Abstract
Migraine attacks increase during the perimenstrual period in approximately half of female migraineurs. There are differences in the pathogenesis and clinical features of menstrually related and non-menstrual migraine attacks. The objective of this study was to compare the characteristics of migraine in patients with menstrually related and non-menstrual migraine, and to investigate the differences between premenstrual, menstrual, and late-menstrual migraine attacks. Three-hundred and thirty-two women with migraine without aura were evaluated using questionnaires and diaries to determine the characteristics of headache, preceding and accompanying symptoms, and the relation of migraine attacks and menstruation. One-hundred and sixty-three women had menstrually related migraine without aura (49.1%). Duration of disease and duration of headache were longer (p = 0.002 and p < 0.001, respectively), and nausea, vomiting, phonophobia, and aggravation of headache with physical activity were more frequent in patients with menstrually related migraine (p = 0.005, p = 0.006, p < 0.001 and p = 0.006, respectively). Premonitory symptoms and allodynia were observed more frequently in the menstrually related migraine group (p = 0.012 and p = 0.004, respectively). Perimenstrual migraine attacks occurred premenstrually (days −2 and −1) in 46 patients (25.3%), menstrually (days 1 to 3) in 90 patients (49.4%), and late menstrually (days 4 to 7) in 19 patients (10.4%). Our results showed that the duration of headache was longer and accompanying symptoms were more frequent and diverse in patients with menstrually related migraine without aura, suggesting that these findings may reflect the increase in excitability or susceptibility of the brain in these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine has a clear relationship with female sex. The prevalence of migraine is similar in prepubertal girls and boys [1, 2], but after menarche and during reproductive years, this rate changes and the female to male ratio increases from 1:1 to 2–3:1 [2,3,4]. Approximately, half of female migraineurs experience increased incidence of attacks during the perimenstrual period [5,6,7,8].
The International Classification of Headache Disorders 3rd Edition (ICHD 3-beta) described pure menstrual migraine and menstrually related migraine in the appendix [9]. A1.1.1 Pure menstrual migraine without aura is defined as attacks of migraine without aura that occur exclusively on day 1 ± 2 of menstruation in at least two out of three menstrual cycles, and at no other times of the cycle, whereas A1.1.2 menstrually related migraine without aura is defined as migraine without aura that occurs on day 1 ± 2 of menstruation in at least two out of three menstrual cycles, and additionally at other times of the menstrual cycle.
The period that extends from 2 days before to 3 days after the onset of menstruation, which corresponds to the late luteal/early follicular phase of the menstrual cycle, is the most risky period for migraine attacks to occur [10, 11]. The abnormal neurotransmitter and neurohormonal responses triggered by estrogen-withdrawal due to the abrupt drop in serum estrogen levels during this period or abnormal release of prostaglandins from the endometrium appear to be involved in the pathophysiology of menstrually related migraine [11, 12]. Fluctuations of estrogen levels may have direct or indirect effects on serotonergic and opiatergic neurotransmitter systems, which are considered to be important regulators of the trigeminal pain pathways [12].
Perimenstrual migraine attacks have been suggested to be longer [5, 14,15,16,17], more severe [5, 13, 16, 18,19,20] and more resistant to treatment [5, 15, 16]. The objective of this study was to compare the characteristics of migraine between patients with menstrually related and non-menstrual migraine, and to investigate the differences between premenstrual, menstrual, and late-menstrual migraine attacks.
Methods
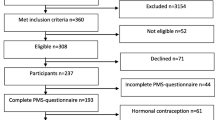
We prospectively recruited 673 consecutive women with migraine with or without aura according to ICHD III-beta criteria [9] who were followed in the headache outpatient clinic between Sept 2016 and Feb 2017. The study was conducted in face to face interview by the investigators. Patients with migraine with aura, chronic migraine with a headache frequency of ≥15 days per month, those with duration of migraine of less than 1 year, patients with primary or secondary headache other than migraine, those with other neurologic disease, patients in menopause, and patients taking hormone therapy or oral contraceptives were excluded from the study. Patients who failed to complete questionnaires and/or headache diaries were also excluded from the study.
