Abstract
Clinical, neuroimaging, and laboratory features are not specific enough to establish the etiological diagnosis of the acute inflammatory myelitis (AIM). Longitudinally extensive transverse myelitis (LETM) seen on magnetic resonance imaging (MRI) has been associated with a poor functional prognosis. The aim of this study was to assess the functional outcomes of a first AIM event comparing patients with LETM vs. no LETM on MRI and to report the differential diagnosis. Clinical, radiological, biochemical aspects were collected, and Winner–Hughes Functional Disability Scale (WHFDS) was performed after 3 and 6 months. Centromedullary lesions were associated with LETM, lateral lesions with partial lesion (PL), and brain MRI lesions with multiple sclerosis and acute encephalomyelitis disseminated. LETM patients were associated with a worse functional outcome as the need of a wheelchair after 3 and 6 months (OR = 7.61 p = 0.01; OR 4.8 p = 0.04, respectively), a walker or cane (OR = 11.0 p = 0.002, OR = 4.3 p = 0.03, respectively). In addition, we found a correlation between LETM and acute complete transverse myelitis and PL with acute partial transverse myelitis (83.3 and 90.9%, respectively; p < 0.0001). In conclusion, AIM is a heterogeneous syndrome from an etiological point of view and LETM patients had worse functional prognosis compared with PL after 3 and 6 months.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute inflammatory myelitis (AIM) are a heterogeneous group of autoimmune spinal cord disorders characterized by disruption of the ascending and descending pathways with wide differential diagnosis. The most common causes of immune-mediated spinal cord injury are acquired demyelinating diseases, such as multiple sclerosis (MS) and neuromyelitis optica (NMO)/NMO spectrum disorders (NMOSD); or due to systemic autoimmune causes, such as systemic lupus erythematous (SLE) and Sjögren syndrome [1, 2], postinfective or postvaccinal are causes to consider as differential diagnosis [3]. After a detailed assessment, in almost 15–30% of the cases, no cause is found (idiopathic myelitis) [4, 5]. Magnetic resonance imaging (MRI) of the spinal cord with and without gadolinium enhancement is the initial investigation of choice in the evaluation of acute myelitis (AM) and it is the best noninvasive tool for identifying patients with longitudinally extensive transverse myelitis (LETM), which is more often observed in NMOSD and less in MS [1]. Therefore, differentiating LETM from partial or short lesion (PL) on MRI is very important from a clinical point of view to guide physicians towards the correct etiological diagnosis because of the different prognosis, these diseases have. Early studies reported that up to one-third of patients with a first episode of AIM remained unable to walk [6]. Several clinical factors are associated with a poor functional outcome, including motor involvement at onset, sphincter dysfunction, number of relapses, spinal shock, severe functional deficit at onset, and LETM [7, 8]. AIM does not have specific diagnostic criteria; therefore, a systematic and comprehensive assessment could help us to recognize the etiology of the AIM, facilitate therapeutic decision, and provide early accurate treatment to prevent future relapses and disability.
The purpose of this retrospective study is to assess the functional outcome of a consecutive series of patients with a first AIM event (LETM vs. no LETM) through Winner–Hughes Functional Disability Scale (WHFDS) after 3 and 6 months and to establish the etiologic spectrum, clinical presentation, and neuroradiological findings in an Argentinean population.
