Abstract
Background
Identifying reliable biomarkers for early detection and prediction of cognitive impairment in Parkinson’s disease (PD) is crucial for optimal patient care. This study set out to investigate the potential of YWHAG as a diagnostic biomarker for cognitive impairment in PD.
Methods
We enrolled a total of 331 PD patients and selected 241 patients that met the criteria for cognitive impairment analysis. The patients were classified into three groups: PD-NC: PD patients with normal cognition, PD-MCI: PD patients with mild cognitive impairment, and PD-D: PD patients with dementia. ELISA was employed to assess YWHAG expression, as well as the neurofilament light chain (NfL). Additionally, cognitive impairment was evaluated using MoCA scores. Correlation analysis and receiver operating curve analysis (ROC) were performed to clarify the relationship between YWHAG expression and cognitive impairment.
Results
Our findings revealed a significant upregulation of YWHAG expression in both the PD-MCI and PD-D groups compared to the PD-NC group. This observation aligned with the elevated expression of NfL in the PD-MCI and PD-D groups. YWHAG and NfL expression levels displayed negative correlations with MoCA scores and positive associations with age. Furthermore, ROC curve analysis demonstrated the diagnostic efficacy of YWHAG expression in distinguishing individuals with PD-NC, PD-MCI, and PD-D.
Conclusions
Our findings indicate that YWHAG could serve as a promising biomarker for cognitive impairment in PD. The upregulation of YWHAG expression in PD-MCI and PD-D groups, its association with cognitive impairment, and its correlations with MoCA scores and NfL levels support its potential clinical utility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) ranks as the second most common neurodegenerative condition on a global scale. Among the major complications afflicting PD patients is cognitive impairment, to which > 75% of PD patients who have had PD for over a decade are susceptible [1]. This cognitive dysfunction, known as PD-related cognitive impairment (PD-CI), and more severe cases known as PD dementia (PD-D), detrimentally impacts a patient’s quality of life, increases healthcare costs, and imposes a substantial burden on caregivers [2, 3]. Therefore, the development of biomarkers for the timely identification and prediction of cognitive impairment in PD holds immense significance. Such biomarkers could facilitate timely interventions and optimize treatment strategies for cognitive decline in PD patients [4].
Recently, there has been an increasing focus on the exploration of biological biomarkers that facilitate the diagnosis and monitoring of cognitive impairment among PD patients. One such target is alpha-synuclein [5], a protein closely linked to the pathogenesis of PD. Alpha-synuclein aggregates are a hallmark of PD and have been implicated in the development of cognitive dysfunction. While alpha-synuclein holds promise as a target for intervention, its detection and quantification remain challenging, as it is predominantly found in insoluble aggregates within the brain. Another target of interest is acetylcholinesterase (AChE) [6], an enzyme involved in the breakdown of acetylcholine, a neurotransmitter crucial for cognitive function. Inhibitors of AChE, such as donepezil, have been tested in the treatment of cognitive impairment among PD patients. However, their efficacy is limited, and they may have adverse effects on other neurotransmitter systems, highlighting the need for more specific and targeted approaches.
Among the numerous candidate biomarkers, the protein YWHAG has emerged as a promising candidate. YWHAG, also known as 14-3-3 gamma, is a crucial gene responsible for encoding the 14-3-3 gamma protein. This protein plays a pivotal role in various biological processes, with a significant focus on its high expression within the brain. Within the intricate network of cellular functions, 14-3-3 gamma is emerging as a key regulator in the brain, orchestrating and fine-tuning multiple essential pathways. In the brain, where it is notably abundant, 14-3-3 gamma acts as a central conductor, coordinating a multitude of vital processes. It is essential in regulating diverse cell signaling pathways, ensuring that neurons and glial cells communicate effectively and that various cellular responses are finely tuned. This regulatory role extends to processes such as cell growth, differentiation, survival, and apoptosis. Furthermore, 14-3-3 gamma plays a role in safeguarding the integrity of the brain's structure and function. It is involved in maintaining synaptic plasticity, a crucial mechanism for learning and memory, by modulating the function of proteins involved in synaptic transmission and long-term potentiation [7]. In previous studies, YWHAG mutation has been linked to epilepsy [8] and patients and intellectual disabilities [9]. Beyond the brain, it is found that 14-3-3 gamma exerts its influence across a wide array of cellular functions, including the cell cycle, apoptosis, and DNA repair. YWHAG has also been investigated as a marker in the cancer [10].
