Abstract
Lead and cadmium are the two widely recognized toxic metals, with known ecological risk to freshwater fish in contaminated ecosystems. However, their effect at environmentally realistic level in a mixture has been rarely investigated. In the present study, serum metabolic responses of Crucian carp exposed to Pb (30 µgL−1), Cd (100 µgL−1) and Pb+Cd (30 + 100 µgL−1) for 21 days were investigated by 1H NMR-based metabolomics. The metabolic responses were compared to control by multivariate techniques (PCA, PLS-DA and OPLS-DA), and metabolites that significantly contributed to the variation were identified. Metal dependent metabolic responses revealed a decline of alanine, lysine and tyrosine in Pb exposed fish, indicating changes in neurotransmitters, and amino acid metabolism, while fish exposed to Cd showed significant decrease in lysine, isoleucine, leucine, alanine and increase in 3-hydroxybutyrate, acetone, lactate, choline, inosine, guanosine and threonine. The coexposure of Pb and Cd had additive effect on metabolic profile with increase in pyruvate, guanosine and inosine. The overall metabolic changes due to Pb and Cd were characterized by disturbed energy metabolism, impaired osmotic regulation and a shift from aerobic to anaerobic respiration. Moreover, 1H NMR-based metabolomics was proved to be a powerful tool in elucidating the toxic effects of environmental pollutants and underlying mechanism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) and cadmium (Cd) are the two nonessential heavy metals that can be highly toxic even at low concentration due to their reactivity with sulfur (S) and nitrogen (N) atoms in the biomolecules and their constituents (Thapa et al. 2012). Excessive accumulation of Pb and Cd in the body can cause serious damage to different organisms at cellular and molecular level. They have the ability to bind with sulfhydryl groups thereby disrupting the structures and functions of proteins, or they can compete with some essential elements from a variety of proteins that serve as enzymes and transcription factors (Qi et al. 2015). In addition, these metals have the characteristics to induce oxidative stress and stimulate the production of reactive oxygen species (ROS) which lead to the impairment of normal redox cycle in cells (Rodriguez-Serrano et al. 2009; Schutzendubel et al. 2001). Studies have shown that both Pb and Cd can affect organs and various systems of an organism and may cause acute and chronic intoxications (Matovic et al. 2015).
The toxicity of Pb and Cd have been comprehensively investigated in various in vivo and in vitro studies, revealing different molecular, cellular and intracellular mechanisms to indicate the toxicological impacts of these two toxic metals (Clemow and Wilkie 2015; Hambach et al. 2013; Zaza et al. 2015). However, the degree of pathogenesis and deleterious health consequences due to Pb and Cd exposure is multifaceted, and the exact mechanisms underlying their toxicity are yet not fully understood (Matović et al. 2011). Recently, Pb and Cd exposure have been found to influence gene expression, apoptosis, DNA damage and repair (Al Bakheet et al. 2013; Banfalvi et al. 2012; Khalaf et al. 2012; Zhou et al. 2013). The knowledge pertaining to the complete understanding of these mechanisms is far from being achieved and has always remained controversial with only the oxidative stress as the most documented molecular mechanism for Pb and Cd toxicity (Çavaş 2008; Maceda-Veiga et al. 2015; Birben et al. 2012).
Beside natural occurrence of Pb and Cd in the ores and racks, their contamination in the ecosystems can greatly depend on the anthropogenic mobilization. The main sources through which they are mobilized to the environment are mining, smelting, industrial settings and agricultural activities (Sfakianakis et al. 2015). Aquatic ecosystem is the ultimate sink among all contaminated systems that constantly receive these metals from the natural and anthropogenic sources (Wang et al. 2012). The most available and reactive form of Pb and Cd in water that effect organisms are their ionic species that impart serious problem via bioaccumulation, biomagnifications through food chain and toxicity to human (Guzinski et al. 2013). Fish, being a dominant creature in aquatic environment can be better used as bio-indicator of toxic metal pollution. Besides their significance in global food supply in terms of nutritious and balance diet, fish also play crucial role in ecological balance and energy flow in aquatic ecosystems (Li et al. 2014). Fish are particularly sensitive to contaminants in water, because they show marked physiological and biochemical changes in contaminated environments, and thus, fish can serve as suitable bioindicator in environmental monitoring and quality assessment of aquatic ecosystem (Gül et al. 2004).
Metabolomics is the key “omic” science in system biology that has been widely used in toxicity assessment, disease diagnosis and safety evaluation (Laghi et al. 2014). Among various analytical techniques (GC–MS, LC–MS, NMR) used for metabolic profiling, NMR has many advantages. It is considered as unbiased, nondestructive, rich in structural information and rapid with little or no separation (Wei et al. 2015; Xu et al. 2015). Recently, metabolomics is more commonly used for biomarkers identification associated with prognosis or diagnosis of disease and toxicity assessment to improve our understanding of cellular response to toxic substances or other environmental stressors (Mamas et al. 2011). During stress or disease, cells respond to adjust themselves to intra-and/or extracellular environment in order to maintain homeostasis. One way to attain homeostasis is the modulation of biofluids in response to disease, medications and exposure to toxic substances. Such regulation is expressed in a specific metabolic pattern which results in biochemical changes that are pertinent to the disease or external stress (Dudka et al. 2014; Lindon et al. 2004).
