Abstract
Mental imagery is a quasi-perceptual experience in the absence of external stimuli. This concept has intrigued psychologists, sportspersons, neurologists and other scientists for over a decade now. Imagery has been used in rehabilitation and the results have been promising. Researchers refer to this as healing the body through the mind. However, the challenge is lack of standardized protocols, homogeneity and consistency in application of mental imagery in different populations. The purpose of this review is to discuss and understand the role of mental imagery in the treatment of central neuropathic pain (CNP). Treatment options of CNP are inadequate and their benefits are short lived. We conducted an extensive search on various databases using combinations of different keywords and reviewed the available literature in this area. We were able to finalize twelve studies where mental imagery was used for treating CNP in spinal cord injury (SCI), stroke and multiple sclerosis. However, the methodology and techniques of mental imagery training used in these studies were non-homogeneous and inconsistent. This review provides a guiding framework to further explore the different techniques of mental imagery and their roles in treating CNP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pain is one of the most debilitating complications, among other neurological conditions. It may lead to fatigue, anxiety, depression, disturbances in sleep and deterioration in the overall quality of life [1]. Over the years, researchers have faced the challenge to define pain but have not been very successful due to its subjective nature. According to the International Association for the Study of Pain (IASP), pain can be defined as any unpleasant sensory and emotional occurrence associated with actual or prospective tissue injury, or described in terms of such damage [2].
Pain can be further classified into three types, namely nociceptive, inflammatory and neuropathic pain (NP). Each of these types is mediated by different mechanisms. The symptoms of NP may be localized or more generalized. Typically, the symptoms may be associated with painful or burning sensation (dysesthesia), hyperalgesia, or the perception of a non-nociceptive stimulus as painful (allodynia) [3]. NP can be subdivided into peripheral neuropathic pain (PNP) that occurs due to involvement of the peripheral nervous system (e.g., painful diabetic neuropathy) and central neuropathic pain (CNP) which occurs after a central nervous system disorder (e.g., post-stroke, multiple sclerosis (MS) or spinal cord injury) [4]. The mechanisms of peripheral and central neuropathic pain are different. PNP occurs as a result of spontaneous firing of damaged nerve fibers and oversensitivity of pain pathways due to denervation [5]. On the other hand, CNP is associated with central sensitization and central re-organization at higher levels in the CNS [6]. Neuropathic pain is refractory and often difficult to treat. The available treatment options include medications (such as pregabalin, opioids), surgery and physical therapy interventions. However, there are side effects associated with the former two approaches and the physical therapy interventions are diverse and not well established [7, 8].
Mental imagery (MI) involves the cognitive processes of imagining something that is not actually present. MI techniques have been proven to reduce pain associated with phantom limb pain, complex regional pain syndrome and other types of pain with unknown pathology [6]. CNP results in maladaptive changes in the brain and existing evidence available regarding the use of MI for CNP in neurological conditions is limited. To the best of our knowledge, this is the first review that examines the different characteristics and techniques of MI used for treating CNP in neurological conditions.
Neuropathic pain
The NP Special Interest Group (NeuPSIG); a task force of the International Association for the Study of Pain (IASP) has redefined NP as pain arising as a direct consequence of a lesion or disease affecting the somatosensory system [9]. In the current definition, the word somatosensory system replaced the word nervous system present in the old definition, so as to differentiate NP from other types of musculoskeletal pain arising indirectly from disorders of the motor system [10]. Examples of NP include peripheral neuropathy, post herpetic neuralgia, central post stroke pain, spinal cord injury (SCI) pain, and trigeminal neuralgia [11]. NP can be divided into two categories: (a) those that are consequences of a peripheral lesion or disease and (b) those that are consequences of a central lesion or disease [12].
Peripheral neuropathic pain
Peripheral neuropathic pain (PNP) results from damage to peripheral nerves. This may occur due to metabolic damage, toxins, medications, cytokines, and other inflammatory mediators, resulting in fiber density changes and neuronal hyper excitability [5]. Pain processing pathways constituting unmyelinated C fibers and thinly myelinated Aδ fibers may undergo fiber degeneration and alterations in voltage-gated sodium channels’ expression resulting in ectopic firing and faulty signal transmission [13]. In addition, there is increased supply of (pro-) inflammatory mediators due to accumulation of infiltrating immune cells (neutrophils, macrophages and mast cells) resulting in nerve fiber sensitization and neuronal damage. The ultimate result of the maladaptive mechanisms is a state of inappropriate signaling from the peripheral neuron to its second-order targets, with multi-factorial errors in both transduction and transmission [14]. PNP may be seen in painful diabetic polyneuropathy, post herpetic neuralgia, HIV-associated NP, cancer pain and post-surgical pain [11].
