Abstract
Psychiatric symptoms and motor impairment are major contributions to the poor quality of life in patients with Parkinson's disease (PD). Here, we applied a novel diffusion-weighted imaging approach, diffusion MRI connectometry, to investigate the correlation of quality of life, evaluated by Parkinson's Disease Questionnaire (PDQ39) with the white matter structural connectivity in 27 non-demented PD patients (disease duration of 5.3 ± 2.9 years, H and Y stage = 1.5 ± 0.6, UPDRS-III = 13.7 ± 6.5, indicating unilateral and mild motor involvement). The connectometry analysis demonstrated bilateral posterior limbs of the internal capsule (PLIC) with increased connectivity related to the higher quality of life (FDR = 0.027) in a multiple regression model. The present study suggests for the first time a neural basis of the quality of life in PD in the light of major determinants of poor quality of life in these patients: anxiety, depression, apathy and motor impairment. Results in our sample of non-demented PD patients with relatively mild motor impairment and no apparent sign of depression/anxiety also identify a unique and inexplicable association of the PLIC to the quality of life in PD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Quality of life (QoL) is a subjective, multidimensional concept of well-being covering personal and interpersonal aspects of life, and a main concern and contributor to morbidity in non-curable disorders such as Parkinson’s disease (PD) [1].
Patients with PD demonstrate a combination of motor and non-motor symptoms (NMS), which severely impair their physical compatibility as well as emotional and social domains of their health. Apart from devastating motor dysfunctions, NMS such as mood disorders and cognitive symptoms are major culprits of poor QoL in PD patients [2]. The cumulative nature of the symptoms, along with the side effects of long-term dopamine replacement therapy, contributes to worsening patient’s quality of life as PD progresses. Early in Parkinson’s disease, the non-motor symptoms, rather than motor disabilities, seem to have the greatest impact on patients’ QoL [3].
Depressive symptoms are common in early PD and a lifelong prevalence as high as 80% is reported for depression in patients diagnosed with PD. Interestingly, physical disability has less impact on QoL than does clinical depression [4], and regression studies have assigned depression as a main independent predictor of worse QoL [4,5,6]. Nevertheless, depressive symptoms in PD are usually overlooked in daily clinical practice and misdiagnosed as motor symptoms (rigidity and mask-like expression) [5]. It is important to note that NMS such as anxiety and depression usually coexist in PD and synergistically interfere with patient’s routine activity. Although hard to distinguish, one study reported that the effect of anxiety remained significant even after controlling for coexisting depressive traits [7]. These neuropsychiatric disorders are believed to be part of non-motor PD manifestations and are mainly attributed to underlying neurobiological mechanisms of PD rather than a mere psychological response to the ailment [8]. Both depression and anxiety can modify a patient’s self-perception of well-being and thus profoundly affect patient’s quality of life.
Cognitive deterioration is a usual morbidity in advanced PD stages, most commonly in the domains of executive function, memory and attention. Poor cognition can, in turn, reduce the confidentiality of QoL assessment and diminish the validity of patient-reported questionnaires in advanced PD [2].
Brain structural changes in early PD are well described in the literature. The diagnostic pathology in PD has conventionally been considered as Lewy neuritis leading to neural death, mainly in the nigrostriatal pathway [9]. Nevertheless, this does not explain the heterogeneous manifestations of NMS in PD. A diverse group of neural circuits has been shown to lose microstructural integrity in newly diagnosed patients concordant with the inter-individual diverse phenotypes in early PD [10].
To date, no study has investigated white matter alterations in patients with PD in relation to QoL, which would alarm the development of symptoms with the highest impact on disability and morbidity in PD patients. Here, we applied a novel diffusion-weighted imaging approach, diffusion MRI connectometry, to investigate the neuropathological disturbances underlying poor quality of life in PD.
Methods
Participants
27 patients clinically diagnosed with Parkinson’s disease (mean disease duration of 5.3 ± 2.9 years) were recruited from a previous study by Ziegler et al. [11]. Hoehn and Yahr scale and Unified Parkinson’s Disease Rating Scale (UPDRS) were measured to assess disease stage and its severity in the “on” state. Only 24 patients of the study group were on a combination of antiparkinsonian drugs: levodopa (immediate and controlled release), non-ergot-derived dopamine receptor agonists (pramipexole, ropinirole), and a monoamine oxidase B inhibitor (rasagiline). Dosages of dopaminergic drugs were pooled and summarized using the levodopa equivalent daily dose (LEDD) [12], which ranged from 0 to 900 mg.