Given the previously reported roles of YWHAG in neurodegenerative disorders and the applicability of using serum YWHAG expression as an assessment, this paper aims to use a retrospective controlled clinical study to shed light on the feasibility and potential clinical significance of YWHAG as a diagnostic biomarker for cognitive impairment in PD. We analyzed the expression and diagnostic potential of YWHAG along with neurofilament light chain (NfL), a well-established biomarker for cognitive impairment, in correlation with PD with mild cognitive impairment (PD-MCI) or dementia (PD-D) [11].
Increased NfL concentrations in cerebrospinal fluid and blood are indicative of neuronal damage and degeneration, since NfL, as a structural protein found in nerve cells, is released into the bloodstream when neurons are injured or degenerate, making it a valuable tool for monitoring neurodegenerative processes. Its utility in assessing cognitive decline extends to various conditions such as Alzheimer’s disease, multiple sclerosis, and Parkinson’s disease, where it serves as a sensitive indicator of disease progression and cognitive impairment. Abundant evidence has established the clinical utility of NfL as an innovative biomarker for cognitive decline in PD [12].
Herein, the aim of this study is to investigate the potential of YWHAG as a diagnostic biomarker for cognitive impairment in PD.
Methods
Study participants
A total of 331 PD patients were recruited for this study. The study was approved by the ethics committee of Cangzhou Central Hospital (#2020/06/451) and informed written consent was obtained before enrollment. Patients were selected based on the following inclusion and exclusion criteria:
Inclusion criteria: Patients diagnosed with PD by the diagnostic criteria for Parkinson’s disease in China (2016 version).
Exclusion criteria: The study excluded individuals with secondary parkinsonism, which arises from various conditions such as head trauma, infectious encephalitis, cerebrovascular accidents, accidental poisoning, and others. Patients presenting with Parkinson’s plus syndromes, including multiple system atrophy and progressive supranuclear palsy, were also not included. Moreover, individuals with significant cardiac, hepatic, renal, or gastrointestinal dysfunctions were excluded. The study also omitted participants who displayed uncooperative behavior during neurological examinations and blood tests. Those with autoimmune diseases, a history of thyroid disorders, individuals undergoing thyroid hormone replacement therapy, or those taking anti-thyroid medications were likewise excluded. Furthermore, individuals with cognitive impairments caused by Alzheimer’s disease, psychiatric disorders, or vascular dementia, potentially affecting cognitive function, were not considered for the study.
Measurement of NfL and YWHAG
Serum samples obtained from PD patients during their prior hospital visits were stored at a temperature of − 80 °C. The levels of NfL and YWHAG were quantified using an enzyme-linked immunosorbent assay (ELISA). The ELISA procedure adhered to the instructions provided by the manufacturer and the optical density of each well was measured using a microplate reader.
MoCA assessment
Cognitive function was assessed using the Montreal Cognitive Assessment (MoCA). This standardized assessment measures various cognitive domains. Trained assessors administered the MoCA test to all participants following standardized procedures. The scores obtained from the MoCA assessment were used to classify participants into the three groups.
Statistical analysis
To explore the relationship between YWHAG expression, NfL levels, and cognitive impairment, correlational analysis was performed. The Pearson correlation analysis was used. Data were expressed as mean ± standard deviation. Group comparisons were conducted using appropriate statistical tests, such as analysis of variance (ANOVA) or non-parametric tests. Bonferroni adjustment was used in pairwise comparisons. A significance level of p < 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS software package.
Results
Study design and cohort characteristics
This retrospective study recruited 331 PD patients and after selecting patients using inclusion and exclusion criteria, we analyzed a total of 241 serum samples from PD patients (Fig. 1). The patients were categorized into PD-NC, PD-MCI, and PD-D groups based on their cognitive impairment status. The characteristics of patients are shown in Table 1. In brief, male patients constitute a slightly larger proportion in all three groups (58.6% in PD-NC, 57.1% in PD-MCI, and 52.4% in PD-D). The mean age of the PD-D was the highest (70.60 years old), followed by PD-MCI (64.20 years old) and PD-NC (57.74 years old), revealing a higher age associated with PD severity (p < 0.05). In line with this, cognitive scores, including MoCA, GDS-30, MMSE, UPDRS-III, and Hoehn-Yahr grades were also significantly different between the groups. The equivalent levodopa dose, a metric indicating the treatment intensity for PD, was also higher with PD severity (p < 0.01).