Central neuropathic pain
Central neuropathic pain (CNP) is associated with central nervous system lesions. Another important term associated with CNP is central sensitization. IASP defines central sensitization as the increased responsiveness of nociceptive neurons in the CNS to their normal or sub-threshold afferent input [15]. Central sensitization occurs when the stimulation is sufficiently intense or repeated. This results in sensitization of spinal and supraspinal nociceptive pathways to subsequent stimuli. If the nociceptive input remains persistent, this central sensitization becomes maladaptive. In supraspinal regions, the resulting imbalance between descending facilitation and inhibition is another major contributor to ongoing pain. Maladaptive sub-cortical and cortical plasticity also contributes to the painful interpretation of incoming signals, resulting in the enhancement of the chronic pain state [16]. CNP can result from vascular (ischemic or hemorrhagic), infectious (abscess, encephalitis, myelitis), demyelinating, traumatic [brain or spinal cord), or neoplastic disorders. However, CNP occurs most commonly in stroke (central post stroke pain (CPSP)], MS and SCI [15]. Hence, the current review highlights the application and treatment effects of mental imagery training for CNP in these three conditions.
CNP in different neurological conditions
Pain in stroke
Stroke as defined by the World Health Organization (WHO) is a clinical syndrome consisting of rapidly developing clinical signs of focal (or global, in case of coma) disturbance of cerebral function lasting more than 24 h or leading to death with no apparent cause other than a vascular origin [17]. CPSP is a chronic syndrome, which may occur due to hemorrhagic or ischemic lesions particularly involving thalamus. It may be spontaneous or in response to an external stimulus [18]. It has a prevalence of about 2–8% in stroke patients [19]. The pain may be continuous or intermittent and is associated with sensory abnormalities such as hyperalgesia or allodynia. The onset usually occurs between 1 and 3 months, and the majority of patients develop symptoms by 6 months [20]. Studies suggest that this type of pain occurs as a result of central inhibition, a decrease of GABAergic inhibition and central sensitization. However, the exact pathophysiology of CPSP remains unclear [21].
Another type of pain common in stroke patients is complex regional pain syndrome (CRPS); commonly known as shoulder hand syndrome. It is a neuropathic pain syndrome characterized by spontaneous or stimulus evoked pain accompanied by autonomic and motor disturbances. CRPS is categorized into type I and type II pains. In type I CRPS, there is an absence of overt nerve damage, whereas type II pain follows peripheral nerve injury. In stroke patients type I is more common than type II. The incidence of CRPS post-stroke ranges from 1.5 to 70% [22]. The pain in CRPS is continuous and intense; sometimes inconsistent with the severity of the injury. It is associated with limited range of motion (ROM), edema, warmth, redness and tenderness to palpation in shoulder and wrist with elbow spared [23].
Pain in spinal cord injury
Pain in SCI can be classified into nociceptive (musculoskeletal or visceral) and neuropathic (above-level, at-level or below-level) pain types [24]. In a meta-analysis on NP in SCI, it was found that the overall pooled point prevalence of neuropathic pain post SCI was 53%. Moreover, pain below the level of the lesion was more common, particularly in older patients and those with tetraplegia and after 1 year of SCI [25]. The characteristics of CNP in SCI include burning (superficial) spontaneous pain, pressing (deep) spontaneous pain, paroxysmal pain, evoked pain and paresthesia/dysesthesia [26]. CNP following SCI is associated with significant changes in the neuroanatomy of multiple brain regions traditionally associated with nociceptive processing, including the ventral posterior region of the thalamus, prefrontal cortex, insular cortex, amygdala, and premotor cortex [27].
Pain in multiple sclerosis
MS is a chronic demyelinating disease of the CNS with a variable disease course. It is characterized by sensory impairments, lack of coordination, spasticity, motor weakness, fatigue and pain [28]. The prevalence of neuropathic pain in early stages of MS has been found to be 28% [29]. Neuropathic pain particularly comprises central neuropathic pain, trigeminal neuralgia and Lhermitte sign [30]. The cause of pain is attributed to demyelinating lesions in areas involved in pain perception [31].
