Abstract
Liners are commonly used in engineered waste disposal landfill to minimize the potential contamination of the aquatic environment. The adsorption behavior of Cu(II) from aqueous solution onto clay admixed with various mix ratios of quarry fines was investigated. The amount of Cu(II) adsorption increases with increase in contact time. The copper removal efficiencies of the composite mixture gradually decrease from 94.53 % (raw clay) to 85.59 % (20 % of quarry fines with clay), and appreciable decrease in percent removal 75.61 % was found with 25 % of quarry fines with clay. The kinetic adsorption data were analyzed by pseudo-first-order, pseudo-second-order, Bhattacharya–Venkobachar and Natarajan–Khalaf kinetic models to classify adsorption process mechanisms. Kinetic experimental data were good agreement with pseudo-second-order kinetic model with the degree of fitness of the data (R 2) 0.9999 for the adsorption of Cu(II). The results revealed that quarry fines can be used with optimum of 20 % replacement of natural clay for removal of Cu(II) as a liner material in landfills.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid waste is disposed off on land in units called solid waste dump yard or landfills which are designed to minimize the impact of the waste on the environment by containment of the waste. Landfills have served a key role in the management of solid wastes and are likely to continue to be an important component of the waste management system for at least the next ten years. The generation of leachate is one of the main hazards to groundwater from the disposal of waste by land filling. Leachate migration from waste dump yard or landfills and the release pollutants from sediment (under certain conditions) pose a high risk to groundwater resources if not adequately managed (Ikem et al. 2002). Groundwater contamination due to the leachate from the landfill could lead to pollution of coastal water and consequently produce toxic effects to marine organisms (Chen and Liu 2006). Treatment method for landfill leachate depends on the characteristics of landfill leachate, technical applicability and constraints, effluent discharge alternatives, cost-effectiveness, regulatory requirements and environmental impact (Kurniawan et al. 2006). To retain medium of pollutants present in the leachate, impermeable materials of natural or processed nature, such as clays, bentonite and geomembranes, are used as hydraulic barriers (Bellir et al. 2005). Metal cation which is an important factor in geological environment exerts influence on clay properties (Cao et al. 2009). The microsilica as additive material used for landfill liners is an abundantly available product, for the construction of hydraulic barrier in landfill. The membrane is composed of the clay and microsilica of different contents (Venkatesan and Swaminathan 2008). Significant research has been conducted to investigate the removal of Cu(II) by different adsorbents such as modified kaolinites (Suraj et al. 1998), modified locally available clay mineral (Vengris et al. 2001), natural clay (Veli and Alyuz 2007), bentonite treated with ammonium chloride (Abdelhamid et al. 2012), montmorillonite clay (Turan and Ozgonenel 2013), sodium bentonite commercial clay, called as fluidgel (de Almeida Neto et al. 2013), calcareous and smectitic clay (Sdiri et al. 2014), Tunisian clay (Boujelben et al. 2015). Quarry fines consist mainly of excess fines generated from crushing, washing and screening operations at quarries. This waste product is left in huge heaps in the neighborhood of the quarry causing serious health hazards. This leads to serious environmental pollution and occupation of vast area of land especially after the slurry dries up. Quarry dust exhibits high shear strength, which is highly beneficial for its use as a geotechnical material (Sarvade and Nayak 2014; Mishra et al. 2014). Low concentration of heavy metals such as nickel, cadmium, chromium, zinc and arsenic is toxic in nature even when they are mixed with water (Ulaganathan and Govindan 2013). These heavy metals may cause impact to the aquifer underlying the landfill and therefore may pose potential risk to human health (Lu et al. 2009). The commercial use of heavy metals compounds reflects their occurrence in the landfill body (Ilgen et al. 2008). There is a long history of association between metals and human development. However, due to mining, mineral, smelting and tannery industry, heavy metal pollution has become serious (Kasassia et al. 2008). Heavy metals in landfill sites represent a minimal pollution hazard in domestic waste leachates. However, they cannot be totally ignored in the investigation of contamination of landfill environments; for instance, retorted heavy metals may become mobile again if they are displaced from clay exchange sites by other contaminants of higher concentration in the leachate (Abu-Rukah and Abu-Aljarayesh 2002). The reduction in heavy metal aqueous solutions is feasible by means of many methods including: ion exchange, chemical precipitation, ultra-filtration, electrochemical deposition, among which adsorption on appropriate adsorbent is one of the most efficient in terms of ease and practicability of operation and little consumption of energy. During adsorption processes, pollutants are fixed at the surface of the adsorbents, separating them from liquid phase (Eba et al. 2010). Thus, the present study was carried out to examine the adsorption capacities and adsorbent efficiencies of natural clay admixed with quarry fines as a liner material for the remove of copper ions from aqueous solution. Kinetic models were applied for evaluating the mechanism and behavior of copper adsorption onto natural clay admixed with quarry fines. This study was carried out from 2013 to 2015 at the Department of Civil Engineering, University College of Engineering, Anna University—BIT Campus, Trichy, Tamil Nadu, India.
