Abstract
Electroencephalographic abnormalities may occur in autistic spectrum disorders (ASD) even in the absence of clinical seizures. These abnormalities may vary from nonspecific changes to epileptiform abnormalities and are more common compared to the overall population. The level of intelligence is a significant risk factor for epilepsy in ASD. However, the relation between the functionality of the individuals with autism and the electroencephalographic (EEG) abnormalities, and the clinical significance of these abnormalities still remain relatively unclear. In this study we investigated the presence of EEG abnormalities in sixteen children diagnosed with high-functioning ASD. EEG recording was performed for at least 2 h and included at least 90 min of sleep activity. While none of the patients had clinical seizures, 5 patients (31.3%) were detected to have EEG abnormalities. Four of these were epileptiform (25%), and one patient developed seizure during follow-up. Our results support the fact that EEG abnormalities are observed at a higher rate also in ASD with a better functionality. The potential impact of EEG abnormalities on cognition and behavior, and the risk of epilepsy should be considered during long-term follow-up of these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Presenting an increasing prevalence rate in the recent years, autism is a highly common childhood disorder [1]. Epilepsy is a significant factor, which increases mortality and morbidity in autism [2]. Electroencephalographic (EEG) abnormalities may occur in autism also without the presence of clinical seizures. Paroxysmal EEG abnormalities in ASD have been reported at rates, ranging between 6 and 60% [3,4,5,6,7]. These changes have a spectrum, ranging from nonspecific changes such as bioelectrical slowing or asymmetry to epileptiform changes. They are more common compared to the population [8]. While seizures are more common among those with a low IQ level, the correlation between EEG abnormalities and level of intelligence has been studied less [3]. However, EEG abnormalities are still higher in ASD with normal intelligence level and better functioning compared to the population [9]. Here the question arises if these abnormalities increase the risk of developing seizures; and if they have a potential impact on cognition, social communication, and behavior additionally [10].
In this study, we investigated the presence of EEG abnormalities in a group of patients diagnosed with high-functioning ASD and observed the presence of seizures in long-term follow-up.
Patients and methods
Sixteen children (14 males and 2 females), within the age range of 7–17, were included in the study. Autism was diagnosed based on clinical interview and DSM-IV criteria. The Childhood Autism Rating Scale (CARS) was used to grade the severity of autism and children with a score between 30 and 36 (mild to moderate autism) were included in the study. The level of functioning was evaluated with Wechsler Intelligence Scale for Children-Revised (WISC-R) and functional status of the child. Children, who had a WISC-R performance score above 65 and/or children, who attended a regular school, had relatively good language skills and communicative abilities and who had no dysmorphic findings were evaluated as high functioning. Informed consent was obtained from the families of all the participants.
The patients underwent sleep deprived video-EEG-monitoring with scalp electrodes placed according to the 10/20 system. Duration of the EEG records was at least 2 h and included at least 90 min of sleep activity. Data assessed during EEG recording included background activity, topographic distribution of the background activity, changes by activation methods, phasic elements of sleep, focal or diffuse slow activity, focal or diffuse suppression, fast activity, periodic patterns and epileptogenic activities. Epileptogenic activities included spikes, spike and waves, multiple spikes and multiple spike and waves. They are further classified either focal or generalized. Focal ones are subgrouped according to the electrode position, which may be frontal (F), centrotemporal (CT), central (C), posterior temporal, parietal or occipital (PTO).
Results
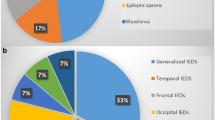
The mean age of the patients was 11.8 ± 3.5 years; mean age at diagnosis was 3.5 ± 1.4 years. While none of the patients had clinical seizures, 5 patients (31.3%) had EEG abnormalities. One abnormality was fast activity in both frontal regions, which was not evaluated as epileptic. Four of the EEG’s showed epileptiform abnormality (25%): three of them were focal and one was generalized. Three of the epileptiform EEG abnormalities were observed only during sleep. One EEG showed abnormality during only wakefulness, which was a generalized pattern. The EEG features of the patients are summarized in Table 1.