Mental imagery: concept
Humans can generate imagery, even in the absence of any triggering external stimulus. Mental imagery (MI) refers to the active process by which humans relive the sensations with or without external stimuli [32]. Richardson defined mental practice as the symbolic rehearsal of a physical activity in the absence of any gross muscular movements. Different terminologies such as visualization, imagery, and mental practice have been used to describe MI [33]. However, MI is not limited to only visualization, but it involves all senses. This cognitive operation utilizes different modalities such as visual, auditory, tactile, kinesthetic, olfactory, gustatory, or any combination of these senses [34]. Suinn maintained that the rich multimodal (utilizing all the senses) processes of imagery rehearsal are holistic, under conscious control, and can closely replicate the original experience, even arousing emotions similar to those associated with the experience [35].
MI and pain
The idea behind as how the brain imagines and how imagery works has intrigued neuroscientists over the decades. The effect of imagery on chronic pain has been explored and it has been suggested that there is shared representation of content and location between imagery and perception [36]. The presence of pain is associated with negative images [37]. The use of a mirror based on the concept of mirror neurons by Ramachandran et al. to train patients with phantom limb pain supports the role of MI in chronic pain [38]. MI modulates pain by altering the activity of the motor cortex, which is related to pain modulation [39]. In an experiment by Fardo et al,. participants were required to imagine a glove or a wound on the forearm, and it was observed that pain perception increased during imagination of a lesion; whereas, it reduced while imagining a glove on the forearm [40]. Therefore, it is imperative to further understand the intricacies of imagery and its effect on pain, so that it can be used as interventions for treating CNP occurring in neurological conditions.
Techniques of MI used in CNP
Researchers have used various forms of MI for treating CNP in multiple neurological conditions. The commonly used techniques include guided imagery, graded motor imagery, visual illusion and hypnosis.
Guided imagery
Fitzgerald and Langevin [41] defined guided imagery (GI) as the interaction of mind and body using the power of imagination to bring about changes in physical, emotional, or spiritual dimensions [42]. It refers to the use of imagination to invoke one or more of the senses. It involves the ‘guiding’ of an individual through experiences in the mind, to access physical, emotional and spiritual dimensions to affect bodily change [43]. Patients listen to audio tapes that combine soothing music with quiet narrations of peaceful images [44]. GI involves the creation and controlled visualization of mental images. It has been successfully used for subsiding chronic pain due to cancer, musculoskeletal and post-operative conditions [45]. Researchers believe that the theoretical basis behind its efficacy is the pain gate theory. If the route of the painful stimuli can be blocked by a pleasant stimulus, perception of pain may be alleviated. Release of endorphins is also seen to be associated with positive cognition associated with GI [46].
Graded motor imagery
Another form of motor imagery used to treat chronic pain is graded motor imagery (GMI). It was described by Butler and Moseley and is based on the principle of “train the brain”. Studies suggest that application of GMI helps in cortical re-organization that eventually results in reduction of pain [47]. It has been used effectively in treating chronic pain arising from CRPS, phantom limb pain, stroke, carpal tunnel syndrome, neck and back pain among others [48, 49]. GMI is graded, i.e., it involves three sequential phases: laterality training, imagined motor imagery (explicit) and mirror therapy. Mirror therapy provides visual feedback that is useful in modulating somatic pain by providing powerful feedback into the cortex. The patients place their affected limb inside a mirror box and watch movements of their non-affected limb in the mirror. This tricks the brain as it visualizes the affected side to be moving in a pain-free normal movement pattern [48]. Mirror therapy has been used in the past to improve motor control and pain in patients with CRPS post stroke [50].
Visual illusion
Studies in SCI patients have utilized visual illusion for treating chronic NP [51, 52]. An illusion is created of normal movement instead of non-functional or painful limb in a manner that the patients get the impression that affected part has the ability to function [53]. Sakamoto et al. [54] have shown that combining observation and imagery of an action enhances corticospinal excitability in comparison to observation or imagery alone. Visual illusion is hypothesized to cause neuroplastic changes that may affect pain. Another proposed mechanism by which they may act is by restoring the body schema that is distorted as a result of disease or trauma [53].