Materials and methods
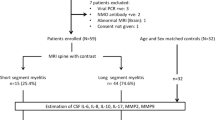
All patients diagnosed with isolated AIM that was admitted at the Neurology Department of the Carlos G. Durand Hospital of Buenos Aires, Argentina, from June 2008 to June 2014 and met the inclusion/exclusion criteria (see Table 1) that were included. According to the medical record database, clinical manifestations of spinal cord dysfunction were classified as acute partial transverse myelitis (APTM, incomplete or patchy involvement of at least one spinal segment with mild-to-moderate weakness, asymmetric, or dissociated sensory symptoms and occasionally sphincter dysfunction) and acute complete transverse myelitis (ACTM, acute or subacute symmetric moderate or severe loss of function distal to that level) and motor, sensory, and autonomic dysfunction were collected. Etiological diagnoses were defined by internationally accepted criteria [1, 4, 9–14]. All patients underwent brain and spinal cord MRI with and without gadolinium contrast on T1-weighted images using a 1.5-T scanner for diagnostic workup. Sagittal and axial T1, T2, fluid-attenuated inversion recovery (FLAIR), and short tau inversion recovery (STIR) sequences were carried out routinely. Lesions on spinal cord MRI included extension (sagittal images) classified as PL (only one lesion <2 vertebral segments in length), LETM (extending over ≥3 vertebral segments), and multisegmental lesions (MSL, two or more lesions <2 vertebral segments in noncontiguous segment) and distribution (axial images) that were classified as peripherally located (lateral/posterior) and centromedullary located. Axial, coronal, and sagittal brain MRI were analyzed and classified as normal, suggestive of MS [15, 16] and white matter non-specific lesions that were suggestive of NMO (“typical lesions” defined as lesions located at sites of high AQP4 expressions) [17, 18]. Contrast enhancement was classified as present or not present. Oligoclonal bands (OCBs) were analyzed using isoelectric focusing. Aquaporin-4 antibodies (AQP4-ab) were analyzed by indirect immunofluorescence (IIF) assay as described originally by Lennon et al. [19]. Other specific tests were done according to the clinical suspicion of the neurologist. Functional outcome was performed retrospectively at 3 and 6 months after presentation of the symptoms with the WHFDS [19]: 0 = normal, 1 = minor symptoms, 2 = able to walk more than 30 ft without assistance, 3 = able to walk more than 30 ft with assistance, 4 = bed bound/wheelchair bound, and 5 = requires assisted ventilation, 6 = death.
Statistic
Categorical variables were presented as proportions, and continuous variables were presented as means and SDs. Measures of risk, such as ORs, are presented with respective 95% CIs. The statistical significance of the differences observed between the variables was analyzed using Chi-square test or Fisher’s exact test for categorical data. The significance level was established with p < 0.05. Data analysis was performed using the Epi Info 7 software program.
Results
Demographic, clinical, and laboratory findings
A total of 46 patients (n = 46) diagnosed as AIM were included. Differential diagnosis, clinical, and paraclinical characteristics are summarized in Table 2. Mean age at onset was 34.76 (±11.19) years, and 82.61% (38/46) were female. All our patients had sensory dysfunction in at least one limb at onset. We identified a clear sensory level in 73.91% (34/46), and the most frequently affected localizations were thoracic 70.5% (24/34) followed by cervical 23.53% (8/34) and finally lumbar 5.88% (2/34). Motor system dysfunction was found in 91.30% (42/46), mostly in the lower limbs (LL) (58.70%). Thirty patients (65.22%) had sphincter dysfunction with bladder (retention or urinary urge incontinence) and/or bowel (constipation or fecal urge incontinence) involvement. From a clinical point of view, APTM was observed in 52.17% (24/46) and ACTM in 47.83% (22/46) of the cases, at onset. OCBs were performed in 66% of patients (32/46), and positive results were obtained in 12 subjects (37.5%). MS patients had 100% positive OCBs (not performed n = 9), while only 10% (1/10) NMO patients had positive results. All idiopathic myelitis patients included in this study were both OCBs and AQP4-ab negative at presentation. Data about AQP4-ab were available in 54.34% being positive in only 13.05%; all these patients were diagnosed as NMO/NMOSD.
Differential diagnosis of AIM
All our patients had symptomatic spinal cord and MRI lesions at onset. After 6 months of follow-up, we identified the following causes: MS = 43.47% (20/46), NMOSD 21.73% (10/46) (AQP4-ab positive or negative patients fulfilled the Wingerchuk revised criteria for NMO: 8 and AIM AQP4-ab positive: 2), idiopathic = 15.21% (7/46), SLE-related myelitis = 13.04% (6/46), and acute encephalomyelitis disseminated (ADEM) = 6.51% (3/46) (see Table 2). None of these patients had relapses during this follow-up period.
MRI lesion characteristics
In spinal cord MRI, we observed that 47.83% (22/46) patients corresponded to LETM, 41.30% (19/46) to MSL, and 10.87% (5/46) to PL. LETM lesions expanded for a mean of 8.04 (±4.56) spinal segments. Centromedullary lesions were associated with LETM (19/22), whereas lateral and/or posterior lesions were associated with PL and MSL (24/24). In addition, we found correlation between LETM and ACTM; and PL or MSL with APTM (83.3 and 90.9%, respectively; p < 0.0001). Contrast enhancement lesions were found in 54.34% of the patients (25/46) at presentation. Brain MRI lesions were associated with MS and ADEM, and non-specific T2-hyperintensities and normal findings were observed in the rest of the differential diagnosis. MRI characteristics of spinal cord and brain lesions are summarized in Table 3.