The study was performed according to the guidelines of the Ethics Committee of the University of Liège and written informed consent was obtained from all participating subjects in accordance with the Declaration of Helsinki.
Clinical measures
The quality of life as the main study variable was evaluated by the Parkinson’s Disease Questionnaire (PDQ39) [13]. Other tests as cofounders included Mattis Dementia Rating Scale, Mini-Mental State Examination and Symbol Digit Modalities test to assess global cognition, Rey auditory verbal learning test to measure episodic memory, Judgment of Line Orientation for visuospatial judgment, as well as Hospital Anxiety and Depression Scale (HADS) for psychiatric evaluation [11].
Imaging data acquisition
This dataset was acquired on a 3 T Siemens scanner, producing 120 DWI images (repetition time = 6800 ms, echo time = 91 ms; voxel size: 2.4 × 2.4 × 2.4 mm3; field of view = 211 × 211 mm) with a twice-refocused spin–echo sequence with EPI readout at b value of 2500 s/mm2 acquired in 54 transverse slices using an 88 × 88 voxel matrix. 2 b0 volumes were also acquired for motion correction. To reduce eddy current distortions in DWI, electrostatic repulsion sequence was used to uniformly distribute 120 gradient vectors in space [11].
Diffusion MRI data processing
Diffusion MRI data were corrected for subject motion, eddy current distortions, and susceptibility artifacts due to the magnetic field inhomogeneity using ExploreDTI toolbox.
Connectometry analysis
Diffusion data were reconstructed in the MNI space using q-space diffeomorphic reconstruction to obtain the spin distribution function (SDF) [14]. A diffusion sampling length ratio of 1.25 was used, and the output resolution was 2 mm.
Diffusion MRI connectometry [15] was used to study the effect of quality of life (PDQ39). Connectometry is a novel approach in the analysis of diffusion MRI signals that simply tracks the difference of white matter tracts between groups, or correlation of white matter fibers with a variable of interest, by searching into subcomponents of the neural pathway. Connectometry approach extracts the SDF in a given fiber orientation as a measure of water density along that direction. Quantitative anisotropy (QA) is a diffusion index derived from spin density, i.e., SDF. QA of each fiber orientation gives the peak value of water density in that direction. A multiple regression model was used to consider the age, sex, HADS (anxiety), HADS (depression), duration of disease, UPRDS-III, Hoehn and Yahr scale, LEDD, cognitive measures and dominant side of motor symptoms as covariates in a total of 27 subjects. A t threshold of 2.5 was assigned to select local connectomes, and the local connectomes were tracked using a deterministic fiber tracking algorithm [16]. Track trimming was conducted with ten iterations. All tracks generated from bootstrap resampling were included. A length threshold of 40 mm was used to select tracks. The seeding density was 50 seeds per mm3. To estimate the false discovery rate (FDR), a total of 2000 randomized permutations were applied to the group label to obtain the null distribution of the track length. The analysis was conducted using DSI Studio (http://dsi-studio.labsolver.org), released in November 2017.
Results
Clinical measures
PD patients showed only mild motor impairment in on-drug status (UPDRS-III = 13.7 ± 6.5). Fifteen patients had unilateral motor involvement, three had unilateral plus axial involvement, eight had bilateral involvement without balance impairment, and only three had mild to moderate disability with impaired postural reflexes. The HADS score revealed only one patient with a definite diagnosis of depression [17]. None of the participants scored below the cutoff thresholds of cognitive tests to be marked as demented (Table 1).
Connectometry
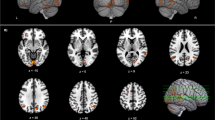
Results from multiple regression models in diffusion MRI connectometry revealed a significant positive association (FDR = 0.027) between PDQ39 scores of PD patients and white matter QA of the posterior limb of the internal capsule tracts bilaterally (Fig. 1). In other words, lower quality of life is correlated to lower connectivity in the mentioned area.
Results of diffusion MRI connectometry multiple regression analysis regarding the quality of life in patients with Parkinson’s disease, using DSI Studio software. Lower quantitative anisotropy in bilateral posterior limbs of the internal capsule is correlated with lower Parkinson’s disease questionnaire scores
To highlight whether this finding is truly bilateral or dependent on the side of the motor symptoms dominancy, we repeated the analysis considering the dominant side as the main variable. The multiple regression model did not reveal any significant association with left or right posterior limb of the internal capsule with the dominant side of the motor symptoms.
