu MM, Vieira RS, Aimoli CG, Santana CC (2007) Crosslinking of chitosan membranes using glutaraldehyde: effect on ion permeability and water absorption. J Membr Sci 301:126–130
Cardenas A, Argueles-Monal W, Goycoolea FM, Higuera-Ciapara I, Peniche C (2003) Diffusion through membranes of the polyelectrolyte complex of chitosan and alginate. Macromol Biosci 3:535–539
Chae K-J, Choi M-J, Lee J-W, Kim K-Y, Kim IS (2009) Effect of different substrates of the performance, bacterial diversity, and bacterial viability in microbial fuel cells. Bioresour Technol 100:3518–3525
Cheng S, Logan BE (2011) Increasing power generation for scaling up single-chamber air cathode microbial fuel cells. Bioresour Technol 102:4468–4473
Dash M, Chiellini F, Ottenbrite RM, Chiellini E (2011) Chitosan—a versatile semi-synthetic polymer in biomedical applications. Prog Polym Sci 36:981–1014
Dhawade PP, Jagtap R (2012) Characterization of the glass transition temperature of chitosan and its oligomers by temperature modulated differential scanning calorimetry. Adv Appl Sci Res 3:1372–1382
Du Z, Li H, Tingyue G (2007) A state of the art review on microbial fuel cells: a promising technology for wastewater treatment and bioenergy. Biotechnol Adv 25:464–482
Erable B, Feron D, Bergel A (2012) Microbial catalysis of the oxygen reduction reaction for microbial fuel cells: a review. ChemSusChem 5:975–987
Feng Y, Wang X, Logan BE, Lee H (2008) Brewery wastewater treatment using air-cathode microbial fuel cells. Appl Microbiol Biotechnol 78:873–880
Ghoreyshi AA, Jafary T, Najafpour GD, Haghparast F (2011) Effect of type and concentration of substrate on power generation in a dual chambered microbial fuel cell. World Renewable Energy Congress, Sweden
Hamel S, Fréchette LG (2011) Critical importance of humidification of the anode in miniature air-breathing polymer electrolyte membrane fuel cells. J Power Sources 196:6242–6248
Heidrich ES, Curtis TP, Dolfing J (2011) Determination of the internal chemical energy of wastewater. Environ Sci Technol 45:827–832
Her N, Amy G, Jarusutthirak C (2000) Seasonal variations of nanofiltration (NF) foulants: identification and control. Desalination 132:143–160
Isıklan N, Şanlı O (2005) Separation characteristics of acetic acid–water mixtures by pervaporation using poly(vinyl alcohol) membranes modified with malic acid. Chem Eng Process Process Intensif 44:1019–1027
Jang JK et al (2013) Electricity generation by microbial fuel cell using microorganisms as catalyst in cathode. J Micro Biotechnol 23:1765–1773
Kim BH et al (2004) Enrichment of microbial community generating electricity using a fuel-cell-type electrochemical cell. Appl Microbiol Biotechnol 63:672–681
Lappin-Scott HM, Costerton JW (1990) Starvation and penetration of bacteria in soils and rocks. Experientia 46:807–812
Lee DH, Oh HJ, Bai SJ, Song YS (2014) Photosynthetic solar cell using nanostructured proton exchange membrane for microbial biofilm prevention. ACS Nano 8:6458–6465
Lefebvre O, Uzabiaga A, Chang IS, Kim B-H, Ng HY (2011) Microbial fuel cells for energy self-sufficient domestic wastewater treatment- a review and discussion from energetic consideration. Appl Microbiol Biotechnol 89:259–270
Logan B (2008) Microbial fuel cells. Wiley, Hoboken
Logan BE, Rabaey K (2012) Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Sci Mag 337:686–690
Logan BE, Regan JM (2006) Microbial fuel cells-challenges and applications. Environ Sci Technol 14:5172–5180
Ma J, Sahai Y (2013) Chitosan biopolymer for fuel cell applications. Carbohydr Polym 92:955–975
Mat NC, Liong A (2009) Chitosan-poly (vinyl alcohol) and calcium oxide composite membrane for direct methanol fuel cell applications. Eng Lett 116:1017–1029
Moilanen DE, Spry DB, Fayer MD (2008) Water dynamics and proton transfer in Nafion fuel cell membranes. Langmuir 24:3690–3698
Oh S, Logan BE (2005) Hydrogen and electricity production from a food processing wastewater using fermentation and microbial fuel cell technologies. Water Res 39:4673–4682