The term environmental metabolomics refers to the study of organism environment interactions and the risk associated with the effects of environmental pollutants on physiological function and health of organism at a molecular level (Bundy et al. 2008). A number of species both from animal and plant kingdom have been subjected to the study of environmental metabolomics (Viant 2008). Recently, some of the freshwater fish including gold fish (Carassius auratus) have been successfully investigated for the environmental risk of organic and inorganic pollutants using 1H NMR-based metabolomics approach (Jordan et al. 2012; Li et al. 2014; Lu et al. 2014; Xu et al. 2015). However, to the best of our knowledge, there is no information available on metabolomics responses of freshwater fish to toxic heavy metals, Pb and Cd. The present study was conducted at Wuhan PR. China, in 2015 to elucidate the individual and combined toxic effects of Pb and Cd at environmentally realistic concentrations using 1H NMR-based metabolomics technique in Crucian carp, an ecologically important freshwater fish species.
Materials and methods
Chemicals and reagents
Lead nitrate and cadmium chloride of purity >99 %, nitric acid, acetic acid (conc. glyacial), sodium thiosulfate, EDTA (disodium salt of EDTA), potassium iodide crystal, magnesium sulfate, ammonium chloride and ammonium hydroxide were purchased from Sinopharm Chemical Reagents Co., Ltd (Beijing, China). The deionized water used for preparation of reagents and elemental stock solutions were passed through Millipore purification apparatus (Millipore, MA, USA) to a resistivity higher than 18.2 MΩ cm. ICP-Multi-element certified reference materials (CRM) were obtained from PerkinElmer No. N9300281, 1 Shelton, Connecticut, USA. All the chemicals were analytical grade and used without any further purification.
Experimental condition and exposure assay
Crucian carp of appropriate body weight (92 ± 4.2 g) and length (12 ± 2.6 cm) were procured from a freshwater fish breeding base in Wuhan, China, and acclimated to the laboratory condition for 1 week in the laboratory tanks (50 cm × 30 cm × 30 cm) containing dechlorinated tap water with continuous supply of oxygen. The important water quality parameters were regularly assessed according to the standard methods of APHA (1992). The optimum condition (total hardness 156.32 ± 4.43 mgL−1 as CaCO3, temp. 22.41 ± 2.11 °C, pH 7.6 ± 0.31, dissolved oxygen 8.26 ± 0.68 mgL−1) for water quality was maintained till the end of the experiment. During acclimation, fish were fed with artificial feed once a day until a day before termination of acclimation period. Half of the aquarium water was renewed everyday to clean the residual feed and ammonia produced by fish. All the experiments were carried out according to the guidelines of Chinese Law for Animal Health Protection and Instructions for Granting Permits for Animal Experimentation for Scientific Purposes [Ethics approval No. SCXK (YU) 2005-0001]. Forty acclimated fish were randomly divided into four different groups: control (n = 10), Pb (n = 10), Cd (n = 10) and Cd+Pb (n = 10), without making any distinction between sexes. Control group was kept under similar experimental condition but without any addition of test chemical, while Pb group was exposed to 30 µgL−1 Pb as Pb(NO3)2. The Cd group was exposed to 100 µgL−1 Cd in the form of CdCl2, whereas Cd+Pb group was exposed to a combination of the two test chemicals at the same rate. The exposure duration was 21 days for all groups. During exposure, the respective concentrations were closely monitored in the aquarium at 2 days interval by ICP-OES to maintain the desired concentration in the tanks. The exposure concentration of Cd and Pb was selected on the basis of previous studies (Khan et al. 2015; Qu et al. 2014), which suggested that exposure to these concentrations might significantly inhibit the activity of antioxidant enzymes and induce a pro-oxidant condition in the various tissues of freshwater fish. Moreover, these concentrations are somewhat related to the contamination levels of rivers and lakes in China (An et al. 2010; Bing et al. 2013; Li et al. 2013; Wang et al. 2012; Yang et al. 2009; Zhou et al. 2007).
Sample collection and preparation for 1H NMR
After 21 days of exposure, blood samples were obtained from the caudal vein of each fish in all groups and allowed to clot for 30 min. The serum was then obtained by centrifugation at 3500 rpm for 5 min. All serum was snap-frozen with liquid nitrogen immediately after collection and stored at −80 °C prior to NMR analysis. For sample preparation, 200 μL of each serum was mixed with 400 μL of phosphate buffer (45 mM, pH 7.40, 50 % D2O) followed by centrifugation at 16,099×g, 4 °C for 10 min. The supernatant (550 μL) from each sample was then transferred into a 5 mm NMR tube and employed directly for NMR analysis.
1H NMR serum analysis
1H NMR spectra of serum were acquired at 298 K on an Agilent DD2-600 MHz spectrometer (599.83 MHz for proton frequency) equipped with an Agilent inverse cryogenic probe. A water-pre-saturated Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence (recycle delay-90°-(τ-180°-τ)n-acquisition) was employed to attenuate NMR signals from macromolecules. For all experiments, 128 transients were collected with 32 k data points and a spectral width of 16 ppm. A 90° pulse length was adjusted to about 10 μs, and recycle delay (RD) was set to 2 s. For all spectra, an exponential window function was applied with a line-broadening factor of 1 Hz prior to Fourier transformation.