YWHAG and NfL Expression are elevated in serum from PD patients with PD-MCI and PD-D
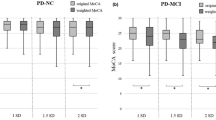
We next compared the YWHAG and NfL expression levels in serum for the three groups. ELISA analysis revealed a significant upregulation of YWHAG expression in both the PD-MCI and PDD groups compared to the PD-NC group (Fig. 2A), and the PD-D group demonstrated an even higher YWHAG level than the PD-MCI patients (p < 0.0001), a trend that is also observed for NfL (Fig. 2B). To validate the cognitive impairment status of the PD samples, we assessed the MoCA scores. As shown in Fig. 2C, the MoCA scores confirmed the presence of cognitive impairment in the PD-MCI and PD-D groups.
Correlation analysis: YWHAG, MoCA scores, and NFL levels
As shown in Fig. 3A, correlation analysis revealed a pronounced negative correlation between YWHAG expression levels and MoCA scores (r = − 0.5490, p < 0.0001), indicating that higher YWHAG expression was associated with worse cognitive impairment. Similarly, a negative correlation between NfL levels and MoCA scores was observed (r = − 0.4413, p < 0.0001), further supporting the relationship between NfL and cognitive impairment (Fig. 3B).
Association of YWHAG and NfL expression with age
YWHAG and NfL expression levels were evaluated concerning the age of PD patients. Interestingly, as shown in Fig. 4A and Fig. 4B, correlation analysis showed that both YWHAG (r = 0.3969, p < 0.0001) and NfL (r = 0.3295, p < 0.0001) expression levels were positively associated with patient age.
Diagnostic potential of YWHAG
ROC curve analysis was performed to assess the potential of YWHAG as a diagnostic biomarker for cognitive impairment in PD. The combined analysis of PD-MCI and PD-D groups showed an AUC greater than 0.5 (Fig. 5A), indicating that YWHAG expression could distinguish individuals with cognitive impairment from those with normal cognition. Similarly, in the individual analyses of PD-MCI (Fig. 5B) and PD-D (Fig. 5C), YWHAG expression also exhibited diagnostic potential, as reflected by AUC values greater than 0.5.
ROC curves for YWHAG levels in PD-MCI to PD-NC. A Evaluation of YWHAG's diagnostic value in identifying PD-MCI and PD-D using ROC curve analysis. B Assessment of YWHAG’s diagnostic value in PD-D diagnosis using ROC curve analysis. C Examination of YWHAG’s diagnostic value in PD-MCI diagnosis using ROC curve analysis
Discussions
The primary aim of the present study was to explore the potential of YWHAG as a biomarker for cognitive impairment in individuals with PD. The findings from our investigation revealed a notable increase in the expression of YWHAG among PD-MCI and PD-D groups when compared to those in the PD-NC group. These observations align with previous studies and provide further evidence supporting the involvement of YWHAG in neurodegenerative conditions [9, 13]. For example, Ramocki et al. suggested that the YWHAG has an important role in cognitive and behavioral function [13]. Similarly, several preclinical studies implicated that alterations in YWHAG are linked to neurological disorders [14, 15]. These findings align with the notion that YWHAG may be involved in the pathogenesis of cognitive dysfunction in PD, and our study is innovative in exploring the diagnostic function of serum YWHAG for cognitive impairment in PD. Our data also expanded our understanding of the regulatory role of YWHAG in the PD domain, underscoring YWHAG as a versatile biomarker.