Treatment
All our patients received high-dose intravenous (i.v.) methylprednisolone, 1 g daily for 5 days. MS patients were also treated with Interferon or Glatiramer acetate at onset; NMO/NMOSD (AQP4-ab seropositive) patients were treated with azathioprine (with or without oral corticosteroids) and 50% of SLE-related myelitis patients with iv cyclophosphamide at presentation. Idiopathic myelitis and ADEM did not receive the long-term therapy during this period.
Functional outcome
When we compared between LETM vs. no LETM (PL and MSL) patients after 3 months, we found that ≥4 WHFDS score (bed bound/wheelchair bound) was seen in 40.91% for LETM vs. 8.33% no LETM (CI 95% OR = 7.61, p = 0.01) patients. In addition, WHFDS ≥3 (able to walk with assistance) was associated with LETM (OR = 11.0, p = 0.002) patients. In addition, WHFDS score at 6 months ≥3 and ≥4 score was associated with LETM (OR = 4.3, p = 0.03 for ≥3 and OR 4.8, p = 0.04 for ≥4). Thus, LETM correlates with a worse functional prognosis compared with PL/MSL after 6 months (see Table 4). In our sample, we identified a WHFDS ≥4 score in MS (15%, 3/20), idiopathic (71.42%, 5/7)m and SLE-related myelitis (50%, 3/6). Differential diagnosis and WHFDS are summarized in Table 2.
Discussion
In the present study, we retrospectively reviewed 46 consecutive patients diagnosed as AIM and found several clinical and MRI features with different functional prognosis. The mean age at presentation was 34.76 (±11.19) years, and females were more commonly affected, in line with other studies [13, 19, 20].
Scott et al. [5] reviewed the utility of distinguishing ACTM from APTM in determining the etiology of AIM, identifying that APTM initially converted to MS in 10.3% of the cases, whereas ACTM suggests a transition to MS in 0–2% of the patients (most commonly observed in NMOSD). In our series, we found that 95% of MS patients and 20% of NMOSD subjects had the initial APTM. ACTM was found in NMO/NMOSD (80%), idiopathic myelitis (85%), SLE-related myelitis (83%), ADEM (66%), and only one MS patient (5%) at presentation. In addition, LETM was seen on ACTM (83.3%) and PL o MSL with APTM (90.9%) at onset on MRI. Regarding to etiological causes, demyelinating diseases (MS 43% and NMOSD 21%) and idiopathic myelitis (15%) were the most frequently found. Thus, during this follow-up period, idiopathic myelitis patients had no history of infection/vaccine and did not fulfill MS/NMO criteria and no systemic inflammatory diseases were found. However, a percentage of these patients may remain undiagnosed (and idiopathic) because of the short follow-up period. Interestingly, we observed a high percentage of SLE-related myelitis (13.04%) cases, but we believe that our results might be biased due the fact that our hospital is a national reference center for the study of SLE.
In recent years, the discovery of AQP4-ab has significantly changed the initial evaluation of AIM [19, 21, 22]. AQP4-ab is present in one-third to one-half of LETM, and seropositive patients are at risk of subsequent optic neuritis or recurrent myelitis [23]. In addition, the Mayo clinic group found that 14% of NMOSD had short lesions on MRI at the first myelitis episode and the authors observed that 92% of these patients presented LETM lesions in subsequent AIM episodes [24]. Therefore, short lesions on MRI do not revoke the need of testing AQP4-ab, or exclude NMOSD diagnosis [24]. In our series, AQP4-ab was performed in 54.34% and positive status was observed in 60% of NMO patients (this technique became available in our hospital in 2010) and 100% of these patients had LETM at presentation. Idiopathic myelitis often shows a spinal cord pattern on MRI that is similar to NMOSD, as seen in the current study [25, 26]. All idiopathic myelitis patients were tested for AQP4-ab, and negative results were found.