Pre-processing of 1H NMR data and peak assignment
All 1H NMR spectra of serum were phased and baseline-corrected manually using MestReNova (V7.0, Mestrelab Research SL). The serum spectra were referenced to the anomeric proton signal of α-glucose (δ5.23). The spectral regions of δ 0.6–9.0 were then integrated into bins with the width of 0.002 ppm using AMIX software package (V3.8.3, Bruker Biospin). The spectral regions contained residual water signals (δ 4.60–5.20) and ethanol signals (δ 1.155–1.199, δ 3.63–3.689) which were discarded prior to data normalization. All spectra were mean-centered, and the integral values of each spectrum were probability quotient normalized to account for different dilutions of samples. NMR signals were assigned by querying publicly accessible metabolomics databases such as Human Metabolome Database (HMDB; http://www.hmdb.ca) (Wishart et al. 2013) and Madison-Qingdao Metabolomics Consortium Database (MMCD; http://mmcd.nmrfam.wisc.edu/) (Cui et al. 2008).
Statistical analysis
Multivariate statistical data analyses including Principal Component Analysis (PCA), Projection to Latent Structure with Discriminant Analysis (PLS-DA) and Orthogonal Projection to Latent Structure with Discriminant Analysis (OPLS-DA) were performed with the SIMCA-P+package (V.12, Umetrics, Sweden). First, the unsupervised pattern recognition method, PCA was used to differentiate the similarities and differences in the serum metabolic profiles of control and exposed groups (Wold et al. 1987). Based on PCA, metabolites differentiating the control group from each of the treated group were identified and integrated. From the integrated data, relative intensity of each of the metabolites was then calculated. Further, a supervised pattern recognition method, PLS-DA, was performed to maximize the separation of control and treated groups based on metabolites identified from PCA with rigorous permutation test (200 permutations) (Gu et al. 2011; Solanky et al. 2003). For clearer separation of the treated groups and improved predictive quality, OPLS-DA was also run for the data (Bylesjö et al. 2006). The validity of the models against over fitting and predictive ability was explained by the parameters R 2 Y and Q 2 Y, respectively. A low or negative value for Q 2 Y indicates no statistically significant difference between the control and exposed groups. To make sure that discrimination in the OPLS-DA model was not due to data over fitting, a validation of the model was performed by rigorous permutation test (100 times) (Brindle et al. 2002; Llorach et al. 2010). The established models were validated by the observed value of P (≤0.05) at 95 % confidence level. Different responses were identified and visualized by S-plots and color-coded loadings plots.
Results and discussion
1H NMR spectroscopy of fish serum
The representative 1H NMR spectra of serum obtained from control and metal exposed (Pb, Cd and Pb+Cd) groups are shown in Fig. 1. A number of metabolites classes were identified including amino acids, energy storage compounds, citric acid cycle intermediates and unsaturated fatty acids. However, the 1H NMR spectra mainly comprised of amino acids, and unsaturated fatty acids were found to be 10 to 100 fold greater in intensity as compared to other metabolites. Although the obtained spectra for different groups seems to be similar in overall appearance and composition, yet marked differences in the spectra can be observed by visual inspection due to the inherent quantitative nature of 1H NMR technique as differences in the 1H NMR signals intensities are directly related to the concentration of metabolites containing the protons.
Typical 600 MHz 1H NMR spectra of serum from Control, Pb, Cd and Pb+Cd exposed fish. The region of δ 5.45–9.0 (in the dashed box) was vertically expanded 8 times compared with the region of δ 0.5–5.45 for the purpose of clarity. Keys: Ala Alanine, Asn asparagine, Arg arginine, Cho choline, Cre creatine, Gln glutamine, Glu glutamate, Gly glycine, GPC glycerolphosphocholine, 3-HB 3-Hydroxybutyrate, His histidine, Ile isoleucine, Leu leucine, Lys lysine, NAG N-acetyl glycoprotein signals, OAG O-acetyl glycoprotein signals, PC phophocholine, Phe phenylalanine, Thr threonine, Tyr tyrosine, Val valine, TG triglyceride, Met methionine, Aceto acetoacetate, Lipid1(L1) R-CH 3(ω-6), Lipid2(L2) CH3(CH2CH=CH) n (ω-3), Lipid3(L3) R-CH 2(ω-6), Lipid4(L4) CH 2CH2C=C, Lipid5(L5) CH 2C=C, Lipid6(L6) C=CCH 2C=C, UFA unsaturated fatty acid
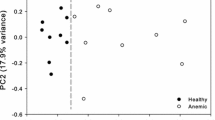
Pb- and Cd-induced altered metabolic profile by PCA
1H NMR spectral data sets obtained from control and exposed groups were first analyzed by PCA for reduction of dimensionality and detection of outliers. The distinct separation of each exposed group from control can be seen in respective PC score plots as given in Fig. 2, except the Pb versus control group, where no obvious separation was observed between serum metabolic profiles of the two groups (P < 0.05). The corresponding PC loadings plots pointed out which metabolites were important in the separation of the two groups and the direction of their changes. The explained variation (R 2) along X-axis between the control and exposed groups were 86.0, 83.6 and 76.4 % with predictability of the PCA model (Q 2 > 0.5) 0.78, 0.70 and 0.63 for Pb versus control, Cd versus control and Pb+Cd versus control group, respectively.
