Abstract
Visual system pathway dysfunction has been postulated in migraineurs. We wanted to investigate if any difference exists interictally in visual attention and visual evoked habituation of frequently attacked migraineurs compared to the healthy control group. The effects of 3-month prophylactic migraine treatment on these parameters were also assessed. The migraineurs at headache-free interval (n = 52) and age, sex-matched healthy controls (n = 35) were compared by habituation response to 10 blocks of repetitive pattern-reversal visual stimuli (each block consisted 100 responses). The amplitude changes of 5th and 10th blocks were further compared with that of block 1 to assess the response of habituation (i.e., decrease) or potentiation (i.e., increase). The level of sustained visual attention was assessed by Cancellation test. Migraineurs were randomized to three different preventive treatments: propranolol 40 mg tid, flunarizine 5 mg bid, or topiramate 50 mg bid. After 3 months of preventive treatment, migraineurs data were compared with their baseline values. The groups did not differ by sex and age. In electrophysiological studies, the habituation ability observed in the healthy group was not observed in migraineurs. However, it was restored 3 months after preventive treatment. In migraineurs, compared to their baseline values, the distorted visual attention parameters also improved after treatment. All drugs were effective. The loss of habituation ability and low visual attention performance in migraineurs can be restored by migraine preventive treatment. This electrophysiological study accompanied by neuropsychological test may aid an objective and quantitative assessment tool for understanding migraine pathophysiology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine is one of the most common and burdensome primary headache disorder [1]. Headache is accompanied by nausea, vomiting, and light/sound sensitivity, and increased by routine physical activity [2]. Research on investigating the pathophysiological mechanisms of migraine is still one of the hottest topics. During an attack, photophobia is reported in 82.5% of the migraineurs [3]. Anatomy and physiology of photophobia have been reviewed in detail [4]. Basically, “visual system pathway dysfunction” has been postulated in migraineurs [5, 6]. Compared to the healthy control, the brains of migraineurs are reported to be hyperresponsive, especially in the occipital cortex, demonstrated by functional MRI studies [7]. Besides, light or glare is known to trigger migraine attacks. Similarly, among the aura symptoms, visual aura is reported to be the most common type of aura compared to the speech/language disturbances, sensory, motor, brainstem, or monocular visual loss [8]. On electrophysiological studies, pattern-reversal repetitive visual evoked potential (rVEP) dominates in migraineurs, supported by the evidence of lack of habituation to the repeated stimuli, interictally [9]. In migraineurs, increased responsiveness in the visual cortex is reported [10].
Some migraineurs report transient cognitive dysfunctions such as concentration and comprehension difficulties during or shortly after an attack [11]. Among the migraineurs with frequent attacks and long history of migraine, disturbances of memory, attention, and low speed of visuomotor processing are reported [12]. However, due to methodological differences, there are some controversial reports about cognitive functioning in migraineurs, either poor or normal cognitive performance [13]. The migraine severity and duration may also be confounding factors for this discrepancy. Children with migraine are reported to have visual attention deficits [14]. Visual attention may be assessed by Cancellation Test [15]. It measures the capacity of sustained attention, the sensory component related with perceptual representations, and motor component related with visual search/scan [15]. The structure of the test includes factors that are labeled as visual search, scan, impulsivity, and response speed.
In this study, we aimed to investigate if any difference exists, interictally, in visual attention and visual habituation of frequently attacked migraineurs compared to the healthy control group. The effects of migraine preventive drugs on these parameters were also assessed.