Brain MRI is a useful diagnostic tool for the initial evaluation of AIM. In our sample, NMOSD patients had normal brain MRI in 50% of the cases, 30% of patients had non-specific results, and 20% were suggestive of NMOSD (periaqueductal and area postrema lesions). However, a recently published consensus showed a 50–85% incidence of brain MRI lesions using the revised NMO diagnostic criteria (2006), and also has been observed in 51–89% of seropositive patients [17, 18]. In contrast, normal brain MRI is associated with a 19% risk of developing MS, while having two or more lesions (typical MS lesions) means up to 88% risk of developing MS after 5 and 20 years of follow-up [27]. In line with these data, 95% of our MS patients had suggestive MS lesions at onset.
As regards prognosis, case series have estimated that approximately 33% of patients with a first episode of AIM remained unable to walk. Others studies disclosed similar poor functional recovery rates, ranging from 11 to 35.8% [6, 8, 28]. When we compared LETM vs. no LETM (PL and MSL) patients, applying the WHFDS at 3 and 6 months, we observed that bed bound/wheelchair bound (≥4 score) was associated with LETM at onset (p = 0.01 at 3 months and p = 0.04 at 6 months). In addition, when we analyze the ability to walk more than 30 ft with assistance (≥3 score), we found that LETM was associated with LETM at presentation (p = 0.002 at 3 months and p = 0.03 at 6 months). Therefore, LETM has a worse functional prognosis compared with PL after 3 and 6 months. However, a multicenter study (Europe) found that LETM is associated with a higher number of relapses but not to a worse functional prognosis [19]. In our sample, the leading causes with ≥4 WHFDS score were idiopathic (71.42%) and SLE-related myelitis (50%). Therefore, these patients may constitute a critical subgroup with a different prognosis and other treatment needs.
We acknowledge several limitations in our study. This is a retrospective study design with failure to obtain OCBs and AQP4-ab in the whole sample, potential recruitment bias due to small population and short-term follow-up. However, OCBs (6/7) and AQP4-ab were performed in all patients for diagnosis of idiopathic myelitis. In addition, we performed IIF to asses AQP4-ab and some idiopathic myelitis patients could have had positive tests for AQP4-ab if more sensitive methods were used (e.g., cell-based assays) [29]. These data serve to increase the international casuistry, as it can be compared with previously published results from Europe and North America.
In conclusion, MS followed by NMOSD were the most frequent causes of AIM. LETM and AQP4-ab helped us to diagnose NMOSD, whereas the OCBs, brain, and PL MRI lesions were associated with MS according to what has been available in other series from different countries. ACTM at onset was associated with LETM on MRI, and these patients had a worse functional prognosis compared with PL after 3 and 6 months. Finally, we believe that functional scales are important in the clinical practice for a better follow-up of these patients and for promoting early specific treatment to improve their quality of life. Future prospective studies with bigger samples are needed to better assess the functional prognosis of these populations.
References
Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG (2007) The spectrum of neuromyelitis Optica. Lancet Neurol 6:805–815
Miller DH, Weinshenker BG, Filippi M, Banwell BL, Cohen JA, Freedman MS et al (2008) Differential diagnosis of suspected multiple sclerosis: a consensus approach. Mult Scler 14:1157–1174
Frohman EM, Wingerchuk DM (2010) Transverse Myelitis. N Engl J Med 363:564–572
Transverse Myelitis Consortium Working Group (TMCW) (2002) Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology 59:499–505
Scott TF, Frohman EM, De Seze J et al (2011) Evidence-based guideline: clinical evaluation and treatment of transverse myelitis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 77(24):2128–2134
Christensen PB, Wermuth L, Hinge HH, Bomers K (1990) Clinical course and long-term prognosis of acute transverse myelopathy. Acta Neurol Scand 8:431–435
Gajofatto A, Monaco S, Fiorini M, Zanusso G, Vedovello M, Rossi F et al (2010) Assessment of outcome predictors in first-episode acute myelitis: a retrospective study of 53 cases. Arch Neurol 67:724–730
Cobo Calvo A, Mañé Martínez MA, Alentorn-Palau A, Bruna Escuer J, Romero Pinel L, Martínez-Yélamos S (2013) Idiopathic acute transverse myelitis: outcome and conversion to multiple sclerosis in a large series. BMC Neurol 3(13):135