Principle component analysis (PCA) sore plots obtained from 1H NMR-based metabolomics data of Crucian carp’s serum in the spectral region of δ 0.6–9.0 representing significant separation between control and Pb (a), Cd (b) and Pb+Cd (c) exposed groups at 30, 100 and 30+100 µg L−1 for 21 days, respectively
Pb- and Cd-induced altered metabolic profile by PLS-DA and OPLS-DA
The 1H NMR spectral data from control and exposed fish serum were also subjected to PLS-DA and OPLS-DA modeling to differentiate serum metabolic pattern between control and exposed groups. Results from the PLS-DA model revealed significant (P < 0.05) demarcation between the control, Cd and Pb+Cd exposed groups as shown in Fig. 3. There was no obvious separation observed for serum metabolic profile of Pb versus control group (Fig. 3a), whereas the predictability of PLS-DA model as explained by Q 2 (proportion of variance in the data predictable by the model) was 0.6 and the explained variation was R 2 X = 30.6 % and R 2 Y = 91.5 % which showed good predictability of the model. The serum metabolic profile of Cd and Pb+Cd groups versus control showed clear separation with good predictability and high value parameters R 2 X, R 2 Y and Q 2 24.9, 98.7, 0.816 % and 28.3, 98.3, 0.851 %, respectively. The Q 2 intercipt for Pb versus control, Cd versus control and Pb+Cd versus control were 0.157, 0.0504 and 0.158, respectively, was <0.5 which further confirmed the validity of the PLS-DA model to predict the metabolomics data.
Based on 1H NMR metabolomics data, the score plots of PLS-DA model (on left) showing separation between the serum metabolites of control verses Pb (a), Cd (c) and Pb + Cd (e) exposed Crucian carps at 30, 100 and 30+100 µgL−1 for 21 days, respectively. The validation plot (on right) of 100 permutation test with Q 2-intercept 0.0, 0.0173 (b), 0.0, 0.0504 (d) and 0.0, 0.158 (f) were <0.5, indicating a valid model
The OPLS-DA analysis was carried out using the 1H NMR data as X matrix and class information as Y matrix with unit variance scaling. The score plots and loadings plots generated from MATLAB (Mathworks, Natick, MA) following back-transformation, which shows color-coded signals with correlation coefficients to reveal significantly altered metabolites of Pb versus control, Cd versus control and Pb+Cd versus control group are presented in Fig. 4. The score plots from OPLS-DA revealed clear separation between control and exposed groups with high predictive and modeling parameters R 2 X and Q 2 30.6, 0.58 for Pb versus control, 24.9, 0.84 for Cd versus control and 28.3, 0.83 for Pb+Cd versus control group, respectively.
Cross-validated OPLS-DA score plots (left) and corresponding color-coded correlation coefficient plots (right) derived from 1H NMR spectra of control verses Pb (a), Cd (b) and Pb + Cd (c) exposed Crucian carps at 30, 100 and 30+100 µgL−1 for 21 days, respectively. The color-coded map indicates significance of metabolite variations between control and exposed group. Red color shows important discriminatory metabolites, and blue color indicates no discrimination at P < 0.05
According to the color-coded correlation coefficient plots in Fig. 4, several metabolites were changed due to Pb, Cd and Pb+Cd exposure in the serum of Crucian carp. Unlike Cd and Pb+Cd exposure, Pb caused significant decrease in few but important metabolites like alanine, lysine and tyrosine as shown in Table 1. Fish serum from Cd exposed group revealed significant decrease in the level of lysine, isoleucine, leucine and alanine, but increase in the level of 3-hydroxybutyrate, acetone, lactate, choline, inosine, guanosine and threonine as depicted in Table 2. The co-exposure of Pb and Cd caused the most significant variation in the serum metabolic profile of fish by enhancing the level of pyruvate and inosine, but declining the level of alanine, lysine, glutamine, asparagine, R-CH 3(ω-6), CH3(CH2CH=CH)n(ω-3), R-CH 2(ω-6), unsaturated fatty acids and O-acetyl glycoprotein as shown in Table 3.
The toxic effect of Pb and Cd in terms of carcinogenesis, histopathology and genotoxicity together with several other toxicological aspects has been extensively studied but their effects caused at metabolite level still remain almost unknown. In the present study, 1H NMR-based metabolomics technique was used to explore changes in the serum metabolic profile of control and exposed (Pb, Cd, Pb+Cd) Crucian carps and to identify the altered metabolites as potential biomarkers of exposure. The data obtained from 1H NMR spectra were analyzed by multivariate statistical techniques including PCA, PLS-DA and OPLS-DA for pattern recognition and metabolite identification. PCA is an unsupervised exploratory pattern recognition tool irresponsive to the status of each sample and serve to reduce dimensionality, identify outliers and indicate similarity and difference between multiple NMR spectral data sets. On the other hand, PLS-DA is a supervised pattern recognition method more commonly employed to classify multiple classes by probing variables at X matrix which are correlated with class variables at Y matrix. In PLS-DA model, the X matrix is the measured matrix (NMR data) and Y matrix consists of dummy variables which indicate the class for each treatment (Zhang et al. 2011). Compare to PLS-DA model, the OPLS-DA model analyzes data with orthogonal filtration of X matrix on a vector Y. It splits variance in the X matrix into predictive variance and orthogonal variance. The structure noise of the data such as age, genetic background and physical activity is filtered and only described by orthogonal components. The predictive component, i.e., the first component, only shows the variation of scientific interests. Thus, the interpretability of the resulting model is increased (Fonville et al. 2010). In our study, both unsupervised and supervised pattern recognition methods showed distinct separation between the control and exposed groups with good predictability and validation of the model.