Materials and methods
Adsorbent and preparation of adsorbate
The natural clay used in this study was taken from a natural deposit, situated in Lalgudi, in Trichy district, and Quarry fines was collected from SRC Projects in Salem district of Tamil Nadu (India). Both the samples were dried in an oven at approximately 105 °C and used in their natural state with no chemical changes. The amount of quarry fines used in mixture was 5, 10, 15, 20 and 25 % of the total weight of composite sample. The mixture of clay (C) and quarry fines (QF) was used to make composite liner, and its mixing compositions are given in Table 1. The microstructures of clay were observed by scanning electron microscope (SEM) with JEOL model (JSM—6390LV), and the chemical composition was determined by SEM-coupled energy dispersive X-ray spectroscopy (EDX) with JEOL model (JED-2300). The copper synthetic solution of 1000 mg/L was prepared by dissolving the appropriate amount of CuSO4·5H2O in double-distilled water. Working solutions of the required concentrations were obtained by successive dilution, and pH adjustments were carried on using 0.1 M hydrochloric acid (HCl) and 0.1 M sodium hydroxide (NaOH).
Batch studies
Batch adsorption experiments were carried out to study the influence of agitation time and initial copper concentration on the adsorbent. Six different series of clay and quarry fines with fixed adsorbent dose (1 g/100 mL) at 100 mL of copper solution of desired concentration (20 mg/L) were taken in 250 mL capacity of Erlenmeyer flasks and agitated at 120 rpm for predetermined time intervals (3, 6, 9, 12, 15, 30, 45, 60, 90 and 120 min) at constant room temperature on an Remi orbital shaker. The solutions were centrifuged (Remi R-4C model digital centrifuge) at a speed 3000 rpm, operating for 5 min, and the supernatant was analyzed for metal ion concentration using atomic absorption spectrophotometer. The adsorption equilibrium and isotherms are discussed separately (Rajagopalan et al. 2016).
The percent adsorption of metal ion was calculated as follows in Eq. (1)
The sorption capacity at time t, q t (mg/g) was obtained as Eq. (2)
where C i and C e (mg/L) are the initial and equilibrium concentrations of Cu(II), V is the solution volume (L) and m is the mass of adsorbent (g).
Results and discussion
Characterization of adsorbent
The EDX spectra (energy dispersive X-ray analysis) were used to analyze the raw clay and is shown in Fig. 1. The chemical compositions of clay are given in Table 2. It could be seen that the clay contains a highest percentage of silica (59.69 %) and followed by aluminum (5.49 %), iron (1.96 %), potassium (0.77 %) and magnesium (0.71 %). Clay is fine-grained, hydrous silicates derived from bedrock. Primary elemental composition clay liner material is O, Si, and Al. Clay structure is closed-packed sheets of O atoms with Si and Al ions in interstices spaces. The scanning electron micrographs of clay are shown in Fig. 2 and it depicts that the particles were found to be aggregated and irregular in shape of crystalline structure. Physical adsorption of minute particles with lumped edges was found on huge background. Structures are minute microns and are edged together.