Hypnosis
Hypnosis has been used for treating chronic pain in fibromyalgia, low back pain, disability-related pain, cancer-related pain, irritable bowel syndrome and headache [55,56,57]. Hypnosis is an altered state of consciousness—a transition from a normal, ordinary state of consciousness [58]. It results in an increased focused attention and lack of attention to external stimuli. The patient is always in control and can stop the process of hypnosis whenever he desires to do so [59].
Search strategy
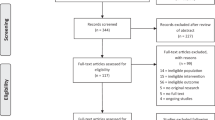
A comprehensive review of the literature was performed covering the period from 2000 to 2018. The electronic search was performed using the following databases: MEDLINE (via OvidSP), EMBASE (via OvidSP), Cumulative Index to Nursing and Allied Health Literature (CINAHL), Scopus, Academic Search Premier, Web of Science, Allied and Complementary Medicine, PubMed, the Cochrane Collaboration, and the Physiotherapy Evidence Database (Pedro). Search terms included combinations of the keywords mental imagery, guided imagery, relaxation, hypnosis, neuropathic pain, pain management, pain relief, visual illusion, mental imagery techniques, stroke, spinal cord injury, multiple sclerosis, central neuropathic pain, peripheral neuropathic pain, mental imagery techniques, visual imagery, kinesthetic imagery and neurological diseases. The references of the articles found were reviewed to identify other relevant research studies. The articles found were also forward-searched to identify related articles. No new articles were retrieved using these procedures. Searches were limited to English language and human studies only. Search results have been elaborated in Table 1.
Discussion
Imagery is not just a visualization technique, but it involves all the senses [42]. This review appraises the evidence related to MI training for CNP in neurological conditions. Although the results were encouraging still this remains as one of the least explored areas. Studies conducted on MI training show a promising future, but the available evidence is heterogeneous. Twelve studies that were identified after reviewing the literature are discussed in Table 1.
Effect of MI post stroke
Pain in stroke is often neglected. We selected four studies where imagery was used for treating post stroke pain. Out of these, three studies reported patients with pain resulting from CRPS type I [50, 60, 61]; while in the fourth study, the cause of pain after stroke was not stated [62]. GMI, which integrates both explicit and implicit forms of imagery in a systematic manner, has shown promising results in the past. Mirror therapy, a simple yet effective treatment can be used alone or as a part of GMI regimen. Evidence shows that mirror therapy enhances proprioceptive inputs leading to sensory–motor reorganization [60]. Significant improvement in pain scores was reported in all the three studies after mirror therapy training. Visual analog scale (VAS) was used as an outcome measure in all the 4 studies; however, in 2 studies, signs of neuropathic pain, namely allodynia, were also assessed [50, 60]. The findings of these studies were based on pain scales only and none of these studies used neuroimaging techniques to corroborate their findings. As the interventional strategies for post stroke pain are limited; more robust studies are needed in this area [50].
Effect of MI in SCI pain
Post SCI, there is a mismatch between sensory feedback and motor commands sent to the affected limb. Evidence shows that after injury, there is a reorganization of primary somatosensory cortex (S1) leading to chronic pain [63]. Reduction in pain scores after mental imagery intervention suggests that it has a potential in producing analgesic-like effects for patients having neuropathic pain after SCI. In most of these studies, visual illusion was created with a mirror. It is believed to modify the disconnect between stimulus and response and aids in the reversal of maladaptive changes due to cortical re-organization [64]. Contrary to the above findings, in a study by Gustin et al. [65], 6 out of 7 patients showed an increase in pain after they were asked to focus on imagined movements of the foot. The authors of this study suggested that SCI leads to enhanced excitability of the neurons in brain areas such as the thalamus and cortex, and movement imagery in the presence of enhanced cortical excitability may have resulted in increased pain and an allodynia-like effect. The conflicting results could be due to low sample size, differences in variables such as the level of injury, time lapse since the injury and the absence of standardized protocol adapted in these studies.