A total of 332 women with natural menstrual cycles who were diagnosed as having migraine without aura according to the ICHD III-beta diagnostic criteria [9] were included in the study. In addition to the demographic characteristics of patients, age at onset of migraine; duration of disease frequency; duration, severity, character, and lateralization of headache; and aggravation of headache with physical activity were recorded. Premonitory symptoms (sleepiness, irritability/anxiety, depressive mood, euphoria/hyperactivity, fatigue, difficulty in concentrating, difficulty in verbal expression, repetitive yawning, blurred vision, nausea/vomiting, sensitivity to light/sound, changes in appetite, neck stiffness), nausea, vomiting, photophobia, phonophobia, cranial autonomic symptoms associated with headache, and family history of migraine were also obtained. Severity of headache was scored on a visual analog scale (0–10). The allodynia symptom checklist was used to determine whether allodynia developed during a migraine attack. The lateralization of headache was defined as unilateral, bilateral, and unilateral + bilateral. Small hyperintense lesions localized in the periventricular or deep white matter, or clinically silent infarct-like lesions identified on brain magnetic resonance imaging were recorded.
Patients were evaluated using questionnaires and headache diaries for 90 days to investigate the association of migraine attacks with menstruation. The diary included questions regarding the presence of menstruation and all headache characteristics including duration, severity, character, and lateralization of headache, preceding and accompanying symptoms. Diaries were collected and reviewed 30, 60, and 90 days after the initial enrollment, and if possible corrected. If migraine attacks occurred in at least 2 out of 3 menstrual cycles, the attacks were considered menstrually related. Those with migraine attacks only in the menstrual period were evaluated as pure menstrual migraine without aura, those with headache in the menstrual period as well as other periods of the menstrual cycle were evaluated as menstrually related migraine without aura, and those without headache in the menstrual period were evaluated as non-menstrual migraine without aura. Menstrual migraine attacks of the patients with menstrually related migraine were compared as premenstrual (defined as days −2 and −1 of menstruation), and menstrual (defined as days 1–3 of menstruation). Although the ICHD 3-beta classification of days −2 to +3 of menstruation was taken as the basis, the menstrual period was extended to day 7 when menstrual bleeding persisted in women who had migraine attacks in this period and were classified as late menstrual (defined as days 4–7 of menstruation). The characteristics of premenstrual, menstrual and late-menstrual groups were compared.
The study was carried out according to the Helsinki Declaration and was approved by the Institutional Ethics Committee. All patients participating in the study provided written informed consent.
Statistical analysis
Statistical analysis was performed using statistical software (Statistical package for the Social Sciences, version 22.0 for Windows, SPSS Inc., Chicago, IL). The distributions of continuous variables were evaluated for normal behavior with Kolmogorov-Smirnov test. Descriptive statistics for continuous variables were expressed as mean ± SD. The number of cases and percentages was used for categorical data. While the differences were not normally distributed, data between two independent group were compared by Mann–Whitney U test, otherwise Kruskal–Wallis test was applied for the comparisons among more than two independent groups. Categorical data were analyzed by Pearson’s Chi-square test. Statistical significance was defined as p < 0.05.
Results
Three-hundred and thirty-two women with migraine without aura were included in the study. Of the 673 patients, 309 were not appropriate for evaluation and, thus, excluded from the study (248 with migraine with aura, 18 with accompanying another headache disorder, 44 with other criteria above mentioned, 31 with inadequate questionnaires and/or headache diaries). The mean age of the patients was 35.3 ± 9.3 years, migraine duration was 9.9 ± 8.6 years, age at onset of migraine was 25.4 ± 8.6 years, frequency of headache was 5.1 ± 3.8 per month, duration of headache was 31.8 ± 22.6 h, and severity of headache was 7.6 ± 1.5.
One-hundred and sixty-three (49.1%) patients had menstrually related migraine without aura, 142 (42.8%) had non-menstrual migraine without aura, 8 (2.4%) had pure menstrual migraine without aura and 19 patients had late-menstrual migraine attacks (5.7%).
In patients with menstrually related migraine, the duration of disease and duration of headache were longer compared to patients who had non-menstrual migraine (p = 0.002 and p < 0.001, respectively). In patients with menstrually related migraine, nausea (p = 0.005), vomiting (p = 0.006), phonophobia (p < 0.001) associated with headache, and aggravation of headache with physical activity (p = 0.006) were more frequent compared to patients who had non-menstrual migraine. In the menstrually related migraine group, the number of patients with premonitory symptoms was higher (p = 0.012) and allodynia developed more frequently during the attack in this group (p = 0.004) (Table 1).