Oh ST, Kim JR, Premier GC, Lee TH, Kim C, Sloan WT (2010) Sustainable wastewater treatment: how might microbial fuel cells contribute. Biotechnol Adv 28:871–881
Pant D, Van Bogaert G, Diels L, Vanbroekhoven K (2010) A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour Technol 101:1533–1543
Paquete CM et al (2014) Exploring the molecular mechanisms of electron shuttling across the microbe/metal space. Front Microbiol 5:1–12
Park JT, Lee SJ, Park JP, Yang MH, Choi JH, Lee SY (2011) Characterization of a bacterial self-assembly surface layer protein and its application as an electrical nanobiosensor. J Nanosci Nanotechnol 11:402–407
Perlman H (2014) A visit to a wastewater-treatment plant: primary treatment of wastewater. http://water.usgs.gov/edu/wwvisit.html. Accessed Aug 2014
Ping Q, Zhang C, Chen X, Zhang B, Huang Z, He Z (2014) Mathematical model of dynamic behavior of microbial desalination cells for simultaneous wastewater treatment and water desalination. Environ Sci Technol 48:1310–1319
Popuri S, Hall C, Wang C-C, Chang C-Y (2014) Development of green/biodegradable polymers for water scaling applications. Int Biodeterior Biodegrad 95:225–231
Prakash GK Surya, Viva FA, Bretschger O, Yang B (2010) Inoculation procedures and characterisation of membrane electrode assemblies for microbial fuel cells. J Power Sources 195:111–117
Rao SP, Sridhar S, Krishnaiah A, Wey MY (2007) Pervaporation separation of ethylene glycol/water mixtures by using cross-linked chitosan membranes. Ind Eng Chem Res 46:2155–2163
Reddy N, Yang Y (2010) Citric acid cross-linking of starch films. Food Chem 118:702–711
Riva R, Ragelle H, Rieux A, Duhem N, Jerome C, Preat V (2011) Chitosan and chitosan derivatives in drug delivery and tissue engineering. Adv Polym Sci 244:19–44
Suginta W, Khunkaewla P, Schulte A (2015) Electrochemical biosensor applications of polysaccharides chitin and chitosan. Chem Rev 113:5458–5479
Vaghari H, Jafarizadeh-Malmiri H, Berenjian A, Anarjan A (2013) Recent advances in application of chitosan in fuel cells. Sustain Chem Process 1:1–16
Wang X, Feng YJ, Lee H (2008) Electricity production from beer brewery wastewater using single chamber microbial fuel cell. Water Sci Technol 57:1117–1121
Werpy T et al (2004) Top value added chemicals from biomass. Results of screening for potential candidates from sugars and synthesis gas, vol 1. Department of Energy, Washington
Winfield J, Ieropoulos I, Rossiter J, Greenman J, Patton D (2013) Biodegradation and proton exchange using natural rubber in microbial fuel cells. Biodegradation 24:733–739
Wu B, Fane AG (2012) Microbial relevant fouling in membrane bioreactors: influencing factors, characterization, and fouling control. Membranes 2:565–584
Wu L, Zhang Z, Ran J, Zhou D, Li C, Tongwen X (2013) Advances in proton-exchange membranes for fuel cells: an overview on proton conductive channels (PCCs). Phys Chem Chem Phys 15:4870–4887
Xu J, Sheng GP, Luo HW, Li WW, Wang LF, Yu HQ (2012) Fouling of proton exchange membrane (PEM) deteriorates the performance of microbial fuel cell. Water Res 46:1817–1824
Zhang F, Ge Z, Grimaud J, Hurst J, He Z (2013) Long-term performance of liter-scale microbial fuel cells treating primary effluent installed in a municipal wastewater treatment facility. Environ Sci Technol 41:4941–4948
Acknowledgements
Authors are thankful to the University of the West Indies, Cave Hill Campus for providing study and travel grants for conducting characterization studies of the membranes.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: M. Abbaspour.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Harewood, A.J.T., Popuri, S.R., Cadogan, E.I. et al. Bioelectricity generation from brewery wastewater in a microbial fuel cell using chitosan/biodegradable copolymer membrane. Int. J. Environ. Sci. Technol. 14, 1535–1550 (2017). https://doi.org/10.1007/s13762-017-1258-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-017-1258-6