Regarding the toxicity of Pb, Cd and their co-exposure at environmental level, significant changes in several metabolites were observed from the color-coded correlation coefficient plots (Fig. 4). Fish exposure to 30 µgL−1 Pb caused significant reduction in alanine, lysine and tyrosine, while the rest of the metabolite did not show any change. Alanine is an aliphatic amino acid and plays important role in glucose-alanine cycle between different tissues and liver. It was observed that alanine level decreased in Ruditapes philippinarum due to Cu exposure because alanine is involved in anaerobic metabolism (Zhang et al. 2011). Both lysine and tyrosine are essential amino acids and a part of the intermediatory metabolism (Fig. 5). Lysine undergoes to produce acetyl-Co A, an intermediatory molecule via transamination with α-ketoglutarate, while tyrosine is a precursor to neurotransmitters and hormones (McCoole et al. 2012).
Scheme representing the perturbed metabolic pathways of Crucian carp exposed to environmental concentration of Pb, Cd and Pb+Cd for 21 days as detected by1H NMR analysis. Citric acid cycle was the junction pathway of energy metabolism, lipid metabolism and amino acids metabolism. Arrows (“↑↓”) in different colors representing significant increase or decrease in metabolites due to Pb (yellow), Cd (blue) and Pb+Cd (red) intoxication in fish as compared to their respective control group. (For interpretation of the references to color in this figure legend, the reader is referred to the webversion of the article)
Significant decrease in lysine, isoleucine, leucine, alanine and increase in 3-hydroxybutyrate, acetone, lactate, choline, inosine, guanosine and threonine were observed in the serum of fish exposed to 100 µgL−l Cd. Isoleucine is glucogenic as well as ketogenic amino acid which can be converted to succinyl Co A to enter into (tricarboxylic acid) TCA cycle or oxaloacetate for gluconeogenesis (Campos-Ferraz et al. 2013). Whereas leucine stimulates the synthesis of protein through activation of intracellular cell signaling pathways especially those which are used by insulin (Lynch et al. 2003). Lactate, acetone and 3-hydroxybutyrate are the intermediatory metabolites that normally occur in the blood and play essential role in energy metabolism (Pavlides et al. 2010). However, increase in these metabolites indicated a switch of the organism to anaerobic respiration that may be due to hypoxia (Li et al. 2014; Xu et al. 2015). Choline is one of the components in phospholipids and plays crucial role in maintaining the structural integrity of membranes (Li et al. 2014). The increased level of choline in fish serum might be due to damage caused by Cd-induced oxidative deterioration of structural membranes in various tissues. An increase in inosine, a metabolite of energy rich molecule ATP, was also noticed in fish exposed to butachlor due to mitochondrial membrane damage by oxidative stress (Xu et al. 2015).
Similar to the altered serum metabolic profiles of fish exposed to Pb and Cd, the co-exposure of Pb and Cd (30+100 µgL−l) resulted in significant increase in pyruvate and inosine, while the level of several other metabolites such as alanine, lysine, glutamine, asparagine, some unsaturated fatty acids and O-acetyl glycoprotein was decreased. Pyruvate is a product of glucose oxidation in aerobic respiration. The increase in pyurvate and acetate was also indicated in amyotrophic lateral sclerosis (ALS), an invariably fatal neurological disorder to meet the excessive requirement of ATP (Kumar et al. 2010). Although the metabolic response for each metal was different in our study, there are still similarities in some of the metabolites which responded in the same manner to Pb, Cd and Pb+Cd exposure. The amino acids alanine, lysine, tyrosine, isoleucine, leucine, glutamine and asparagine are the intermediatory compounds in TCA cycle (Fig. 5) (Kokushi et al. 2012). The decrease in these amino acids may be due to lesser production under stress or being depleted in mitigating oxidative stress posed by metal toxicity (Nagato et al. 2013). Depletion of alanine in particular was not only observed for Pb, Cd and Pb+Cd exposure in our study but also indicated for several other metals (Cu, Li, Ar, Ni) in Mytilus galloprovicialis (Jones et al. 2012). In our study, increase in pyruvate and decrease of alanine might suggest an increase in energy metabolism because alanine can be converted to pyruvate in TCA cycle (Ekman et al. 2007; Southam et al. 2008). It was observed that organism utilize greater proportion of their energy as coping mechanism against toxins which resulted in growth impairment and compromise of other physiological activities (Nagato et al. 2013; Spann et al. 2011). On the other hand, some amino acids like lysine, leucine, isoleucine and tyrosine under specific metabolic condition can be converted to ketone bodies, that’s why in our study acetone was found higher in Pb+Cd exposed fish serum (Fasulo et al. 2012). In addition to the disturbance in energy metabolism, toxic metals exposure may affect osmoregulatory status of the fish as previous studies shown that free amino acids in the blood are also required to maintain osmoregulation in aquatic organisms (Nagato et al. 2013; Shinji et al. 2012). Interestingly, it was observed that the total number of 3 metabolites significantly altered during Pb exposure, but in Cd and Pb+Cd exposure, 11 and 13 metabolites were significantly changed. This further showed that the effect of Pb and Cd on fish metabolic profile was metal specific, concentration dependant and synergistic for Pb and Cd exposure in combination.