Eight patients had pure menstrual migraine without aura. The mean age of the patients was 39.1 ± 6.2 years, migraine duration was 6.7 ± 4.8 years, age at onset of migraine was 32.4 ± 8.2 years, duration of headache was 32.2 ± 25.7 h, and severity of headache was 7.7 ± 1.2. Of the eight patients, six had nausea, four had vomiting, six had photophobia, seven had phonophobia, three had cranial autonomic symptoms, one had allodynia and four had premonitory symptoms during migraine attacks.
Perimenstrual migraine attacks occurred during the premenstrual period (days −2 and −1) in 46 patients (25.3%), during the menstrual period (days 1–3) in 90 patients (49.4%), and during the late-menstrual period (days 4–7) in 19 patients (10.4%). No definitive distinction could be made in 27 patients having migraine attacks both in premenstrual and menstrual period and these patients were not included in the evaluation. With the exception of vomiting, which was more frequent in the menstrual group than the premenstrual group (p < 0.001), there were no differences in the clinical features of migraine among 3 groups (Table 2).
Discussion
In the present study, 49.1% of the patients had menstrually related migraine without aura and 2.4% had pure menstrual migraine without aura. Previous studies reported that menstrually related migraine without aura was seen in 40–70% of female patients with migraine [5,6,7, 10, 21, 22]. The prevalence of pure menstrual migraine without aura ranged from 5 to 12% [10, 20,21,22]. Perimenstrual migraine attacks have been reported to be longer [5, 14,15,16,17], more severe [5, 13, 16, 18,19,20], and more resistant to treatment [5, 15, 16]. However, no difference between perimenstrual attacks and non-menstrual attacks was found in some studies [23,24,25]. It has been suggested that the reported differences in prevalence and clinical characteristics may be due to the method of evaluation of the attacks and their association with menstruation, or because the studies were conducted with population-based patients or patients selected from tertiary clinics [26].
In our study, the duration of headache was longer in patients with menstrually related migraine compared with patients who had non-menstrual migraine. Long headache duration may be associated with a more pronounced suppression of inhibitory neurotransmitter systems responsible for the modulation of pain, along with a decrease in estrogen levels during menstruation [12]; it may also be explained by the fact that the attacks in this period are more unresponsive to terminating therapies.
In our study, allodynia during attacks was more frequent in patients with menstrually related migraine. This result suggested that hormonal factors may have a facilitating effect on the development of central sensitization. Menstrually related migraine has been reported to be more frequent than non-menstrual migraine in patients who develop cutaneous allodynia during migraine attacks [27, 28]. Gonadal hormones affect central pain modulation, which causes changes in the pain threshold of trigeminal neurons. In a study where trigeminal sensitization was induced by intradermal capsaicin injection, the area of allodynia in female subjects was found wider, more so in the menstrual phase than in the luteal phase [29]. In our study, headache was found aggravated by physical activity in a larger number of patients in the menstrually related migraine group than in the non-menstrual migraine group. This result may be related to the fact that peripheral sensitization that developed during the migraine attack was stronger in patients with menstrually related migraine. The increase of prostaglandins in the perimenstrual period, which are peripheral mediators of pain, may be responsible for this. It has been suggested that prostaglandins released from the endometrium during the perimenstrual period may play a role in the development of migraine attacks [12].
In our study, nausea, vomiting, and phonophobia were more frequently associated with headache in patients with menstrually related migraine. Likewise, some studies reported that nausea and vomiting [13, 17, 19], and phonophobia and photophobia [22] were more frequent in menstrually related migraine attacks. In other studies, no differences were found between menstrually related and non-menstrual migraine attacks in terms of symptoms associated with headache [15, 16, 19, 21]. Activation of the nucleus tractus solitarius neurons, which receive input from the trigeminal nucleus caudalis, is one of the possible mechanisms responsible for the development of nausea and vomiting during migraine attacks [30,31,32]. The reason for the more frequent occurrence of nausea and vomiting during headache in menstrually related migraineurs, like in allodynia, may be central sensitization of the trigeminal nucleus caudalis neurons. On the other hand, the likely presence of nausea and vomiting also in the premonitory period in which hypothalamic activity is shown in migraineurs [33] points toward a role of the hypothalamus in the development of nausea and vomiting during migraine attacks owing to the links revealed between the nucleus tractus solitarius and the hypothalamus [34]. Given that the hypothalamus has an important role in maintaining hormonal and autonomic functions, we thought that the more frequent occurrence of nausea and vomiting in patients with menstrually related migraine may also be related to the hypothalamic activation developed during migraine attacks. Furthermore, the more frequent presence of premonitory symptoms in patients with menstrually related migraine compared with patients who had non-menstrual migraine in our study may indicate the presence of a stronger hypothalamic activation in this patient group.