The effect of visual illusion was compared with other modalities used for electrical stimulation in these studies. Visual illusion when combined with transcranial direct current stimulation (tDCS) showed more effective pain reduction than any of the modalities used alone [52]. It has been suggested that both imagery and tDCS enhance corticospinal excitability, hence reversing the effect of intracortical inhibition. Additionally, it was found that activation of mirror neuron system may contribute to reduced pain in visual illusion group. In another study, transcutaneous electrical nerve stimulation (TENS) was compared with visual illusion in a crossover study and it was found that both techniques were effective in reducing neuropathic pain symptoms [66]. In this review, we did not include articles related to virtual reality where computer-based technology was used to connect users to an artificial virtual environment. Another technique of mental imagery used in SCI for pain relief was GI. When compared with massage, it was effective in reducing pain in SCI by enhancing the parasympathetic responses in the patients [67].
Effect of MI in MS
The prevalence rate of pain in MS ranges between 29 and 83% of the population [30]. Studies related to the use of MI as treatment of MS pain were limited and the most common technique recognized was self-hypnosis. The use of self-hypnosis was identified in three studies [68,69,70] but the methodology adopted in all the studies was not similar. In one study, Jensen et al. [68] compared hypnosis with progressive muscle relaxation and found that pain scores improved with hypnotic treatment. However, since progressive muscle relaxation has similar mechanism of pain relief as hypnosis, it behaved like an active condition rather than a control for comparison. In another study, each patient underwent 4 sessions of 4 different treatment modules; hence, isolated effects of each intervention could not be ascertained [69]. Although most available studies indicate the beneficial effects of self-hypnosis in MS pain, there was a wide range of hypnotic suggestions used in these studies.
Limitations, relevance and conclusions
Although the findings of studies in this area are intriguing, but there are certain limitations. The most important limitation is that MI protocol was not standardized. The studies reviewed in the present article have used various techniques of imagery training, henceforth making it difficult to reach a conclusion regarding which technique will be more effective for the patients. Nonetheless, there were significant improvements in most of the studies with MI training in one form or the other. Secondly, the number of subjects was less in most of the studies, which directly affected the power of the study and limited its reliability and generalizability. Thirdly, even though the basic pathology of NP was same but depending on the condition, there were variations among different clinical conditions with respect to the nature and characteristics of CNP.
In spite of these limitations, the present review highlights some interesting findings. This is the first review to focus on MI techniques for CNP in neurological conditions. It helps to bring about the similarity and differences in the nature and treatment of MI techniques for treating CNP. The available techniques presently used for MI and outcomes are so varied that they could not be confined to a single inclusion criterion. The studies are limited in this area; hence, this narrative review can form a basis of further systematic reviews. In future, systematic reviews and meta-analysis can be conducted for an in-depth understanding of the various aspects of imagery in management of pain in these conditions. In conclusion, MI techniques have tremendous potential in treating chronic, painful NP conditions. They are innovative, effective, easy to use and cost effective, and should be used as an adjunct or independently as a treatment modality for CNP.
Abbreviations
- CNS:
-
Central nervous system
- CPSP:
-
Central post stroke pain
- CRPS:
-
Complex regional pain syndrome
- DRG:
-
Dorsal root ganglion
- GMI:
-
Graded motor imagery
- IASP:
-