Methods
Participants
Female and males aged between 18 and 60 years, who had an adequate level of understanding the questionnaires with written consent, were enrolled prospectively between July, 2009 and July, 2011. The Local Ethical Committee approved the study. The migraine group composed of headache patients admitted to Neurology Department who were diagnosed with migraine based on ICHD-II criteria [16]. The inclusion criteria were as follows: to have >4 migraine attacks/months for more than 3 months, each attack severity of VAS ≥ 3/10 (in a scale of 0–10, in which “0” is no pain and “10” worst pain imaginable), eligible to take prophylactic treatment either with propranolol, flunarizine, or topiramate. The control group composed of age- and sex-matched healthy volunteers. Exclusion criteria for the participants were as follows: Migraine patients currently taking or have received any medications ≤3 months ago for migraine prophylaxis; patients who have any contraindication to take the study drugs; patients having any type of headaches other than migraine; patients with any type of painful conditions, the presence of any systemic, metabolic, endocrinologic, neurologic, or psychiatric disease; patients taking any type of drugs which may affect cognitive and electrophysiological studies, history of optic neuritis, or any pathology of the visual system, pregnancy, history of substance/drug abuse, history of neurosurgery. Migraine history, neurological examination, laboratory evaluations, and when needed neuroradiological examinations were performed. All participants had a baseline VEP and Cancellation test interictally (headache-free interval, at least 3 days before or after a migraine attack). Migraine patients were randomized into 3 subgroups; propranolol 40 mg tid, flunarizine 5 mg bid, and topiramate 50 mg bid. Baseline and 3 months after prophylactic treatment, pattern-reversal repetitive VEP recordings, and Cancellation test were performed.
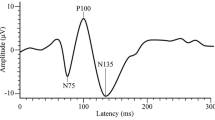
The repetitive pattern-reversal VEP (rVEP) Recording Method
The subjects were asked to focus on the red spot in the center of the checkerboard, with one eye closed. Medelec Synergy EMG/EP unit (MEDELEC Synergy, USA) was used for VEP recordings. The sweep speed was 250 ms, filters 1–100 Hz, sensitivity 5 μV, and contrast 100%. In a standard VEP recording protocol, a minimum of 2 VEP waves are averaged by 200 stimuli recorded from Oz–Cz electrodes. For the repetitive pattern-reversal VEP technique, the responses to uninterrupted visual stimuli at 3.1 Hz were recorded during 10 sequential blocks. Each block consisted of 100 averaged VEP responses. The peak-to-peak amplitudes of N1-P1 and P1-N2 peaks were calculated [10]. The amplitude changes of 5th and 10th blocks were further compared with that of block 1 to assess the response of habituation (i.e., decrease) or potentiation (i.e., increase) as described before [17]. All data were analyzed offline, blinded to the study groups.
The Cancellation test
The Cancellation test (CT) involves searching and scanning for target letter or symbols against a background of distractor [15]. The test has four sheets: organized letters, organized symbols, random letters, and random symbols (Fig. 1). In organized sets, the distribution of stimuli arranged in rows and columns follows a sequence. In the random sets, stimuli are distributed randomly on sheets without any logical sequence. Each set contains 60 target stimuli (15 targets located in each quadrant) embedded in 300 items. Subjects are required to circle the target stimuli. After every 10 correct responses, the colored pencil is changed. The test was performed at hospital by one the authors (FI). The total scores for correct target responses (CT1), number of omission errors (CT2), number of incorrect responses (CT3), total number of errors (CT4), and time to complete/duration (CT5) were calculated. The test–retest reliability coefficients of CT scores in healthy adults changed between 0.32 and 0.81 [18]. The data were analyzed by an experienced clinical psychologist (EEB) who was blinded to the groups.
Statistical analysis
The data were analyzed with the PASW Statistics version 18 software package. The normal distribution of variables was verified by the Kolmogorov–Smirnov test and Shapiro–Wilk test. Comparisons of the dependent groups were made either with Student’s t test (parametric) or Wilcoxon signed-rank test (non-parametric). Comparisons of independent groups were made either with Student’s t test (parametric) or Mann–Whitney U test (non-parametric). Chi-square (X 2) test was used to investigate whether distributions of categorical variables differ within groups. Data are shown as mean ± SD for continuous variables and absolute numbers for dichotomous variables. p value less than 0.05 was considered statistically significant.