Conclusion
This study investigated the serum metabolic responses of Crucian carp to environmental Pb and Cd by 1H NMR-based metabolomics approach. The PCA, PLS-DA and OPLS-DA analyses of 1H NMR spectral data sets revealed significant changes in the serum metabolic profiles of control and exposed fish. A different metabolic response was observed for each metal and its co-exposure with distinct similarities in some of metabolic biomarkers (alanine, lysine). Based on metal specific metabolic biomarkers response, changes in neurotransmitters and amino acid metabolism were induced by Pb, while impaired energy metabolism and disturbed osmotic regulation with a shift from aerobic to anaerobic respiration were observed due to Pb and Cd exposure, whereas the co-exposure of Pb and Cd in a mixture had an additive effect on metabolic disturbance. Moreover, Crucian carp was found to be a preferable bioindicator for heavy metal pollution in freshwater ecosystem and 1H NMR-based metabolomics as remarkable tool to elucidate the effect of environmental pollutants and underlying mechanism for the discovery of novel metabolic biomarkers.
References
Al Bakheet SA, Attafi IM, Maayah ZH, Abd-Allah AR, Asiri YA, Korashy HM (2013) Effect of long-term human exposure to environmental heavy metals on the expression of detoxification and DNA repair genes. Environ Pollut 181:226–232
An Q, Wu Y, Wang J, Li Z (2010) Assessment of dissolved heavy metal in the Yangtze River estuary and its adjacent sea, China. Environ Monit Assess 164:173–187
Banfalvi G, Sarvari A, Nagy G (2012) Chromatin changes induced by Pb and Cd in human cells. Toxicol In Vitro 26:1064–1071
Bing H, Wu Y, Liu E, Yang X (2013) Assessment of heavy metal enrichment and its human impact in lacustrine sediments from four lakes in the mid-low reaches of the Yangtze River, China. J Environ Sci 25:1300–1309
Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O (2012) Oxidative stress and antioxidant defense. World Allergy Organ J 5:9–19
Brindle JT, Antti H, Holmes E, Tranter G, Nicholson JK, Bethell HW, Clarke S, Schofield PM, McKilligin E, Mosedale DE (2002) Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1HNMR-based metabonomics. Nat Med 8:1439–1445
Bundy JG, Davey MP, Viant MR (2008) Environmental metabolomics: a critical review and future perspectives. Metabolomics 5:3–21
Bylesjö M, Rantalainen M, Cloarec O, Nicholson JK, Holmes E, Trygg J (2006) OPLS discriminant analysis: combining the strengths of PLS-DA and SIMCA classification. J Chemom 20:341–351
Campos-Ferraz PL, Bozza T, Nicastro H, Lancha AH Jr (2013) Distinct effects of leucine or a mixture of the branched-chain amino acids (leucine, isoleucine, and valine) supplementation on resistance to fatigue, and muscle and liver-glycogen degradation, in trained rats. Nutrition 29:1388–1394
Çavaş T (2008) In vivo genotoxicity of mercury chloride and lead acetate: micronucleus test on acridine orange stained fish cells. Food Chem Toxicol 46:352–358
Clemow YH, Wilkie MP (2015) Effects of Pb plus Cd mixtures on toxicity, and internal electrolyte and osmotic balance in the rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 161C:176–188
Cui Q, Lewis IA, Hegeman AD, Anderson ME, Li J, Schulte CF, Westler WM, Eghbalnia HR, Sussman MR, Markley JL (2008) Metabolite identification via the madison metabolomics consortium database. Nat Biotechnol 26:162–164
Dudka I, Kossowska B, Senhadri H, Latajka R, Hajek J, Andrzejak R, Antonowicz-Juchniewicz J, Gancarz R (2014) Metabonomic analysis of serum of workers occupationally exposed to arsenic, cadmium and lead for biomarker research: a preliminary study. Environ Int 68:71–81
Ekman DR, Teng Q, Jensen KM, Martinovic D, Villeneuve DL, Ankley GT, Collette TW (2007) NMR analysis of male fathead minnow urinary metabolites: a potential approach for studying impacts of chemical exposures. Aquat Toxicol 85:104–112
Fasulo S, Iacono F, Cappello T, Corsaro C, Maisano M, D’Agata A, Giannetto A, De Domenico E, Parrino V, Lo Paro G, Mauceri A (2012) Metabolomic investigation of Mytilus galloprovincialis (Lamarck 1819) caged in aquatic environments. Ecotoxicol Environ Saf 84:139–146
Fonville JM, Richards SE, Barton RH, Boulange CL, Ebbels T, Nicholson JK, Holmes E, Dumas ME (2010) The evolution of partial least squares models and related chemometric approaches in metabonomics and metabolic phenotyping. J Chemom 24:636–649
Gu H, Pan Z, Xi B, Asiago V, Musselman B, Raftery D (2011) Principal component directed partial least squares analysis for combining nuclear magnetic resonance and mass spectrometry data in metabolomics: application to the detection of breast cancer. Anal Chim Acta 686:57–63