Effect of contact time on adsorption of copper
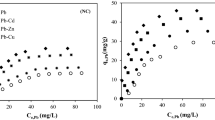
In order to find the equilibrium time for maximum uptake of copper, the adsorption of copper on six different series of clay and quarry fines at fixed concentration was studied as a function of contact time. The effect of initial concentration with the effect of agitation time on adsorption of copper was observed (Fig. 3). Figure 3 shows that the uptake of the metal was very fast in the first 15 min; however, after 15 min the amount of metal ion adsorbed remaining approximately constant and equilibrium time attained was found to be 15 min. The adsorption of unit mass of copper increased from 1.372 to 1.891 mg/g with increase in time for CQF1. Similarly, the unit mass increased from 1.33 to 1.84 mg/g, for CQF2; 1.32–1.80 mg/g for CQF3; 1.26–1.77 mg/g for CQF4; 1.16–1.71 mg/g for CQF5; 1.06–1.51 mg/g for CQF6 when the concentration of copper was 20 (mg/L). The copper removal efficiencies of the composite mixture gradually decrease from 94.53 to 85.59 % between CQF1 and CQF5, whereas CQF6 mixture shown appreciable decreases as 75.61 % with increase in quarry fines in the mixture shown in (Fig. 4).
Adsorption kinetics
For the precise design of adsorption process, it is necessary to consider adsorption kinetics (Ciesielczyk et al. 2015). Adsorption kinetics is used in determining the adsorbate–adsorbent interaction, adsorption characteristics and adsorption mechanism. In order to investigate the controlling mechanism of adsorption process, the equations of pseudo-first-order kinetic model, pseudo-second-order kinetic model, Bhattacharya and Venkobachar kinetic model and Natarajan and Khalaf kinetic model were applied to test the experimental data.
Pseudo-first-order equation
The adsorption of metal ions from an aqueous solution onto the adsorbents (clay and clay–quarry fines admixture) can be considered as a reversible process with equilibrium being established between the solution and the adsorbent (Senthil Kumar et al. 2011). Lagergren’s first-order rate equation has been so-called pseudo-first-order kinetics (Lagregren 1898):
where q e and q t refer to the sorption capacity at equilibrium (mg/g) and at time t, respectively, and k 1 is the pseudo-first-order rate constant (1/min).
By integrating Eq. (3) and applying conditions, q t = 0 at t = 0 and q t = q t at t = t, Eq. (3) becomes
The values of q e and k 1 were calculated from the slope and intercept of the line obtained by the plot of log (q e − q t ) versus t for clay and clay–quarry fines admixture (Fig. 5).
Pseudo-second-order equation
The adsorption data from experiment were also interpreted, using Ho’s pseudo-second-order kinetic model. This model is created on the assumption that the adsorption process follows second-order chemisorption. It is expressed as Ho and McKay (1999):
where q e and q t are the adsorption capacity at equilibrium and at time, t, respectively (mg/g), and k 2 is the pseudo-second-order rate constant (g/mg/min). By integrating Eq. (5) and applying boundary conditions, the Eq. (5) becomes
Equation (6) can be algorithmic rearranged to obtain the linear form:
The values of q e and k 2 were calculated from the slope and intercept of the line obtained by the plot of t/q t versus t for clay and clay–quarry fines admixture (Fig. 6).
Bhattacharya and Venkobachar (1984)
Bhattacharya and Venkobachar presented a simple first-order reversible kinetic model, based on solution concentration to study the mechanism of sorption and characteristic constants of sorption (Ho and McKay 1999). This model has been used by several researchers to study the sorption mechanisms (Ekwumemgbo et al. 2010).
where k B = B–V constant (min−1); C o = initial concentration(mg/L); C t and C e = concentration (mg/L) at time t and at equilibrium. The value of k B was calculated from the slope of the line obtained by the plot of log [1 − (U)T] versus t for clay and clay–quarry fines admixture as shown in (Fig. 7).
Natarajan and Khalaf (Kannan and Vanangamudi 1991)
Natarajan and Khalaf developed a model based on initial concentration and the concentration at any time t (Yilmaz et al. 2015). The equation is expressed as:
where C o = initial concentration (mg/L); C t = concentration (mg/L) at time t. The value of k N was calculated from the slope of the line obtained by the plot of log (C o/C t ) versus t for clay and clay–quarry fines admixture (Fig. 8).