In the present study, menstrual migraine attacks occurred in the first 3 days of menstruation in half of the patients with menstrually related migraine. There was no difference in the clinical characteristics of migraine except for the more frequent occurrence of vomiting in patients with migraine attacks on days 1–3 of menstruation compared with those with premenstrual attacks. Previous studies also reported that migraine attacks were more frequent in the first 3 days of the menstrual period [13, 19, 35, 36]. Only one study compared premenstrual, menstrual, and late-menstrual migraine attacks, and found no difference between them [15].
Our results showed that the duration of headache was longer and the prevalence and diversity of accompanying symptoms were more frequent in patients with menstrually related migraine without aura. These findings may reflect the characteristics of menstrual migraine attacks, as well as the differences of clinical features between patients with menstrually related and non-menstrual migraine. It is still unclear why some migraineurs have attacks triggered by menstruation and others do not. We thought that the increase in excitability of the brain in some patients with migraine may be more pronounced, and attacks triggered by hormonal changes during the menstrual period may be regarded as a part of this condition.
References
Bille B (1962) Migraine in school children. A study of the incidence and short-term prognosis, and a clinical, psychological and electroencephalographic comparison between children with migraine and matched controls. Acta Paedatr Suppl 136:1–151
Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB (2010) Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia 30(9):1065–1072
Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M (2001) Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 41(7):646–657
Stewart WF, Wood C, Reed ML, Roy J, Lipton RB, AMPP Advisory Group (2008) Cumulative lifetime migraine incidence in women and men. Cephalalgia 28(11):1170–1178
Couturier EG, Bomhof MA, Neven AK, van Duijn NP (2003) Menstrual migraine in a representative Dutch population sample: prevalence, disability and treatment. Cephalalgia 23(4):302–308
Granella F, Sances G, Pucci E, Nappi RE, Ghiotto N, Napp G (2000) Migraine with aura and reproductive life events: a case control study. Cephalalgia 20(8):701–707
Wöber C, Holzhammer J, Zeitlhofer J, Wessely P, Wöber-Bingöl C (2006) Trigger factors of migraine and tension-type headache: experience and knowledge of the patients. J Headache Pain 7(4):188–195
MacGregor EA (2012) Classification of perimenstrual headache: clinical relevance. Curr Pain Headache Rep 16(5):452–460
Headache Classification Committee of the International Headache Society (IHS) (2013) The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 33(9):629–808
MacGregor EA, Chia H, Vohrah RC, Wilkinson M (1990) Migraine and menstruation: a pilot study. Cephalalgia 10(6):305–310
Somerville BW (1972) The role of estradiol withdrawal in the etiology of menstrual migraine. Neurology 22(4):355–365
Martin VT, Behbehani M (2006) Ovarian hormones and migraine headache: understanding mechanisms and pathogenesis—part 2. Headache 46(3):365–386
MacGregor EA, Hackshaw A (2004) Prevalence of migraine on each day of the natural menstrual cycle. Neurology 63(2):351–353
MacGregor EA, Victor TW, Hu X, Xiang Q, Puenpatom RA, Chen W, Campbell JC (2010) Characteristics of menstrual vs nonmenstrual migraine: a post hoc, within-woman analysis of the usual-care phase of a nonrandomized menstrual migraine clinical trial. Headache 50(4):528–538
Granella F, Sances G, Allais G, Nappi RE, Tirelli A, Benedetto C, Brundu B, Facchinetti F, Nappi G (2004) Characteristics of menstrual and nonmenstrual attacks in women with menstrually related migraine referred to headache centres. Cephalalgia 24(9):707–716
Pinkerman B, Holroyd K (2010) Menstrual and nonmenstrual migraines differ in women with menstrually-related migraine. Cephalalgia 30(10):1187–1194
Vetvik KG, Benth JŠ, MacGregor EA, Lundqvist C, Russell MB (2015) Menstrual versus non-menstrual attacks of migraine without aura in women with and without menstrual migraine. Cephalalgia 35(14):1261–1268