International Association for the Study of Pain
- NP:
-
Neuropathic pain
- CNP:
-
Central neuropathic pain
- PNP:
-
Peripheral neuropathic pain
- ROM:
-
Range of motion
- SCI:
-
Spinal cord injury
- tDCS:
-
Transcranial direct current stimulation
- TENS:
-
transcutaneous electrical nerve stimulation
- WHO:
-
World Health Organization
References
Woo AK (2010) Depression and anxiety in pain. Rev Pain 4(1):8–12
Cohen M, Quintner J, van Rysewyk S (2018) Reconsidering the International Association for the study of pain definition of pain. Pain Rep 3(2):1–7
Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson A, Yarnitsky D, Freeman R, Truini A et al (2017) Neuropathic pain. Nat Re v Dis Prim 3:17002
Teixeira MJ, Almeida DB, Yeng LT (2016) Concept of acute neuropathic pain. The role of nervi nervorum in the distinction between acute nociceptive and neuropathic pain. Revista Dor 17:5–10
Meacham K, Shepherd A, Mohapatra DP, Haroutounian S (2017) Neuropathic pain: Central vs. peripheral mechanisms. Curr Pain Headache Rep 21(6):28
Watson JC, Sandroni P. Central neuropathic pain syndromes. In: Mayo clinic proceedings 91(3): 372–385
Chong MS, Bajwa ZH (2003) Diagnosis and treatment of neuropathic pain. J Pain Symptom Manage 25(5):S4–S11
Vrinten DH, Kalkman CJ, Adan RA, Gispen WH (2001) Neuropathic pain: a possible role for the melanocortin system? Eur J Pharmacol 429(1–3):61–69
Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW et al (2008) Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 70(18):1630–1635
Haanpää M, Attal N, Backonja M, Baron R, Bennett M, Bouhassira D et al (2011) NeuPSIG guidelines on neuropathic pain assessment. PAIN® 152(1):14–27
Meyer HP (2008) Neuropathic pain—current concepts. South Afr Fam Pract 50(3):40–49
Cruccu G, Sommer C, Anand P, Attal N, Baron R, Garcia-Larrea L et al (2010) Assessment of Neuropathic Pain. Eur Handb Neurol Manag Second Ed. 1(6):91–100
Fields HL, Rowbotham M, Baron R (1998) Postherpetic neuralgia: irritable nociceptors and deafferentation. Neurobiol Dis 5(4):209–227
Costigan M, Scholz J, Woolf CJ (2009) Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 32:1–32
van den Broeke EN, Lambert J, Huang G, Mouraux A (2016) Central sensitization of mechanical nociceptive pathways is associated with a long-lasting increase of pinprick-evoked brain potentials. Front Hum Neurosci 10:531
Ro L, Chang K (2005) Neuropathic pain: mechanisms and treatments. Chang Gung Med J 28(9):597–605
Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A et al (2013) An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 44(7):2064–2089
Siniscalchi A, De Sarro G, Gallelli L (2014) Central Post-stroke Pain and Pharmacological Treatment: work in progress. SOJ Neurol 1(1):1–2
Henry JL, Lalloo C, Yashpal K (2008) Central poststroke pain: an abstruse outcome. Pain Res Manage 13(1):41–49
Şahin-Onat Ş, Ünsal-Delialioğlu S, Kulaklı F, Özel S (2016) The effects of central post-stroke pain on quality of life and depression in patients with stroke. J Phys Ther Sci 28(1):96–101
Klit H, Finnerup NB, Jensen TS (2009) Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurol 8(9):857–868
Pertoldi S, Di Benedetto P (2005) Shoulder-hand syndrome after stroke. Complex Regional Pain Syndr 41(4):283–292
Kim YW, Kim Y, Kim JM, Hong JS, Lim HS, Kim HS (2016) Is poststroke complex regional pain syndrome the combination of shoulder pain and soft tissue injury of the wrist?: a prospective observational study: STROBE of ultrasonographic findings in complex regional pain syndrome. Medicine 95(31):e4388
Mahnig S, Landmann G, Stockinger L, Opsommer E (2016) Pain assessment according to the International Spinal Cord Injury Pain classification in patients with spinal cord injury referred to a multidisciplinary pain center. Spinal cord 54(10):809
Burke D, Fullen BM, Stokes D, Lennon O (2017) Neuropathic pain prevalence following spinal cord injury: a systematic review and meta-analysis. Eur J Pain 21(1):29–44
Lee S, Zhao X, Hatch M, Chun S, Chang EY (2013) Central neuropathic pain in spinal cord injury. Crit Rev Phys Rehabil Med 25(3–4):159–172
Gustin SM, Wrigley PJ, Siddall PJ, Henderson LA (2009) Brain anatomy changes associated with persistent neuropathic pain following spinal cord injury. Cereb Cortex 20(6):1409–1419