In addition to YWHAG, our study confirmed the significant elevation of NfL expression in PD-MCI and PD-D groups, which is consistent with previous reports highlighting NfL as a reliable biomarker for cognitive impairment in PD [16]. Moreover, the concurrent increase in both YWHAG and NfL levels suggests their complementary roles in assessing cognitive decline in PD patients, providing further support for their combined use as innovative biomarkers in clinical practice and another arsenal to combating cognitive impairment among PD patients. The new biomarker for cognitive impairment also has significant clinical implications for achieving early detection of cognitive decline in PD. They can also facilitate monitoring of cognitive decline, in which frequent and long-term evaluation is imperative, allowing for timely interventions and optimizing treatment strategies. Notably, the use of serum biomarkers obviates the need for expensive difficult-to-access imaging instrument and allow for long-term monitoring of PD patients [17]. Our approach concurs with previous endeavors that characterize YWHAG as a serum biomarker for diagnosing cancer [18]. Furthermore, the use of innovative biomarkers like YWHAG and NfL can contribute to personalized medicine approaches, facilitating targeted interventions and improved patient outcomes in PD [19] [20].
Our study is paralleled by the growing interest in elucidating the mechanism of YWHAG on neuronal function and cognitive ability. For example, as illustrated in Fig. 6, it has been shown that YWHAG interacts with key synaptic proteins and contributes to the maintenance of synaptic plasticity [13]. Moreover, YWHAG's involvement in cellular signaling pathways, particularly those related to cell survival and apoptosis, implicates its potential significance in cognitive processes [21]. It has also been suggested that haploinsufficiency of YWHAG in the developing cerebral cortex may lead to abnormal neuronal migration [22].
Despite the strengths of our study, there are limitations to consider. First, the sample size was relatively small, and the study focused on serum levels of YWHAG and NfL without considering combining them with other potential biomarkers or imaging techniques. Future studies with larger sample sizes are warranted to validate the diagnostic and prognostic value of YWHAG, considering the heterogeneity of cognitive impairment in PD. Additionally, longitudinal studies are needed to assess the longitudinal changes in YWHAG and NfL levels and their associations with disease progression and cognitive decline in PD.
Conclusions
To summarize, our investigation has shed new light on the potential clinical significance of YWHAG as a cutting-edge biomarker for cognitive impairment in PD. The observed upregulation of YWHAG, its association with cognitive decline, and the concurrent increase in NfL levels underscore their potential value in the evaluation of cognitive impairment. These findings contribute to the expanding knowledge base and lay the groundwork for further exploration of YWHAG and NfL as pioneering biomarkers in PD. Ultimately, the discovery of dependable biomarkers for cognitive decline in PD holds substantial implications for clinical practice, facilitating early intervention and enhancing patient care.
References
Aarsland D, Batzu L, Halliday GM, Geurtsen GJ, Ballard C, Ray Chaudhuri K, Weintraub D (2021) Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers 7:47
Brandão PRP, Munhoz RP, Grippe TC, Cardoso FEC, e Castro BM, Titze-de-Almeida R, Tomaz C, Tavares MCH (2020) Cognitive impairment in Parkinson’s disease: a clinical and pathophysiological overview. J Neurol Sci 419:117177
Weintraub D, Comella CL, Horn S (2008) Parkinson’s disease–Part 1: pathophysiology, symptoms, burden, diagnosis, and assessment. Am J Manag Care 14:S40–S48
Svenningsson P, Westman E, Ballard C, Aarsland D (2012) Cognitive impairment in patients with Parkinson’s disease: diagnosis, biomarkers, and treatment. Lancet Neurol 11:697–707
Fan T-S, Liu SC-H, Wu R-M (2021) Alpha-synuclein and cognitive decline in Parkinson disease. Life 11:1239