Results
In this study, 87 subjects were eligible. Age (35.6 ± 9.1 versus 34.2 ± 9.6 years) and sex (83 versus 77% female) did not differ between the migraineurs (n = 52) and healthy control group (n = 35) (p > 0.05). Four migraineurs were diagnosed with migraine with aura. The migraineurs had 7.6 ± 4.3 attacks/month and disease duration was 6.9 ± 5.8 years. For prophylaxis, the migraineurs were randomized to flunarizine (n = 16), propranolol (n = 19), and topiramate (n = 17) treatment groups. The migraine attack frequency and disease duration did not differ between the treatment groups (all p > 0.05).
In migraineurs, sustained visual attention parameters, assessed by the Cancellation test, were statistically distorted compared to the healthy control group (Table 1a). Compared to the healthy control group, before preventive treatment, the number of correct targets was less (59.33 ± 0.63 versus 58.53 ± 1.28 p < 0.05), omission errors were more (0.67 ± 0.63 versus 1.46 ± 1.28, p < 0.05), total number of errors were more (0.69 ± 0.69 versus 1.62 ± 1.38 p < 0.05), and time to complete/duration of the tests were longer (80.36 ± 17.16 versus 105.3 ± 44.06 p < 0.05). These results suggest attention deficit and prolonged reaction time in frequently attacked migraineurs compared to the age- and sex-matched healthy control group.
Three months after preventive treatment, compared to their baseline values, visual attention parameters in migraineurs improved. The number of migraineurs completed the study was less than recruited due to loss of follow-up. However, the comparisons were made according to the pre- and post-treatment data of the same group of patients who completed the study. Basically, in migraineurs, compared to their baseline values, 3 months after migraine preventive treatment, the number of correct targets was more (58.53 ± 1.24 versus 59.20 ± 0.94, p < 0.05), omission errors were less (1.47 ± 1.24 versus 0.80 ± 0.94, p < 0.05), total number of errors were less (1.55 ± 1.20 versus 0.85 ± 0.98, p < 0.05), and time to complete/duration of the tests were shorter (103.61 ± 44.73 versus 96.15 ± 39.29, p < 0.05) (Table 1b).
To assess habituation or potentiation response in migraineurs and healthy control group, sub-averaged peak-to peak amplitudes at blocks 5 and 10 were compared with that of block 1. In healthy control, the amplitudes of the 1st and the 10th blocks were 8.91 ± 2.73 and 7.72 ± 2.71 µV on the right side and 8.39 ± 2.29, 8.02 ± 3.72 µV on left side, respectively. There was a decrease in amplitude and habituation ability was statistically significant in the healthy control (all p < 0.05) (Table 2). However in migraineurs before treatment, the amplitudes of the 1st and the 10th blocks were 8.94 ± 3.63 and 8.78 ± 3.55 µV on the right side and 8.67 ± 3.62, 8.55 ± 3.62 µV on left side, respectively. Habituation ability was not detected in migraineurs (all p > 0.05) (Table 2). Three months after migraine preventive treatment, loss of habituation ability was restored (Table 3). The amplitudes of the 1st and the 10th blocks were 8.93 ± 2.99, 7.96 ± 2.54 µV on the right and 9.23 ± 3.33, 8.30 ± 2.76 µV on the left side, respectively (all p < 0.05).
When each study drugs were analyzed separately, all drugs were effective in restoring the habituation ability in migraineurs (Table 3). The percentage changes (% mean ± SD) are also included in Table 3. For propranolol, the amplitudes of the 1st and the 10th blocks were 9.32 ± 2.67, 7.82 ± 2.57 µV on the right and 9.37 ± 2.88, 8.03 ± 2.56 µV on the left side, respectively. For flunarizine, the amplitudes of the 1st and the 10th blocks were 8.63 ± 3.86, 7.83 ± 3.34 µV on the right and 9.78 ± 4.28, 8.82 ± 3.48 µV on the left side, respectively. For topiramate, the amplitudes of the 1st and the 10th blocks were 8.82 ± 2.53, 8.29 ± 1.56 µV on the right and 8.48 ± 2.81, 8.08 ± 2.30 µV on the left side, respectively (Table 3).
Discussion
In this study, we aimed to identify if any difference exists in visual attention and visual evoked habituation of frequently attacked migraineurs compared to age- and sex-matched healthy control group. The effects of migraine preventive treatment on these parameters were also assessed.