There was no significant difference between the neurological examinations of subjects with normal EEGs and of subjects with EEG abnormalities. All patients had clinical data based on 5 years of follow-up. The patients with normal EEG did not have any seizure during follow-up. Four patients with EEG abnormality didn’t also have any seizures or worsened clinically. However, patient 1, who had frontal fast activity at the age of 14, began to have seizures at the age of 17. He had generalized tonic–clonic seizures lasting 3–4 min and with postictal confusion. They occurred both awake and during sleep. Following EEGs showed spike and wave activity at left frontotemporal region during wakefulness and spike-multiple spike and wave activity at right frontotemporal regions during sleep. His seizures were controlled with carbamazepine.
Discussion
In our study, we looked for EEG abnormalities in a group of patients with high-functioning ASD and found epileptiform abnormalities in four patients, which correspond to 25% of the patients. During 5-year follow-up, one patient developed seizures.
The relationship between epilepsy and autism is well established [9,10,11]. Various studies report epilepsy prevalence in ASD, ranging between 5 and 46%, depending on the sample being studied [2,3,4,5,6,7,8]. EEG abnormalities may occur in autism also without the presence of clinical seizures at rates ranging between 6 and 60% [3,4,5,6,7,8,9]. Whether the study group includes patients with seizures or not, determines partly the variation between the rates of what. Moreover, sleep records, particularly overnight sleep, tend to show abnormalities in EEG more often [12]. However, the rates are still higher compared to healthy children. As a matter of fact, paroxysmal EEG changes may occur also in healthy children at a rate of 1–3.5% [10, 13]. Indeed, our results displayed that EEG abnormalities occurred at a higher rate in ASD compared to that in typically developing children, and were in compliance with the prevalence limits, reported in the literature. However, our study group is a sample group and too small to predict an exact prevalence.
Of the four patients with EEG abnormalities, findings were detected in the parietal region in two, in the bilateral frontal region in one patient and one patient had diffuse asymmetrical discharges. There is no common established pattern of localization among the studies, describing EEG abnormalities. Various localizations were reported; including focal, multifocal, generalized, unilateral and bilateral [5, 6, 8, 14]. A number of studies also reported temporal [7, 15], centrotemporal [4] or frontal [16] localizations. Different findings in four patients in our study involved both focal and generalized characteristics.
Three of our patients had EEG abnormality either only during sleep or they tend to increase during sleep. However, our records included only a 90 min of sleep, which is a limitation of our study. Overnight records may give more precise results.
While a lower level of functioning is more clearly known to be associated with increased epilepsy prevalence in autism [17], the mechanism of the correlation between IQ and the EEG changes in autism remains relatively unclear. There are studies demonstrating [4, 16] and not demonstrating [15] a lower IQ level in patients with EEG abnormalities.
The potential impact of these EEG abnormalities is unclear. It is difficult to demonstrate that epileptiform abnormality causes cognitive social and behavioral impairment [18]. However, there is a hypothesis that epileptiform activity may disturb synaptic plasticity cortical networks, which may lead to cognitive and behavioral problems [18]. And it was previously reported by several studies that autistic symptoms may be more severe in patients with epileptiform abnormalities. In a recent report, EEG abnormalities have been found to be associated with the stereotypes and aggressive behavior [9]. So, the potential effect of these abnormalities should be kept in mind during evaluation of children with ASD and epileptiform discharges. The type of EEG abnormality is also important. Many studies do not differentiate between different types of EEG abnormalities [10]. The abnormalities in EEG may lead to subtle problems in cognition, learning and communicative process and interfere with the educational process. Indeed, a detailed neuropsychological assessment and a comprehensive neuropsychiatric evaluation of mental and behavioral status may display several abnormalities that may be of clinical significance in the long-term prognosis. The question if these EEG abnormalities should be treated or not, is the subject of a more comprehensive research [18].
Another significance of these EEG abnormalities is their potential relation to the risk of developing seizures. One of our patients began to have seizures 3 years after EEG was performed. There were no provocative factors for the onset of seizures. There is no prospective study looking at the risk of developing seizures in ASD. And there is no routine recommendation of EEG for ASD without seizures. Here the type of the EEG abnormality may also be important, because some abnormalities may be nonspecific such as slowing, and in contrast some may show epileptogenicity such as spike and sharp waves [10]. So, it may be important to consider the risk of developing seizure any time during long-term follow-up of a patient with abnormal EEG.