Gül Ş, Belge-Kurutaş E, Yıldız E, Şahan A, Doran F (2004) Pollution correlated modifications of liver antioxidant systems and histopathology of fish (Cyprinidae) living in Seyhan Dam Lake, Turkey. Environ Int 30:605–609
Guzinski M, Lisak G, Kupis J, Jasinski A, Bochenska M (2013) Lead(II)-selective ionophores for ion-selective electrodes: a review. Anal Chim Acta 791:1–12
Hambach R, Lison D, D’Haese PC, Weyler J, De Graef E, De Schryver A, Lamberts LV, van Sprundel M (2013) Co-exposure to lead increases the renal response to low levels of cadmium in metallurgy workers. Toxicol Lett 222:233–238
Jones OAH, Swain SC, Svendsen C, Griffin JL, Sturzenbaum SR, Spurgeon DJ (2012) Potential new method of mixture effects testing using metabolomics and Caenorhabditis elegans. J Proteome Res 11:1446–1453
Jordan J, Zare A, Jackson LJ, Habibi HR, Weljie AM (2012) Environmental contaminant mixtures at ambient concentrations invoke a metabolic stress response in goldfish not predicted from exposure to individual compounds alone. J Proteome Res 11:1133–1143
Khalaf AA, Moselhy WA, Abdel-Hamed MI (2012) The protective effect of green tea extract on lead induced oxidative and DNA damage on rat brain. Neurotoxicology 33:280–289
Khan SA, Liu X, Li H, Fan W, Shah BR, Li J, Zhang L, Chen S, Khan SB (2015) Organ specific antioxidant defenses and FT-IR spectroscopy of muscles in Crucian carp (Carassius auratus gibelio) exposed to environmental Pb2+. Turk J Biol 39:427–437
Kokushi E, Uno S, Harada T, Koyama J (2012) 1H NMR-based metabolomics approach to assess toxicity of bunker a heavy oil to freshwater carp, Cyprinus carpio. Environ Toxicol 27:404–414
Kumar A, Bala L, Kalita J, Misra UK, Singh RL, Khetrapal CL, Babu GN (2010) Metabolomic analysis of serum by (1) H NMR spectroscopy in amyotrophic lateral sclerosis. Clin Chim Acta 411:563–567
Laghi L, Picone G, Capozzi F (2014) Nuclear magnetic resonance for foodomics beyond food analysis. TrAC Trends Anal Chem 59:93–102
Li F, Huang J, Zeng G, Yuan X, Li X, Liang J, Wang X, Tang X, Bai B (2013) Spatial risk assessment and sources identification of heavy metals in surface sediments from the Dongting Lake, Middle China. J Geochem Explor 132:75–83
Li M, Wang J, Lu Z, Wei D, Yang M, Kong L (2014) NMR-based metabolomics approach to study the toxicity of lambda-cyhalothrin to goldfish (Carassius auratus). Aquat Toxicol 146:82–92
Lindon JC, Holmes E, Bollard ME, Stanley EG, Nicholson JK (2004) Metabonomics technologies and their applications in physiological monitoring, drug safety assessment and disease diagnosis. Biomarkers 9:1–31
Llorach R, Garrido I, Monagas M, Urpi-Sarda M, Tulipani S, Bartolome B, Andres-Lacueva C (2010) Metabolomics study of human urinary metabolome modifications after intake of almond (Prunus dulcis (Mill.) DA Webb) skin polyphenols. J Proteome Res 9:5859–5867
Lu Z, Wang J, Li M, Liu Q, Wei D, Yang M, Kong L (2014) H NMR-based metabolomics study on a goldfish model of Parkinson’s disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Chem Biol Interact 223C:18–26
Lynch CJ, Halle B, Fujii H, Vary TC, Wallin R, Damuni Z, Hutson SM (2003) Potential role of leucine metabolism in the leucine-signaling pathway involving mTOR. Am J Physiol Endocrinol Metab 285:E854–E863
Maceda-Veiga A, Figuerola J, Martinez-Silvestre A, Viscor G, Ferrari N, Pacheco M (2015) Inside the Redbox: applications of haematology in wildlife monitoring and ecosystem health assessment. Sci Total Environ 514:322–332
Mamas M, Warwick BD, Ludwig N, Royston G (2011) Clinical application of wave intensity for the treatment of essential hypertension. Heart Vessels 85:5–17
Matovic V, Buha A, Ethukic-Cosic D, Bulat Z (2015) Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem Toxicol 78:130–140
Matović V, Buha A, Bulat Z, Đukić-Ćosić D (2011) Cadmium toxicity revisited: focus on oxidative stress induction and interactions with zinc and magnesium. Arch Ind Hyg Toxicol 62:65–76
McCoole MD, Atkinson NJ, Graham DI, Grasser EB, Joselow AL, McCall NM, Welker AM, Wilsterman EJ, Baer KN, Tilden AR, Christie AE (2012) Genomic analyses of aminergic signaling systems (dopamine, octopamine and serotonin) in Daphnia pulex. Comp Biochem Physiol D: Genomics Proteomics 7:35–58
Nagato EG, D’Eon JC, Lankadurai BP, Poirier DG, Reiner EJ, Simpson AJ, Simpson MJ (2013) 1H NMR-based metabolomics investigation of Daphnia magna responses to sub-lethal exposure to arsenic, copper and lithium. Chemosphere 93:331–337
Pavlides S, Tsirigos A, Migneco G, Whitaker-Menezes D, Chiavarina B, Flomenberg N, Frank PG, Casimiro MC, Wang C, Pestell RG, Martinez-Outschoorn UE, Howell A, Sotgia F, Lisanti MP (2010) The autophagic tumor stroma model of cancer: role of oxidative stress and ketone production in fueling tumor cell metabolism. Cell Cycl 9:3485–3505