Discussion
In this study, we investigated the association between white matter microstructure and quality of life in PD patients using diffusion MRI connectometry. It was demonstrated that higher PDQ39 scores in PD patients are significantly correlated with higher QA values of the posterior limb of the internal capsule (PLIC), while controlling for age, gender, depression, anxiety, cognitive disturbances, side of the motor symptoms dominancy, duration and severity of the disease and the treatment dosages.
Internal capsule is an important subcortical structure where a high concentration of motor and sensory fibers project in a vertical manner to and from cortical areas. Besides the well-known corticospinal tract, PLIC encompasses the thalamic radiation relaying somatosensory signals to cortical levels of interoceptive processing networks [18], as well as corticofugal fibers. Structural disruption of the PLIC is implicated in executive dysfunction, attention deficit, visuospatial deficit, memory loss and cognitive impairment [10]. Disruption of PLIC is also observed in mood disturbances associated with differential emotional stimuli interpretation such as anxiety, depression and apathy, top listed in neuropsychiatric disturbances contributing to lower QoL in PD [19].
There exists high-level evidence for anxiety and depression as prodromal markers for PD. Meanwhile, hardships of measurement, the low positive likelihood of depression and uncertain lead time prior to initiation of PD discourage using depression and anxiety as prodromal PD markers [20]. Anxiety, as an entity and apart from other mood disorders such as depression and apathy, has proved the strongest indicator of poor QoL, in cognitively intact PD patients [21]. In an exploratory track-based spatial statistics study, Liao et al. demonstrated significantly reduced FA in the right posterior limb of the internal capsule in adolescent patients suffering general anxiety disorder compared to matched controls [22]. Kim and his colleagues have shown that integrity of the optic radiations emerging from the posterior thalamus passing through the posterior limb and the retrolenticular part of the internal capsule is significantly correlated with the presence and severity of anxiety sensation in PD patients [23]. Moreover, gray matter alterations occur in consort to the white matter disruptions, where Tinaz and colleagues [24] well discussed cortical atrophy in the orbitofrontal, ventrolateral prefrontal and occipitoparietal cortices and striatal volume loss in MRI of early PD patients with regard to anxiety [24]. Disruptions of the described somatosensory loop from subcortical pathways that regulate the external sensory information before they reach the cortex, including predominant sensory pathway in the PLIC, may evolve into psychomotor and psychiatric manifestations.
The pallidomesencephalic pathway, partly crossing the PLIC, is the major neural circuit of goal-directed locomotor behavior. These fibers originate from the internal globus pallidus and project onto the pedunculopontine nucleus of the midbrain and are directly implicated in loss of goal orientation and apathy in striatal strokes [25]. Apathy, defined as a reduction in interest for aimed motivations, is the core mood disturbance in PD, with a relatively high predictive value in upcoming severe executive and motor impairment. Apathy is, therefore, a predictive marker of poor QoL in PD patients [26]. Post-stroke mood symptoms are described following PLIC disruptions in the literature. In a prospective study, Starkstein and colleagues revealed a strong association between apathy in post-stroke patients and lesion in the PLIC while controlling the effect of minor and major depression. Apathetic patients in this study showed higher cognitive and physical impairments in comparison with patients without apathy [27]. Cognitive dysfunction is a comorbid feature of apathy, and the emergence of apathy in PD patients strongly predicts upcoming dementia [28]. Cognitive deterioration in advanced PD might reflect disruption of frontal cortical projections, penetrating the internal capsule to reach the subcortical regions [29]. This is in line with decreased FA seen in the internal capsule with the implication of executive dysfunction and the attention domains of cognition in PD patients [10].
Several studies have explored neural pathways in depressed PD patients. Cortico-limbic network, projections of the left amygdala to the bilateral mediodorsal thalamus, and prefrontal and posterior cingulate cortices are among the circuits shown to be related to depression and its severity in PD [29]. Similar to the other described mood disorders, depression shares many structural pathologies with other psychomotor features of neurodegenerative disorders. Frontal–striatal–thalamic and limbic–thalamic–frontal loops are shown to underlie the neurobiology of depression [30]. PLIC plays a role in the regulation of emotion, cognition and behavior encompassing parts of the fronto-limbic-striatal circuit and is implicated in major depression or post-stroke depression [31]. An increased fractional anisotropy (FA) in bilateral posterior limbs of the internal capsule in patients with early-onset major depressive disorder further supports this fact [32]. Apathy and depression have a bimodal pattern of emergence in PD patients. Thus, prodromal clinical signs of PD might point to depressive or apathetic symptoms present at the time or prior to the diagnosis of PD. These early symptoms might herald the initiation of PD by years and subside upon initiation of dopaminergic therapy and finally might reemerge later in the course of the disease [33]. Results of the mentioned studies thereby shed light over neural disruptions associated with prodromal signs rather than late ones.