Migraine is a common primary headache with a prevalence of 15–17% in females and 6% in males [19]. In our study, the female migraineurs were also 4.8 times more than the male migraineurs. Age and sex did not differ between the groups. The pathophysiology of migraine is complex [20]. The migrainous brain is structurally, anatomically, and functionally altered, and very sensitive to fluctuations in homeostasis [20]. It is both hypersensitive to external stimuli and hyperresponsive to repeated stimuli [21]. In a repetitive pattern-reversal VEP study, loss of habituation was proposed to be related with a functional disconnection of the thalamus leading to decreased intracortical lateral inhibition in migraineurs [22]. Altered processing of sensory stimuli and altered synchronicity in migraine patients were also proposed to be related with thalamocortical dysrhythmia [23]. In a recent human imaging study, the presence of abnormal low-frequency oscillations in thalamocortical networks in the interictal phase of migraineurs was demonstrated [24].
Although migraine is known to be relatively a benign and episodic neurological disease, some neuroimaging findings [25] and neuropsychological impairments are reported in frequently attacked migraineurs and with long disease duration [13]. Frequently attacked migraineurs are reported to have compromised physical, mental, and social functioning [26]. In a healthy brain, the normal selective attention function requires attenuation of sensory responses in the cortex [27]. It is suggested that loss of habituation might affect daily cognition in migraineurs such as difficulties in attention [28]. In migraineurs, interictally, neurocognitive processing of visual stimuli is reported to be dysfunctional, called as “lack of cognitive-level visual habituation” [28]. In a neurophysiological study, 232 unfamiliar images were viewed and event-related potentials were recorded in the healthy control group and migraineurs [28]. The amplitude of late positive potentials was found to be increased across trial blocks in migraineurs [28]. In the literature, compared to the healthy control group, slower reaction times are reported in migraineurs assessed by the Stroop test [29]. Cancellation Test (CT) measures the capacity of selective sustained attention, the sensory component related with perceptual representations, motor component related with visual search and scan, and motivational component related with affect. In our study, we found distorted visual attention performance in migraineurs assessed by CT. Compared to the healthy control group, the number of correct targets was less, omission errors were more, total number of errors were more, and time to complete/duration of the tests were longer in migraineurs. These results suggest attention deficits and prolonged reaction time in migraineurs compared to the age- and sex-matched healthy control group. In a previous study, children with migraine were also shown to have selective and alternate attention difficulties compared to the healthy control group [14]. Abnormal neuronal oscillations, mainly thalamocortical dysrhythmia and misguided striatal oscillations, were also proposed in children with attention deficit/hyperactivity disorder [30].
In our study, the migraineurs were randomized to flunarizine (n = 16), propranolol (n = 19), and topiramate (n = 17) treatment groups. The migraine attack frequency and disease duration did not differ between the treatment groups (all p > 0.05). Three months after migraine preventive treatment, compared to their baseline values, visual attention parameters improved. The number of correct targets was more, omission errors were less, total number of errors was less, and time to complete/duration of the tests were shorter. Similar to our findings, children with migraine reported to have visual attention deficits and migraine preventive treatment (sodium valproate, amitriptyline, flunarizine) reversed their performance assessed by CT [31]. We do not think this is a sole training effect, because CT was performed 3 months apart and it does not test the long-term memory, but only attention. Although propranolol, flunarizine, and topiramate may produce memory disturbances and behavioral side effects (e.g., depression) [32, 33], our results suggest that migraine itself might have deleterious effects on attention. Migraine preventive treatment may restore visual attention.