Martin VT, Wernke S, Mandell K, Ramadan N, Kao L, Bean J, Liu J, Zoma W, Rebar R (2005) Defining the relationship between ovarian hormones and migraine headache. Headache 45(9):1190–1201
MacGregor EA, Frith A, Ellis J, Aspinall L, Hackshaw A (2006) Incidence of migraine relative to menstrual cycle phases of rising and falling estrogen. Neurology 67(12):2154–2158
Pavlović JM, Stewart WF, Bruce CA, Gorman JA, Sun H, Buse DC, Lipton RB (2015) Burden of migraine related to menses: results from the AMPP study. J Headache Pain 16:24
Granella F, Sances G, Zanferrari C, Costa A, Martignoni E, Manzoni GC (1993) Migraine without aura and reproductive life events: a clinical epidemiological study in 1300 women. Headache 33(7):385–389
Dzoljic E, Sipetic S, Vlajinac H, Marinkovic J, Brzakovic B, Pokrajac M, Kostic V (2002) Prevalence of menstrually related migraine and nonmigraine primary headache in female students of Belgrade University. Headache 42(3):185–193
Stewart WF, Lipton RB, Chee E, Sawyer J, Silberstein SD (2000) Menstrual cycle and headache in a population sample of migraineurs. Neurology 55(10):1517–1523
Silberstein SD, Massiou H, McCarroll KA, Lines CR (2002) Further evaluation of rizatriptan in menstrual migraine: retrospective analysis of long-term data. Headache 42(9):917–923
Diamond ML, Cady RK, Mao L, Biondi DM, Finlayson G, Greenberg SJ, Wright P (2008) Characteristics of migraine attacks and responses to almotriptan treatment: a comparison of menstrually related and nonmenstrually related migraines. Headache 48(2):248–258
Vetvik KG, Russell MB (2011) Are menstrual and nonmenstrual migraine attacks different? Curr Pain Headache Rep 15(5):339–342
Güven H, Çilliler AE, Çomoğlu SS (2013) Cutaneous allodynia in patients with episodic migraine. Neurol Sci 34(8):1397–1402
Baykan B, Ekizoglu E, Karli N, Kocasoy-Orhan E, Zarifoglu M, Saip S, Siva A, Ertas M (2016) Characterization of migraineurs having allodynia: results of a large population-based study. Clin J Pain 32(7):631–635
Gazerani P, Andersen OK, Arendt-Nielsen L (2005) A human experimental capsaicin model for trigeminal sensitization. Gender-specific differences. Pain 118(1–2):155–163
Kaube H, Keay KA, Hoskin KL, Bandler R, Goadsby PJ (1993) Expression of c-Fos-like immunoreactivity in the caudal medulla and upper cervical spinal cord following stimulation of the superior sagittal sinus in the cat. Brain Res 629(1):95–102
Ruggiero DA, Underwood MD, Mann JJ, Anwar M, Arango V (2000) The human nucleus of the solitary tract: visceral pathways revealed with an “in vitro” postmortem tracing method. J Auton Nerv Syst 79(2–3):181–190
Kelman L, Tanis D (2006) The relationship between migraine pain and other associated symptoms. Cephalalgia 26(5):548–553
Maniyar FH, Sprenger T, Monteith T, Schankin C, Goadsby PJ (2014) Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain 137(Pt 1):232–241
Kannan H, Yamashita H (1985) Connections of neurons in the region of the nucleus tractus solitarius with the hypothalamic paraventricular nucleus: their possible involvement in neural control of the cardiovascular system in rats. Brain Res 329(1–2):205–212
Johannes CB, Linet MS, Stewart WF, Celentano DD, Lipton RB, Szklo M (1995) Relationship of headache to phase of the menstrual cycle among young women: a daily diary study. Neurology 45(6):1076–1082
Wöber C, Brannath W, Schmidt K, Kapitan M, Rudel E, Wessely P, Wöber-Bingöl C, PAMINA Study Group (2007) Prospective analysis of factors related to migraine attacks: the PAMINA study. Cephalalgia 27(4):304–314
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Ethics approval
The study was carried out according to 1964 Helsinki Declaration and was approved by the Institutional Ethics Committee.
Informed consent
All patients participating in the study provided informed consent.
Rights and permissions
About this article
Cite this article
Güven, B., Güven, H. & Çomoğlu, S. Clinical characteristics of menstrually related and non-menstrual migraine. Acta Neurol Belg 117, 671–676 (2017). https://doi.org/10.1007/s13760-017-0802-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-017-0802-y