Finally, PLIC plays a pivotal role in movement regulation with pyramidal and extrapyramidal motor pathways passing through [34]. Damage to the PLIC as a white matter tract comprising corticofugal fibers might mirror early disruptions in motor function and anticipate a more severe motor decline in later PD stages, as it has been linked to poor motor outcomes in PD or other movement disorders [35, 36].
Besides minor clinical signs, PD patients in this study, except one, did not hit the cutoff point for the diagnosis of anxiety or depression as measured by HADS score, nor did they have signs of severe motor impairment by the part three UPDRS score. Mild cognitive disturbances, but not any evidence of dementia, were demonstrated in these patients.
Diffusion MRI connectometry is a novel approach to investigate connectivity patterns of fiber bundles. This method empowers the analysis by using the notion of “local connectomes” and probing only the significantly associated fiber bundle to the study variable instead of pre-allocating regions or tracks unavoidably encompassing unrelated branches [15]. Furthermore, connectometry measures the density of water diffusion for any given direction of a voxel, which represents the “local connectome fingerprint” of each individual [37], reflecting its higher sensitivity and specificity than conventional diffusivity indices [15]. Association of PLIC with poor QoL in this group of PD patients may, therefore, shed light on signature tract disruptions as possible biomarkers to predict exacerbation of psychomotor and cognitive symptoms later in the course of the disease.
A limited number of patients and slight deviation toward patients with advanced motor impairments—11 patients with H and Y stage 2 or above—prevents extrapolation of these results to early Parkinson’s disease. Longitudinal studies investigating a larger number of prodromal or early PD patients with other anatomical and functional imaging techniques are needed to explore whether these results are reproducible and further explain our findings in related to motor and non-motor symptoms of PD.
Conclusions
White matter microstructural disruption of the posterior limb of the internal capsule in correlation with quality of life impairment in PD patients is in line with previous studies investigating major determinants of poor quality of life in these patients; anxiety, depression, apathy and motor impairment. Results in our sample of PD patients with relatively mild motor impairment and no apparent sign of depression/anxiety identify a unique and inexplicable association of the PLIC to the QoL in PD patients.
References
Opara JA et al (2012) Quality of life in Parkinson’s disease. J Med Life 5(4):375–381
Chaudhuri KR, Schapira AH (2009) Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol 8(5):464–474
Santos-Garcia D, de la Fuente-Fernandez R (2013) Impact of non-motor symptoms on health-related and perceived quality of life in Parkinson’s disease. J Neurol Sci 332(1–2):136–140
Scalzo P et al (2009) Depressive symptoms and perception of quality of life in Parkinson’s disease. Arq Neuropsiquiatr 67(2A):203–208
Martinez-Martin P (2011) The importance of non-motor disturbances to quality of life in Parkinson’s disease. J Neurol Sci 310(1):12–16
Montel S, Bonnet A-M, Bungener C (2009) Quality of life in relation to mood, coping strategies, and dyskinesia in Parkinson’s disease. J Geriatr Psychiatry Neurol 22(2):95–102
Elbers RG et al (2014) Impact of fatigue on health-related quality of life in patients with Parkinson’s disease: a prospective study. Clin Rehabil 28(3):300–311
Menza MA, Robertson-Hoffman DE, Bonapace AS (1993) Parkinson’s disease and anxiety: comorbidity with depression. Biol Psychiatry 34(7):465–470
Jellinger KA (2014) The pathomechanisms underlying Parkinson’s disease. Expert Rev Neurother 14(2):199–215
Hall JM et al (2016) Diffusion alterations associated with Parkinson’s disease symptomatology: a review of the literature. Parkinsonism Relat Disord 33:12–26
Ziegler E et al (2014) Mapping track density changes in nigrostriatal and extranigral pathways in Parkinson’s disease. Neuroimage 99:498–508
Hobson DE et al (2002) Excessive daytime sleepiness and sudden-onset sleep in Parkinson disease: a survey by the Canadian Movement Disorders Group. JAMA 287(4):455–463
Peto V et al (1995) The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual Life Res 4(3):241–248