Repetitive stimulation is associated with selective activation of anaerobic glycolysis. The amplitude of evoked cortical response to repetitive stimuli normally decreases in healthy brain, which is known as “habituation” [34]. This habituation phenomenon is known to protect brain against over-excitation and lactate accumulation due to repetitive stimulation [35]. However, in migraineurs, this protective phenomenon is shown to be dysfunctional and loss of habituation and even potentiation have been reported [36]. In our study, we evaluated interictal habituation response by repetitive visual stimulation as described before [17]. In the healthy control group, the habituation ability was preserved; however, it was not detected in migraineurs. There are some conflicting reports against the loss of habituation in migraineurs [37]. We may assume methodological differences such as repetitive VEP technique, migraine severity, and disease duration may affect these negative reports. As reported before, we suggest comparing the N1-P1 amplitudes of the 1st block to the 10th block of 100 VEPs each, in frequently attacked migraineurs [17]. In a recent test–retest reliability study of visual habituation, loss of habituation was confirmed in migraineurs [38]. Similarly, both in blinded and non-blinded analyses, VEP habituation was reported to be deficient in migraineurs interictally [39].
Three months after migraine preventive treatment, loss of habituation ability was restored in our migraineurs. When each study drugs were analyzed separately, all drugs were effective in restoring the habituation ability. In a transcranial magnetic stimulation study of 29 migraineurs, cortical excitability of the visual cortex was also shown to be modulated by propranolol preventive treatment [40]. The action mechanism of flunarizine in migraine prophylaxis is not completely understood. In a whole-cell patch clamp recordings of cultured rat cortical neurons, flunarizine was shown to block sodium and calcium currents, a potential mechanism of cortical hyperexcitability [41]. In our clinical study, we were able to show that flunarizine restored loss of habituation in migraineurs, probably through the same mechanism. Topiramate was also able to restore habituation in our migraineurs. Similar to our result, in a study of migraine patients without aura, 2-month treatment with topiramate was shown to reverse loss of habituation assessed by acoustic stimulation [42]. In another study conducted by magnetoencephalography, visual cortex excitability was shown to be associated with remission in chronic migraineurs after topiramate preventive treatment [43]. One of the limitations of our study might be the limited number of migraine patients in each group to demonstrate a statistically significant difference among three different migraine preventive drugs. The number of subjects who were retested after 3 months of treatment was lower than the recruited. However, the comparisons and analyses were made according to the pre- and post-treatment data of the same patients who completed the study. The other limitation of our study was that we were not able to identify a possible correlation between the VEP habituation parameters, Cancellation Test items, and the clinical response to the prophylactic treatments because of insufficient clinical data. However, our study was designed only to assess the effects of 3 months of migraine prophylactic treatment on visual attention and electrophysiological (visual habituation) parameters in migraineurs. Propranolol, flunarizine, and topiramate are drugs that are approved to be clinically effective on migraine prophylaxis. In our study, we did not design to collect data in detail of the clinical outcome measures such as more than 30 or 50% of reduction in migraine attack frequency or migraine severity.
In this study, we demonstrated that 3 different preventive drugs (propranolol, flunarizine, and topiramate) restored visual attention and evoked cortical hyperresponsiveness in migraineurs. We may assume that visual attention deficit in migraineurs may be due to the same pathophysiological mechanisms underlying VEP habituation deficit such as “thalamocortical dysrhythmia”. This proposal may require further detailed investigations.
Conclusions
In summary, this is a prospective, randomized, electrophysiological, and neurophysiological study to assess the effects of preventive treatment in frequently attacked migraineurs compared to the healthy control group. It demonstrates an objective data on how frequent migraine attacks affect brain responsivity and attention. It also provides how migraine preventive drugs restore these brain functions. The loss of habituation ability and low visual attention performance in migraine patients may be restored by migraine preventive treatments. We hope this electrophysiological study accompanied by neuropsychological tests may provide an objective and quantitative assessment tool for our understanding of migraine pathophysiology and response to different treatment modalities.