Solaro C, Trabucco E, Uccelli MM (2013) Pain and multiple sclerosis: pathophysiology and treatment. Curr Neurol Neurosci Rep 13(1):320
Foley PL, Vesterinen HM, Laird BJ, Sena ES, Colvin LA, Chandran S et al (2013) Prevalence and natural history of pain in adults with multiple sclerosis: systematic review and meta-analysis. PAIN® 154(5):632–642
Heitmann H, Biberacher V, Tiemann L, Buck D, Loleit V, Selter RC et al (2016) Prevalence of neuropathic pain in early multiple sclerosis. Multiple Sclerosis J 22(9):1224–1230
Feketova S, Waczulikova I, Kukumberg MJ, Mares J (2016) Pain in multiple sclerosis: prevalence and characteristics of various pain conditions. J Mult Scler 3(187):2376-0389
Jackson PL, Lafleur MF, Malouin F, Richards C, Doyon J (2001) Potential role of mental practice using motor imagery in neurologic rehabilitation. Arch Phys Med Rehabil 82(8):1133–1141
Johnson P (1982) The functional equivalence of imagery and movement. Quar J Exp Psychol Sect A 34(3):349–365
Heiland TL, Rovetti R, Dunn J (2012) Effects of visual, auditory, and kinesthetic imagery interventions on dancers’ plié arabesques. J Imagery Res Sport Phys Activity. https://doi.org/10.1515/1932-0191.1065
Suinn RM (1997) Mental practice in sport psychology: where have we been, where do we go? Clin Psychol Sci Pract 4(3):189–207
Cichy RM, Heinzle J, Haynes JD (2011) Imagery and perception share cortical representations of content and location. Cereb Cortex 22(2):372–380
Berna C, Tracey I, Holmes EA (2012) How a better understanding of spontaneous mental imagery linked to pain could enhance imagery-based therapy in chronic pain. J Exp Psychopathol 3(2):258–273
Ramachandran VS, Rogers-Ramachandran D (1996) Synaesthesia in phantom limbs induced with mirrors. Proc R Soc London Ser B Biol Sci 263(1369):377–386
Volz MS, Suarez-Contreras V, Portilla ALS, Fregni F (2015) Mental imagery-induced attention modulates pain perception and cortical excitability. BMC Neurosci 16(1):15
Fardo F, Allen M, Jegindø EME, Angrilli A, Roepstorff A (2015) Neurocognitive evidence for mental imagery-driven hypoalgesic and hyperalgesic pain regulation. Neuroimage 120:350–361
Fitzgerald M, Langevin M (2010) Imagery. In: Snyder M, Lindquist R (eds) Complementary and alternative therapies in nursing, 6th edn. Springer, New York, NY, pp 63–89
Carpenter JJ, Hines SH, Lan VM (2017) Guided imagery for pain management in postoperative orthopedic patients: an integrative literature review. J Holistic Nurs 35(4):342–351
King K (2010) A review of the effects of guided imagery on cancer patients with pain. Complement Health Pract Rev 15(2):98–107
Van Tilburg MA, Chitkara DK, Palsson OS, Turner M, Blois-Martin N, Ulshen M, Whitehead WE (2009) Audio-recorded guided imagery treatment reduces functional abdominal pain in children: a pilot study. Pediatrics 124(5):e890–e897
Mannix LK, Chandurkar RS, Rybicki LA, Tusek DL, Solomon GD (1999) Effect of guided imagery on quality of life for patients with chronic tension-type headache. Headache J Head Face Pain 39(5):326–334
Elsegood KJ, Wongpakaran N (2012) The effects of guided imagery on affect, cognition, and pain in older adults in residential care: a randomized controlled study from Thailand. Res Gerontol Nurs 5(2):114–122
Priganc VW, Stralka SW (2011) Graded motor imagery. J Hand Ther 24(2):164–169
Bowering KJ, O’Connell NE, Tabor A, Catley MJ, Leake HB, Moseley GL, Stanton TR (2013) The effects of graded motor imagery and its components on chronic pain: a systematic review and meta-analysis. J Pain 14(1):3–13
Walz AD, Usichenko T, Moseley GL, Lotze M (2013) Graded motor imagery and the impact on pain processing in a case of CRPS. Clin J Pain 29(3):276–279
Cacchio A, De Blasis E, Necozione S, Orio FD, Santilli V (2009) Mirror therapy for chronic complex regional pain syndrome type 1 and stroke. N Engl J Med 361(6):634–636
Moseley GL (2007) Using visual illusion to reduce at-level neuropathic pain in paraplegia. Pain 130(3):294–298
Soler MD, Kumru H, Pelayo R, Vidal J, Tormos JM, Fregni F, Navarro X, Pascual-Leone A (2010) Effectiveness of transcranial direct current stimulation and visual illusion on neuropathic pain in spinal cord injury. Brain 133(9):2565–2577