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302
Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG (2006) Revised diagnostic criteria for neuromyelitis optica. Neurology 66:1485–1489
Krupp LB, Tardieu M, Amato MP, Banwell B, Chitnis T, Dale RC et al (2013) International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler 19(10):1261–1267
Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF et al (1982) The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25:1271–1277
Jeffery DR, Mandler RN, Davis LE (1993) Transverse myelitis retrospective analysis of 33 cases, with differentiation of cases associated with multiple sclerosis and parainfectious events. Arch Neurol 50:532–535
De Seze J, Lanctin C, Lebrun C, Malikova I, Papeix C, Wiertlewiski S et al (2005) Idiopathic acute transverse myelitis: application of the recent diagnostic criteria. Neurology 65:1950–1953
Swanton JK, Fernando K, Dalton CM, Miszkiel KA, Thompson AJ, Plant GT et al (2006) Modification of MRI criteria for multiple sclerosis in patients with clinically isolated syndromes. J Neurol Neurosurg Psychiatry 77:830–833
Swanton JK, Rovira A, Tintore M, Altmann DR, Barkhof F, Filippi M et al (2007) MRI criteria for multiple sclerosis in patients presenting with clinically isolated syndromes: a multicentre retrospective study. Lancet Neurol 6:677–686
Kim HJ, Paul F, Lana-Peixoto MA, Tenembaum S, Asgari N, Palace J et al (2015) MRI characteristics of neuromyelitis optica spectrum disorder: an international update. Neurology 84:1–9
Wingerchuk DM, Banwell B, Bennett J, Cabre P, Carroll W, Chitnis T et al (2015) International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 85:1–13
Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K et al (2004) A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 364:2106–2112
Sepúlveda M, Blanco Y, Rovira A, Rio J, Mendibe M, Llufriu S et al (2013) Analysis of prognostic factors associated with longitudinally extensive transverse myelitis. Mult Scler 19(6):742–748
Collongues N, Marignier R, Zéphir H, Papeix C, Blanc F, Ritleng C et al (2010) Neuromyelitis optica in France: a multicenter study of 125 patients. Neurology 74:737–742
Costa C, Arrambide G, Tintore M, Castilló J, Sastre-Garriga J, Tur C et al (2012) Value of NMO-IgG determination at the time of presentation as CIS. Neurology 78(20):1608–1611
Weinshenker BG, Wingerchuk DM, Vukusic S, Linbo L, Pittock SJ, Lucchinetti CF et al (2006) Neuromyelitis óptica IgG predicts relapse after longitudinally extensive transverse myelitis. Ann Neurol 59:566–569
Flanagan EP, Weinshenker BG, Krecke KN, Lennon VA, Lucchinetti CF, McKeon A et al (2015) Short Myelitis Lesions in Aquaporin-4-IgG—positive neuromyelitis optica spectrum disorders. JAMA Neurol 72(1):81–87
Harzheim M (2004) Discriminatory features of acute transverse myelitis: a retrospective analysis of 45 patients. J Neurol Sci 217:217–223
Desanto J, Ross JS (2011) Spine infection/inflammation. Radiol Clin North Am 49(1):105–127
Scott TF, Kassab SL, Singh S (2005) Acute partial transverse myelitis with normal cerebral magnetic resonance imaging: transition rate to clinically definite multiple sclerosis. Mult Scler 11:373–377
Ropper AH, Poskanzer DC (1978) The prognosis of acute and subacute transverse myelopathy based on early signs and symptoms. Ann Neurol 4:51–59
Ruiz-Gaviria R, Baracaldo I, Castañeda C et al (2015) Specificity and sensitivity of aquaporin 4 antibody detection tests in patients with neuromyelitis optica: a meta-analysis. Mult Scler Relat Disord. 4(4):345
Acknowledgements
We thank Dr. Carmen Lessa and Edson Chiganer (Immunology and Histocompatibility Unit, Hospital Carlos G. Durand, Buenos Aires, Argentina) for their valuable recommendations in the clinical practice. In addition, we thank Dr. Florencia Ocariz and Adrian Rubstein for his critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any potential financial conflict of interest related to this manuscript.
Ethical standards
The study was approved by the Hospital Ethics Committee.
Informed consent
Prior to study entry, they provided their informed consent.
Funding
This research received no specific Grant.
Rights and permissions
About this article
Cite this article
Carnero Contentti, E., Hryb, J.P., Diego, A. et al. Etiologic spectrum and functional outcome of the acute inflammatory myelitis. Acta Neurol Belg 117, 507–513 (2017). https://doi.org/10.1007/s13760-016-0742-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-016-0742-y