References
Robbins MS, Lipton RB (2010) The epidemiology of primary headache disorders. Semin Neurol 30:107–119
Headache Classification Committee of the International Headache Society (IHS) (2013) The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 33:629–808
Choi JY, Oh K, Kim BJ, Chung CS, Koh SB, Park KW (2009) Usefulness of a photophobia questionnaire in patients with migraine. Cephalalgia 29:953–959
Digre KB, Brennan KC (2012) Shedding light on photophobia. J Neuroophthalmol 32:68–81
Chronicle EP, Mulleners WM (1996) Visual system dysfunction in migraine: a review of clinical and psychophysical findings. Cephalalgia 16:525–535
Kowacs PA, Utiumi MA, Piovesan EJ (2015) The visual system in migraine: from the bench side to the office. Headache 55(Suppl 1):84–98
Martin H, del Rio MS, de Silanes CL, Alvarez-Linera J, Hernandez JA, Pareja JA (2011) Photoreactivity of the occipital cortex measured by functional magnetic resonance imaging-blood oxygenation level dependent in migraine patients and healthy volunteers: pathophysiological implications. Headache 51:1520–1528
Delange JM, Cutrer FM (2014) Our evolving understanding of migraine with aura. Curr Pain Headache Rep 18:453
Ambrosini A, Schoenen J (2003) The electrophysiology of migraine. Curr Opin Neurol 16:327–331
Sand T, Zhitniy N, White LR, Stovner LJ (2008) Visual evoked potential latency, amplitude and habituation in migraine: a longitudinal study. Clin Neurophysiol 119:1020–1027
Meyer JS, Thornby J, Crawford K, Rauch GM (2000) Reversible cognitive decline accompanies migraine and cluster headaches. Headache 40:638–646
Calandre EP, Bembibre J, Arnedo ML, Becerra D (2002) Cognitive disturbances and regional cerebral blood flow abnormalities in migraine patients: their relationship with the clinical manifestations of the illness. Cephalalgia 22:291–302
Suhr JA, Seng EK (2012) Neuropsychological functioning in migraine: clinical and research implications. Cephalalgia 32:39–54
Villa TR, Correa Moutran AR, Sobirai Diaz LA et al (2009) Visual attention in children with migraine: a controlled comparative study. Cephalalgia 29:631–634
Weintraub S, Mesulam MM (2000) Neuropsychological assessment of mental state. In: Mesulam MM (ed) Principles of behavioral and cognitive neurology. Oxford University Press, New York, pp 121–173
Headache Classification Subcommittee of the International Headache Society (2004) The international classification of headache disorders: 2nd edition. Cephalalgia 24(Suppl 1):1–160
Unal-Cevik I, Yildiz FG (2015) Visual snow in migraine with aura: further characterization by brain imaging, electrophysiology, and treatment-case report. Headache 55:1436–1441
Karakas S, Erdogan Bakar E, Dogutepe Dincer E (2006) Handbook of BILNOT battery: research and development of neuropsychological tests, 2nd edn. Eryilmaz Offset, Ankara
Stewart WF, Shechter A, Rasmussen BK (1994) Migraine prevalence. A review of population-based studies. Neurology 44:S17–S23
Burstein R, Noseda R, Borsook D (2015) Migraine: multiple processes, complex pathophysiology. J Neurosci 35:6619–6629
Coppola G, Pierelli F, Schoenen J (2007) Is the cerebral cortex hyperexcitable or hyperresponsive in migraine? Cephalalgia 27:1427–1439
Coppola G, Ambrosini A, Di CL et al (2007) Interictal abnormalities of gamma band activity in visual evoked responses in migraine: an indication of thalamocortical dysrhythmia? Cephalalgia 27:1360–1367
De Tommaso M, Ambrosini A, Brighina F et al (2014) Altered processing of sensory stimuli in patients with migraine. Nat Rev Neurol 10:144–155
Hodkinson DJ, Wilcox SL, Veggeberg R et al (2016) increased amplitude of thalamocortical low-frequency oscillations in patients with migraine. J Neurosci 36:8026–8036
Schmitz N, Admiraal-Behloul F, Arkink EB et al (2008) Attack frequency and disease duration as indicators for brain damage in migraine. Headache 48:1044–1055
Terwindt GM, Ferrari MD, Tijhuis M, Groenen SM, Picavet HS, Launer LJ (2000) The impact of migraine on quality of life in the general population: the GEM study. Neurology 55:624–629