Kinetic parameters for the adsorption of copper ions onto clay and clay–quarry fines admixture were calculated from above plots and are presented in Table 3. Table 3 shows that the obtained coefficient of determination R 2 values between 0.904 and 0.996 for the pseudo-first-order equation, coefficient of determination R 2 values between 0.904 and 0.999 for Bhattacharya and Venkobachar equation and coefficient of determination R 2 values between 0.937 and 0.999 for Natarajan and Khalaf equation are lower than that of pseudo-second-order kinetic model since the coefficient of determination R 2 values for pseudo-second-order kinetic model ranges between 0.9991 and 0.9999. The rate constants and coefficient of determination R 2 values obtained for Lagergren first-order model and Bhattacharya and Venkobachar models were found to be approximately equal. These results indicated that the pseudo-second-order sorption mechanism is most important and that the overall rate constant of ion appears to be controlled by the chemisorptions process. However, coefficient of determination R 2 values between 0.9991 and 0.9999 for the pseudo-second-order kinetic model are very high, which specifies the applicability of the pseudo-second-order kinetic model as a better selection to describe the adsorption of metal ions onto the adsorbents clay and clay–quarry fines admixture and also the calculated q e values and experimental q e values are more compliable for pseudo-second-order model than pseudo-first-order model.
Conclusion
This study proved that Cu(II) could be adsorbed and thus removed in substantial amount by clay admixed with quarry fines, as a liner material in landfill. Effect of contact time (3–120 min) and metal concentration 20 mg/L on adsorption of Cu (II), and the amount of Cu(II) adsorption increase with increase in contact time (15 min of contact time are sufficient). The percentage metal removal of the quarry fines–clay composites decreases with increasing quarry fines. The kinetic models pseudo-first-order, pseudo-second-order, Bhattacharya and Venkobachar kinetics and Natarajan and Khalaf kinetics were used to study the kinetic data and it reveals that kinetic data are in good agreement with pseudo-second-order kinetic model for the adsorption of Cu(II) on clay admixed with quarry fines in different mixture ratios. The quarry fines are a potential material to modify the properties of clay liners with optimum of 20 % replacement to be used in the landfill sites. However, the clay replacement level shall be restricted to 20 %, as percentage removal of copper decreases adversely beyond this replacement level. Moreover, research on landfill liner materials are limited and so far investigated only with conventional materials like clay soil; this study would be a gateway to explore the conservation of natural resource clay and also finding ways to minimize the environmental impacts associated with quarry fines. Since it is an abundant and beneficially utilizes available waste material to use as adsorbent with natural clay for copper removal.
References
Abdelhamid B, Ourari A, Ouali MS (2012) Copper(II) ions removal from aqueous solution using bentonite treated with ammonium chloride. Am J Phys Chem 1:1–10
Abu-Rukah Y, Abu-Aljarayesh I (2002) Thermodynamic assessment in heavy metal migration at El-Akader landfill site, North Jordan. Waste Manag 22:727–738
Bellir K, Lehoeikh MB, Meniai H, Gherbi N (2005) Study of the retention of heavy metals by natural material used as liners in landfills. Desalination 185:111–119
Bhattacharya AK, Venkobachar C (1984) Removal of cadmium by low cost adsorbents. J Environ Eng 110:110–116
Boujelben N, Gharab S, Bouhamed F, Elouear Z, Bouzid J (2015) Removal of copper from aqueous solution using tunisian clay. Am J Environ Sci 11(2):90–98
Cao L, Zhang J, Wang Y, Lian X, Hong L (2009) Experimental research on geotechnical behaviors of compacted clay influenced by metal cation. Procedia Earth Planet Sci 1:1016–1023
Chen C-M, Liu M-C (2006) Ecological risk assessment on a cadmium contaminated soil landfill—a preliminary evaluation based on toxicity tests on local species and site-specific information. Sci Total Environ 359:120–129
Ciesielczyk F, Bartczak P, Jesionowski T (2015) A comprehensive study of Cd(II) ions removal utilizing high surface-area binary Mg–Si hybrid oxide adsorbent. Int J Environ Sci Technol 12:3613–3626