Plumbe L, Peters S, Bennett S, Vicenzino B, Coppieters MW (2013) Mirror therapy, graded motor imagery and virtual illusion for the management of chronic pain (Protocol). Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD010329
Sakamoto M, Muraoka T, Mizuguchi N, Kanosue K (2009) Combining observation and imagery of an action enhances human corticospinal excitability. Neurosci Res 65(1):23–27
Hammond DC (2007) Review of the efficacy of clinical hypnosis with headaches and migraines. Int J Clin Exp Hypnosis 55(2):207–219
Picard P, Jusseaume C, Boutet M, Dualé C, Mulliez A, Aublet-Cuvellier B (2013) Hypnosis for management of fibromyalgia. Int J Clin Exp Hypn 61(1):111–123
Tan G, Fukui T, Jensen MP, Thornby J, Waldman KL (2009) Hypnosis treatment for chronic low back pain. Int J Clin Exp Hypnosis 58(1):53–68
Ardigo S, Herrmann FR, Moret V, Déramé L, Giannelli S, Gold G, Pautex S (2016) Hypnosis can reduce pain in hospitalized older patients: a randomized controlled study. BMC Geriatr 16(1):14
Dillworth T, Mendoza ME, Jensen MP (2011) Neurophysiology of pain and hypnosis for chronic pain. Transl Behav Med 2(1):65–72
Cacchio A, De Blasis E, De Blasis V, Santilli V, Spacca G (2009) Mirror therapy in complex regional pain syndrome type 1 of the upper limb in stroke patients. Neurorehabil Neural Repair 23(8):792–799
Vural SP, Yuzer GFN, Ozcan DS, Ozbudak SD, Ozgirgin N (2016) Effects of mirror therapy in stroke patients with complex regional pain syndrome type 1: a randomized controlled study. Arch Phys Med Rehabil 97(4):575–581
Polli A, Moseley GL, Gioia E, Beames T, Baba A, Agostini M, Turolla A (2017) Graded motor imagery for patients with stroke: a non-randomized controlled trial of a new approach. Eur J Phys Rehabil Med 53(1):14–23
Wrigley PJ, Press SR, Gustin SM, Macefield VG, Gandevia SC, Cousins MJ et al (2009) Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain 141(1–2):52–59
Chi B, Chau B, Yeo E, Ta P (2019) Virtual reality for spinal cord injury-associated neuropathic pain: systematic review. Ann Phys Rehabil Med. 62(1):49–57
Gustin SM, Wrigley PJ, Gandevia SC, Middleton JW, Henderson LA, Siddall PJ (2008) Movement imagery increases pain in people with neuropathic pain following complete thoracic spinal cord injury. PAIN 137(2):237–244
Özkul Ç, Kılınç M, Yıldırım SA, Topçuoğlu EY, Akyüz M (2015) Effects of visual illusion and transcutaneous electrical nerve stimulation on neuropathic pain in patients with spinal cord injury: a randomised controlled cross-over trial. J Back Musculoskeletal Rehabil 28(4):709–719
Lovas J, Tran Y, Middleton J, Bartrop R, Moore N, Craig A (2017) Managing pain and fatigue in people with spinal cord injury: a randomized controlled trial feasibility study examining the efficacy of massage therapy. Spinal Cord 55(2):162
Jensen MP, Barber J, Romano JM, Molton IR, Raichle KA, Osborne TL et al (2009) A comparison of self-hypnosis versus progressive muscle relaxation in patients with multiple sclerosis and chronic pain. Int J Clin Exp Hypn 57(2):198–221
Jensen MP, Ehde DM, Gertz KJ, Stoelb BL, Dillworth TM, Hirsh AT et al (2011) Effects of self-hypnosis training and cognitive restructuring on daily pain intensity and catastrophizing in individuals with multiple sclerosis and chronic pain. Int J Clin Exp Hypn 59(1):45–63
Hosseinzadegan F, Radfar M, Shafiee-Kandjani AR, Sheikh N (2017) Efficacy of self-hypnosis in pain management in female patients with multiple sclerosis. Int J Clin Exp Hypn 65(1):86–97
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to declare. None of the authors received any financial support to conduct this study or to prepare the manuscript. The people who have made substantial contributions towards the work reported in this manuscript are included in the list of authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaur, J., Ghosh, S., Sahani, A.K. et al. Mental imagery training for treatment of central neuropathic pain: a narrative review. Acta Neurol Belg 119, 175–186 (2019). https://doi.org/10.1007/s13760-019-01139-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-019-01139-x