Mathew B, Parambi DG, Mathew GE, Uddin MS, Inasu ST, Kim H, Marathakam A, Unnikrishnan MK, Carradori S (2019) Emerging therapeutic potentials of dual-acting MAO and AChE inhibitors in Alzheimer’s and Parkinson’s diseases. Arch Pharm 352:1900177
Han L, Tang J, Zhu S, Zhu J (2023) LncRNA SNHG7 knockdown aggravates hepatic ischemia-reperfusion injury and promotes apoptosis in hemorrhagic shock pregnant rats by modulating miR-34a-5p/YWHAG Axis. Mol Biotechnol 65:983–996
Zhu L, Chen L, Xu P, Lu D, Dai S, Zhong L, Han Y, Zhang M, Xiao B, Chang L (2020) Genetic and molecular basis of epilepsy-related cognitive dysfunction. Epilepsy Behav 104:106848
Ye X-G, Liu Z-G, Wang J, Dai J-M, Qiao P-X, Gao P-M, Liao W-P (2021) YWHAG mutations cause childhood myoclonic epilepsy and febrile seizures: molecular sub-regional effect and mechanism. Front Genet 12:632466
Yanmei G, Daijun W, Xiaomei L, Yingying W, Meixia D, Li Y (2022) YWHAG promotes gastric cancer proliferation and migration via PI3K/AKT pathway. https://doi.org/10.21203/rs.3.rs-1402042/v1
Aamodt WW, Waligorska T, Shen J, Tropea TF, Siderowf A, Weintraub D, Grossman M, Irwin D, Wolk DA, Xie SX (2021) Neurofilament light chain as a biomarker for cognitive decline in Parkinson disease. Mov Disord 36:2945–2950
Lin C-H, Li C-H, Yang K-C, Lin F-J, Wu C-C, Chieh J-J, Chiu M-J (2019) Blood NfL: a biomarker for disease severity and progression in Parkinson disease. Neurology 93:e1104–e1111
Ramocki MB, Bartnik M, Szafranski P, Kołodziejska KE, Xia Z, Bravo J, Miller GS, Rodriguez DL, Williams CA, Bader PI (2010) Recurrent distal 7q11. 23 deletion including HIP1 and YWHAG identified in patients with intellectual disabilities, epilepsy, and neurobehavioral problems. Am J Hum Genet 87:857–865
Weiner S, Junkkari A, Sauer M, Luikku A, Rauramaa T, Kokkola T, Herukka S-K, Blennow K, Zetterberg H, Leinonen V (2023) Novel cerebrospinal fluid biomarkers correlating with shunt responsiveness in patients with idiopathic normal pressure hydrocephalus. Fluids Barriers CNS 20:1–16
Sathe G, Na CH, Renuse S, Madugundu AK, Albert M, Moghekar A, Pandey A (2019) Quantitative proteomic profiling of cerebrospinal fluid to identify candidate biomarkers for Alzheimer’s disease. Proteomics Clin Appl. https://doi.org/10.1002/prca.201800105
Dong L, Chang Q, Ma J, Liu C, Guo D, Li X, Yang D, Fan Y, Liang K, Li D (2023) Associations of blood UCH-L1 and NfL levels with cognitive dysfunction in Parkinson’s disease patients. Neurosci Lett 804:137219
Politis M (2014) Neuroimaging in Parkinson disease: from research setting to clinical practice. Nat Rev Neurol 10:708–722
Liu P, Kong L, Liang K, Wu Y, Jin H, Song B, Tan X (2020) Identification of dissociation factors in pancreatic Cancer using a mass spectrometry-based proteomic approach. BMC Cancer 20:1–9
Ng ASL, Tan YJ, Yong ACW, Saffari SE, Lu Z, Ng EY, Ng SYE, Chia NSY, Choi X, Heng D (2020) Utility of plasma neurofilament light as a diagnostic and prognostic biomarker of the postural instability gait disorder motor subtype in early Parkinson’s disease. Mol Neurodegener 15:1–8
Buhmann C, Lezius S, Pötter-Nerger M, Gerloff C, Kuhle J, Choe Cu (2022) Age-adjusted serum neurofilament predicts cognitive decline in Parkinson’s disease (MARK-PD). Mov Disord 37:435–436
Zhou X, Wang Z, Xu B, Ji N, Meng P, Gu L, Li Y (2021) Long non-coding RNA NORAD protects against cerebral ischemia/reperfusion injury induced brain damage, cell apoptosis, oxidative stress and inflammation by regulating miR-30a-5p/YWHAG. Bioengineered 12:9174–9188
Kanani F, Titheradge H, Cooper N, Elmslie F, Lees MM, Juusola J, Pisani L, McKenna C, Mignot C, Valence S (2020) Expanding the genotype–phenotype correlation of de novo heterozygous missense variants in YWHAG as a cause of developmental and epileptic encephalopathy. Am J Med Genet A 182:713–720
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they had no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, Y., Zhu, L., Bai, Q. et al. Serum level of YWHAG as a diagnostic marker of cognitive impairment in Parkinson’s disease patients. Acta Neurol Belg 124, 879–885 (2024). https://doi.org/10.1007/s13760-023-02441-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-023-02441-5