de Almeida Neto AF, Vieira MGA, da Silva MGC (2013) Fluid dynamic study for copper removal onto modified clay in fixed bed. Chem Eng Trans 32:1555–1559
Eba F, Gueu S, Eya’A-Mvongbote A, Ondo JA, Yao BK, Ndong NJ, Kouya BR (2010) Evaluation of the absorption capacity of the natural clay from Bikougou (Gabon) to remove Mn(II) from aqueous solution. Int J Eng Sci Technol 2(10):5001–5016
Ekwumemgbo PA, Kagbu JA, Nok AJ, Omoniyi KI (2010) Kinetics of gamma globulin adsorption onto titanium, Rasayan. J Chem 3(2):221–231
Ho YS, McKay G (1999) Pseudo-second-order model for sorption processes. Process Biochem 34:451–465
Ikem A, Osibanjo O, Sridhar MKC, Sobande A (2002) Evaluation of groundwater quality characteristics near two waste sites in Ibadan and Logos, Nigeria. Water Air Soil Pollut 140:307–333
Ilgen G, Glindemann D, Herrmann R, Hertel F, Huang JH (2008) Organo metals of tin, lead and mercury compounds in landfill gases and leachates from Bavaria, Germany. Waste Manag 28:1518–1527
Kannan N, Vanangamudi A (1991) A study on removal of chromium VI by adsorption on lignite coal. Indian J Environ Prot 114:241–245
Kasassia A, Rakimbei P, Karagiannidis A, Zabaniotoua A, Tsiouvaras K, Nastis A, Tzafeiropoulou K (2008) Soil contamination by heavy metals: measurements from a closed unlined landfill. Bioresour Technol 99:8578–8584
Kurniawan TA, Lo W, Chan GYS (2006) Physico-chemical treatments for removal of recalcitrant contaminants from landfill leachate. J Hazard Mater B129:80–100
Lagregren S (1898) About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetenskapsakademiens Handlingar 24:1–39
Lu H, Luan M, Zhang J (2009) A kinetic study on the adsorption of chromium(VI) onto a natural material used as landfill liner. Electron J Geotech Eng 14:1–10
Mishra J, Yadav RK, Singhai AK (2014) Effect of granite dust on index properties of lime stabilized black cotton soil. Int J Eng Res Sci Tech 3(1):19–23
Rajagopalan V, Venkatesan G, Swaminathan G (2016) Removal of copper using clay admixed with quarry fines as a landfills liners. Pol J Environ Stud 25(1):377–384
Sarvade PG, Nayak S (2014) Studies on the utilization of quarry dust to improve the geotechnical properties of lithomargic clay. Int J Adv Struct Geotech Eng 3(1):54–59
Sdiri T, Higashi T, Jamoussi F (2014) Adsorption of copper and zinc onto natural clay in single and binary systems. Int J Environ Sci Technol 11:1081–1092
Senthil Kumar P, Ramalingam S, Sathyaselvabala V, Dineshkirupha S, Sivanesan S (2011) Removal of copper(II) ions from aqueous solution by adsorption using cashew nut shell. Desalination 266:63–71
Suraj G, Iyer CSP, Lalithambika M (1998) Adsorption of cadmium and copper by modified kaolinites. Appl Clay Sci 13:293–306
Turan NG, Ozgonenel O (2013) Study of montmorillonite clay for the removal of copper(II) by adsorption: full factorial design approach and cascade forward neural network. Sci World J 2013:1–11
Ulaganathan S, Govindan V (2013) Removal of chromium from aqueous solutions using derris indica wood based activated carbon. Adsorpt Batch Stud Environ Prot Eng 39(3):21–29
Veli S, Alyuz B (2007) Adsorption of copper and zinc from aqueous solutions by using natural clay. J Hazard Mater 149:226–233
Vengris T, Binkiene R, Sveikauskaite A (2001) Nickel, copper and zinc removal from waste water by a modified clay sorbent. Appl Clay Sci 18:183–190
Venkatesan G, Swaminathan G (2008) Microsilica as novel admixture used as municipal solid waste landfill liner material. Int J Nat Appl Sci 4(2):124–130
Yilmaz MS, Ozdemir OD, Kasap S, Piskin S (2015) The kinetics and thermodynamics of nickel adsorption from galvanic sludge leachate on nanometer titania powders. Res Chem Intermed 41:1499–1515
Acknowledgments
The authors thank the authorities of the Technical Education Quality Improvement Programme (TEQIP) for sponsoring research tuition fee to carry out this study and Sophisticated Test and Instrumentation Centre, Cochin University of Science and Technology, Kerala, India, for providing SEM and EDAX results.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Venkatesan, G., Rajagopalan, V. Adsorption kinetic models for the removal of Cu(II) from aqueous solution by clay liners in landfills. Int. J. Environ. Sci. Technol. 13, 1123–1130 (2016). https://doi.org/10.1007/s13762-016-0951-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-016-0951-1