Qi W, Zhang L, Wang L, Xu H, Jin Q, Jiao Z (2015) Pretreatment with low-dose gamma irradiation enhances tolerance to the stress of cadmium and lead in Arabidopsis thaliana seedlings. Ecotoxicol Environ Saf 115:243–249
Qu R, Wang X, Wang Z, Wei Z, Wang L (2014) Metal accumulation and antioxidant defenses in the freshwater fish Carassius auratus in response to single and combined exposure to cadmium and hydroxylated multi-walled carbon nanotubes. J Hazard Mater 275:89–98
Rodriguez-Serrano M, Romero-Puertas MC, Pazmino DM, Testillano PS, Risueno MC, Del Rio LA, Sandalio LM (2009) Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol 150:229–243
Schutzendubel A, Schwanz P, Teichmann T, Gross K, Langenfeld-Heyser R, Godbold DL, Polle A (2001) Cadmium induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in scots pine roots. Plant Physiol 127:887–898
Sfakianakis DG, Renieri E, Kentouri M, Tsatsakis AM (2015) Effect of heavy metals on fish larvae deformities: a review. Environ Res 137C:246–255
Shinji J, Okutsu T, Jayasankar V, Jasmani S, Wilder MN (2012) Metabolism of amino acids during hyposmotic adaptation in the whiteleg shrimp, Litopenaeus vannamei. Amino acid 43:1945–1954
Solanky KS, Bailey NJC, Beckwith-Hall BM, Davis A, Bingham S, Holmes E, Nicholson JK, Cassidy A (2003) Application of biofluid 1H nuclear magnetic resonance-based metabonomic techniques for the analysis of the biochemical effects of dietary isoflavones on human plasma profile. Anal Biochem 323:197–204
Southam AD, Easton JM, Stentiford GD, Ludwig C, Arvanitis TN, Viant MR (2008) Metabolic changes in flatfish hepatic tumours revealed by NMR-based metabolomics and metabolic correlation networks. J Proteome Res 7:5277–5285
Spann N, Aldridge DC, Griffin JL, Jones OA (2011) Size-dependent effects of low level cadmium and zinc exposure on the metabolome of the Asian clam, Corbicula fluminea. Aquat Toxicol 105:589–599
Thapa G, Sadhukhan A, Panda SK, Sahoo L (2012) Molecular mechanistic model of plant heavy metal tolerance. Biometals 25:489–505
Viant MR (2008) Recent developments in environmental metabolomics. Mol BioSyst 4:980–986
Wang F, Wang WX, Huang XP (2012) Spatial distribution of gut juice extractable Cu, Pb and Zn in sediments from the Pearl River Estuary, Southern China. Mar Environ Res 77:112–119
Wei DD, Wang JS, Li MH, Guo PP, Dong G, Yang MH, Kong LY (2015) A pilot study of the onset of hepatic encephalopathy (OHE) in mice induced by thioacetamide and the protective effect of taurine by holistic metabolic characterization. Metabolomics 11:559–570
Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, Djoumbou Y, Mandal R, Aziat F, Dong E, Bouatra S, Sinelnikov I, Arndt D, Xia J, Liu P, Yallou F, Bjorndahl T, Perez-Pineiro R, Eisner R, Allen F, Neveu V, Greiner R, Scalbert A (2013) HMDB 3.0—the human metabolome database in 2013. Nucleic Acid Res 41:D801–807
Wold S, Esbensen K, Geladi P (1987) Principal component analysis. Chemom Intell Lab Sys 2:37–52
Xu HD, Wang JS, Li MH, Liu Y, Chen T, Jia AQ (2015) 1H NMR based metabolomics approach to study the toxic effects of herbicide butachlor on goldfish (Carassius auratus). Aquat Toxicol 159:69–80
Yang Z, Wang Y, Shen Z, Niu J, Tang Z (2009) Distribution and speciation of heavy metals in sediments from the mainstream, tributaries, and lakes of the Yangtze River catchment of Wuhan, China. J Hazard Mater 166:1186–1194
Zaza S, de Balogh K, Palmery M, Pastorelli AA, Stacchini P (2015) Human exposure in Italy to lead, cadmium and mercury through fish and seafood product consumption from Eastern Central Atlantic Fishing Area. J Food Compos Anal 40:148–153
Zhang L, Liu X, You L, Zhou D, Wu H, Li L, Zhao J, Feng J, Yu J (2011) Metabolic responses in gills of Manila clam Ruditapes philippinarum exposed to copper using NMR-based metabolomics. Mar Environ Res 72:33–39
Zhou J, Ma D, Pan J, Nie W, Wu K (2007) Application of multivariate statistical approach to identify heavy metal sources in sediment and waters: a case study in Yangzhong, China. Environ Geol 54:373–380
Zhou Z, Wang C, Liu H, Huang Q, Wang M, Lei Y (2013) Cadmium induced cell apoptosis, DNA damage, decreased DNA repair capacity, and genomic instability during malignant transformation of human bronchial epithelial cells. Int J Med Sci 10:1485–1496
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 31471655, 31171694).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: X. Liu.
Rights and permissions
About this article
Cite this article
Khan, S.A., Liu, X., Li, H. et al. 1H NMR-based serum metabolic profiling of Carassius auratus gibelio under the toxicity of Pb2+ and Cd2+ . Int. J. Environ. Sci. Technol. 13, 2597–2608 (2016). https://doi.org/10.1007/s13762-016-1086-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-016-1086-0