Yeh F-C, Tseng W-YI (2011) NTU-90: a high angular resolution brain atlas constructed by q-space diffeomorphic reconstruction. Neuroimage 58(1):91–99
Yeh FC, Badre D, Verstynen T (2016) Connectometry: a statistical approach harnessing the analytical potential of the local connectome. Neuroimage 125:162–171
Yeh FC et al (2013) Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One 8(11):e80713
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67(6):361–370
Kim YH et al (2008) Corticospinal tract location in internal capsule of human brain: diffusion tensor tractography and functional MRI study. NeuroReport 19(8):817–820
Castrioto A et al (2016) Emotional manifestations of PD: neurobiological basis. Mov Disord 31(8):1103–1113
Postuma RB, Berg D (2016) Advances in markers of prodromal Parkinson disease. Nature Reviews Neurology 12(11):622–634
Jones JD et al (2015) Anxiety and depression are better correlates of Parkinson’s disease quality of life than apathy. J Neuropsychiatry Clin Neurosci 27(3):213–218
Liao M et al (2014) White matter abnormalities in adolescents with generalized anxiety disorder: a diffusion tensor imaging study. BMC Psychiatry 14:41
Kim MK et al (2017) White matter correlates of anxiety sensitivity in panic disorder. J Affect Disord 207:148–156
Tinaz S, Courtney MG, Stern CE (2011) Focal cortical and subcortical atrophy in early Parkinson’s disease. Mov Disord 26(3):436–441
Haber S (2016) Perspective on basal ganglia connections as described by Nauta and Mehler in 1966: where we were and how this paper effected where we are now. Brain Res 1645:4–7
Barone P et al (2009) The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord 24(11):1641–1649
van Almenkerk S et al (2015) Apathy among institutionalized stroke patients: prevalence and clinical correlates. Am J Geriatr Psychiatry 23(2):180–188
Dujardin K et al (2009) Apathy may herald cognitive decline and dementia in Parkinson’s disease. Mov Disord 24(16):2391–2397
Zheng Z et al (2014) DTI correlates of distinct cognitive impairments in Parkinson’s disease. Hum Brain Mapp 35(4):1325–1333
Seminowicz DA et al (2004) Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage 22(1):409–418
Xiao J et al (2015) Altered white matter integrity in individuals with cognitive vulnerability to depression: a tract-based spatial statistics study. Sci Rep 5:9738
Sacchet MD et al (2014) Structural abnormality of the corticospinal tract in major depressive disorder. Biol Mood Anxiety Disord 4:8–8
Schrag A, Jahanshahi M, Quinn NP (2001) What contributes to depression in Parkinson’s disease? Psychol Med 31(1):65–73
Schnitzler A et al (1997) Involvement of primary motor cortex in motor imagery: a neuromagnetic study. Neuroimage 6(3):201–208
Iseki K et al (2015) Freezing of gait and white matter changes: a tract-based spatial statistics study. J Clin Mov Disord 2:1
Wang M et al (2016) Alterations of functional and structural connectivity of freezing of gait in Parkinson’s disease. J Neurol 263(8):1583–1592
Yeh F-C et al (2016) Quantifying differences and similarities in whole-brain white matter architecture using local connectome fingerprints. PLoS Comput Biol 12(11):e1005203
Acknowledgements
This dataset was supported by the Belgian National Fund for Scientific Research, the University of Liège, the Queen Elisabeth Medical Foundation, the Léon Fredericq Foundation, the Belgian Inter-University Attraction Program, the Walloon Excellence in Life Sciences and Biotechnology program, and the Marie Curie Initial Training Network in Neurophysics (PITN-GA-2009-238593).
Author information
Authors and Affiliations
Contributions
FGS and MHA contributed to the conception and design of the study; MHA, FGS, MMZ and MH contributed to data collection and analysis. FGS and MHA contributed to writing the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed here including human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Availability of data and material
Data used in this study is accessible via: https://www.nitrc.org/ir/data/projects/parktdi.
Conflict of interests
The authors declare that they have no competing interests.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Ghazi Sherbaf, F., Mojtahed Zadeh, M., Haghshomar, M. et al. Posterior limb of the internal capsule predicts poor quality of life in patients with Parkinson’s disease: connectometry approach. Acta Neurol Belg 119, 95–100 (2019). https://doi.org/10.1007/s13760-018-0910-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-018-0910-3