In conclusion, EEG abnormalities may be observed also in ASD patients with a good level of functioning. Although there are still unanswered questions about their clinical significance, potential impact on cognition and behavior as well as the risk of epilepsy should be considered during long-term follow-up of these patients.
References
Van Naarden Braun K, Christensen D, Doernberg N, Schieve L, Rice C, Wiggins L et al (2015) Trends in the prevalence of autism spectrum disorder, cerebral palsy, hearing loss, intellectual disability, and vision impairment, metropolitan Atlanta, 1991–2010. PLoS One 10:e0124120. doi:10.1371/journal.pone.0124120
Tuchman R, Cuccaro M (2011) Epilepsy and autism: neurodevelopmental perspective. Curr Neurol Neurosci Rep 11:428–434
Volkmar FR, Nelson DS (1990) Seizure disorders in autism. J Am Acad Child Adolesc Psychiatry 29:127–129
Tuchman RF, Rapin I (1997) Regression in pervasive developmental disorders: seizures and epileptiform electroencephalogram correlates. Pediatrics 99:560–566
Rossi PG, Posar A, Parmeggiani A (2000) Epilepsy in adolescents and young adults with autistic disorder. Brain Dev 22:102–106
Canitano R, Luchetti A, Zappella M (2005) Epilepsy, electroencephalographic abnormalities, and regression in children with autism. J Child Neurol 20:27–31
Chez MG, Chang M, Krasne V, Coughlan C, Kominsky M, Schwartz A (2006) Frequency of epileptiform EEG abnormalities in a sequential screening of autistic patients with no known clinical epilepsy from 1996 to 2005. Epilepsy Behav 8:267–271
Spence SJ, Schneider MT (2009) The role of epilepsy and epileptiform EEGs in autism spectrum disorders. Pediatr Res 65:599–606
Mulligan CK, Trauner DA (2014) Incidence and behavioral correlates of epileptiform abnormalities in autism spectrum disorders. J Autism Dev Disord 44:452–458
El Achkar CM, Spence SJ (2015) Clinical characteristics of children and young adults with co-occurring autism spectrum disorder and epilepsy. Epilepsy Behav 47:183–190
Jokiranta E, Sourander A, Suominen A, Timonen-Soivio L, Brown AS, Sillanpää M (2014) Epilepsy among children and adolescents with autism spectrum disorders: a population-based study. J Autism Dev Disord 44:2547–2557
Ghacibeh GA, Fields C (2015) Interictal epileptiform activity and autism. Epilepsy Behav 47:158–162
Capdevila OS, Dayyat E, Kheirandish-Gozal L, Gozal D (2008) Prevalence of epileptiform activity in healthy children during sleep. Sleep Med 9:303–309
Hara H (2007) Autism and epilepsy: a retrospective follow-up study. Brain Dev 29:486–490
Baird G, Robinson RO, Boyd S, Charman T (2006) Sleep electroencephalograms in young children with autism with and without regression. Dev Med Child Neurol 48:604–608
Yasuhara A (2010) Correlation between EEG abnormalities and symptoms of autism spectrum disorder (ASD). Brain Dev 32:791–798
Amiet C, Gourfinkel-An I, Bouzamondo A, Tordjman S, Baulac M, Lechat P et al (2008) Epilepsy in autism is associated with intellectual disability and gender: evidence from a meta-analysis. Biol Psychiatry 64:577–582
Trauner DA (2015) Behavioral correlates of epileptiform abnormalities in autism. Epilepsy Behav 47:163–166
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any conflict of interest to disclose.
Ethical standards
This study was approved by the ethical committee of Istanbul University Cerrahpasa Faculty of Medicine and it was in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the families of all the participants.
Rights and permissions
About this article
Cite this article
Ertürk Çetin, Ö., Korkmaz, B., Alev, G. et al. EEG abnormalities and long term seizure outcome in high functioning autism. Acta Neurol Belg 117, 729–732 (2017). https://doi.org/10.1007/s13760-017-0785-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-017-0785-8