Mangun GR, Hillyard SA (1991) Modulations of sensory-evoked brain potentials indicate changes in perceptual processing during visual-spatial priming. J Exp Psychol Hum Percept Perform 17:1057–1074
Mickleborough MJ, Chapman CM, Toma AS, Chan JH, Truong G, Handy TC (2013) Interictal neurocognitive processing of visual stimuli in migraine: evidence from event-related potentials. PLoS One 8:e80920
Annovazzi P, Colombo B, Bernasconi L, Schiatti E, Comi G, Leocani L (2004) Cortical function abnormalities in migraine: neurophysiological and neuropsychological evidence from reaction times and event-related potentials to the Stroop test. Neurol Sci 25(Suppl 3):S285–S287
Sukhodolsky DG, Leckman JF, Rothenberger A, Scahill L (2007) The role of abnormal neural oscillations in the pathophysiology of co-occurring Tourette syndrome and attention-deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry 16(Suppl 1):51–59
Villa TR, Agessi LM, Moutran AR, Gabbai AA, Carvalho DS (2016) Visual attention in children with migraine: the importance of prophylaxis. J Child Neurol 31(5):569–572. doi:10.1177/0883073815601498
Mulleners WM, McCrory DC, Linde M (2015) Antiepileptics in migraine prophylaxis: an updated Cochrane review. Cephalalgia 35:51–62
Vecsei L, Majlath Z, Szok D, Csati A, Tajti J (2015) Drug safety and tolerability in prophylactic migraine treatment. Expert Opin Drug Saf 14:667–681
Thompson RF, Spencer WA (1966) Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev 73:16–43
Sappey-Marinier D, Calabrese G, Fein G, Hugg JW, Biggins C, Weiner MW (1992) Effect of photic stimulation on human visual cortex lactate and phosphates using 1H and 31P magnetic resonance spectroscopy. J Cereb Blood Flow Metab 12:584–592
Schoenen J, Wang W, Albert A, Delwaide PJ (1995) Potentiation instead of habituation characterizes visual evoked potentials in migraine patients between attacks. Eur J Neurol 2:115–122
Omland PM, Uglem M, Hagen K, Linde M, Tronvik E, Sand T (2016) Visual evoked potentials in migraine: is the “neurophysiological hallmark” concept still valid? Clin Neurophysiol 127:810–816
Rauschel V, Ruscheweyh R, Krafczyk S, Straube A (2016) Test-retest reliability of visual-evoked potential habituation. Cephalalgia 36:831–839
Ambrosini A, Coppola G, Iezzi E, Pierelli F, Schoenen J (2016) Reliability and repeatability of testing visual evoked potential habituation in migraine: a blinded case-control study. Cephalalgia. doi:10.1177/0333102416648656
Gerwig M, Niehaus L, Stude P, Katsarava Z, Diener HC (2012) Beta-blocker migraine prophylaxis affects the excitability of the visual cortex as revealed by transcranial magnetic stimulation. J Headache Pain 13:83–89
Ye Q, Yan LY, Xue LJ et al (2011) Flunarizine blocks voltage-gated Na(+) and Ca(2+) currents in cultured rat cortical neurons: a possible locus of action in the prevention of migraine. Neurosci Lett 487:394–399
De Tommaso M, Guido M, Sardaro M et al (2008) Effects of topiramate and levetiracetam vs placebo on habituation of contingent negative variation in migraine patients. Neurosci Lett 442:81–85
Chen WT, Wang SJ, Fuh JL et al (2012) Visual cortex excitability and plasticity associated with remission from chronic to episodic migraine. Cephalalgia 32:537–543
Acknowledgements
The authors report no conflict of interest and funding related with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors report no conflict of interest related with this manuscript.
Ethical Approval
The study was approved by the Local Ethical Commmitte.
Informed consent
Informed consent was obtained from participants.
Rights and permissions
About this article
Cite this article
Ince, F., Erdogan-Bakar, E. & Unal-Cevik, I. Preventive drugs restore visual evoked habituation and attention in migraineurs. Acta Neurol Belg 117, 523–530 (2017). https://doi.org/10.1007/s13760-017-0749-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-017-0749-z