Abstract
Palpita forficifera Munroe, 1959 (Lepidoptera: Crambidae) is considered the main pest of the olive tree (Olea europaea L., Oleaceae) in Brazil and Uruguay. The aim of this work was to study the mating and oviposition behavior of P. forficifera in the field and laboratory. In the field, the sex emitting the mating pheromone was determined and in the laboratory, the rate of emergence of males and females; the age, time and duration of mating; number of copulations and oviposition time of P. forficifera were recorded. The field results showed that it was possible to capture up to five males per trap in just one night in traps with the presence of female P. forficifera. Copulation occurs between the seventh and twenty-third day of life and is most frequent during the third and sixth hours of scotophase. The average duration of the first copulation was 174 min, with 35% of couples recopulating, and there were cases of up to five copulations. Oviposition times were concentrated between 20:00 and 02:00. The results obtained provide insight into the reproductive behavior of P. forficifera and are useful for future studies aimed at identifying the sex pheromone to improve monitoring of the pest in olive orchards.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The olive tree (Olea europaea L., Oleaceae) is grown mainly in subtropical and temperate climate regions for the production of olive oil and table olives. In 2017, world production reached 20.8 million tons (t), in a cultivated area of 10.8 million hectares (FAOSTAT 2019).
Brazilian and Uruguayan olive cultivation is recent, but this scenario is changing due to the opportunities to fill this market niche. Orchards have been established in the south and southeast of Brazil (Coutinho et al. 2015) and in the south of Uruguay (Paullier 2013). The production of olive oil and olives in Brazil represents approximately 1% of domestic consumption, with imports amounting to around 71,000 and 105,800 tons, respectively (FAOSTAT 2019). In the case of Uruguay, production is 6.3 thousand tons and currently serves the domestic market and exports the surplus to other countries, mainly Brazil and the United States (ASOLUR 2019).
One of the main phytosanitary problems of the crop in both countries is the caterpillar of olive tree Palpita forficifera Munroe, 1959 (Lepidoptera: Crambidae) (Paullier 2013; Ricalde et al. 2015). Palpita forficifera is considered a multivoltine species, and the damage is caused by caterpillars that feed on shoots, and when in high infestation can attack senescent leaves, flowers and fruit (Ricalde et al. 2014; Coutinho et al. 2015; Scheunemann et al. 2017). As a result of the damage caused by the loss of leaf area, the plants reduce their fruit production next year because the caterpillars feed on the shoots. When the damage occurs to the fruit, there is a reduction in the quantity and quality of the oil and olives, compromising profitability. In addition to the damage caused by the pest, there is a risk that P. forficifera could be introduced into other countries. Currently, the pest's location is restricted to the South American continent, but there is a risk of it being introduced to other regions, as recently happened with the sympatric species Palpita persimilis Munroe, 1959, which was detected in the United States (Hayden and Buss 2013).
With the increase in the area under olive cultivation in Brazil and Uruguay, P. forficifera has been gaining importance and the management of P. forficifera has been difficult, since there are no insecticide records (Agrofit 2018). In addition, monitoring techniques based on visual assessment of plants infested by the pest and the use of light traps have not been efficient (Scheunemann et al. 2017). In this sense, studies to characterize the reproductive behaviour of P. forficifera could help in the discovery of sex pheromones that could be used for monitoring and control for sexual confusion in the field. Previous studies have shown that the mating and oviposition behavior of the species P. indica (Saunders, 1851) and P. unionalis Hübner 1796 (Lepidoptera: Crambidae) occurs in scotophase (Mazomenos et al. 2002; Hegazi et al. 2007; Choi et al. 2009). However, for P. forficifera, we have no information on reproductive behavior. Nevertheless, based on information on the capture of P. forficifera in light traps (Scheunemann et al. 2017), there is an indication that this species follows the behavior of others species in the same genus. Given the need to better understand the reproductive behavior of P. forficifera and the need to advance studies into the isolation and characterization of the species' sex pheromone, this study aimed to study the mating and oviposition behavior of P. forficifera in field and laboratory conditions.
Material and Methods
The study was carried out in orchards in the experimental field area and in the Entomology Laboratory of Embrapa Clima Temperado (Pelotas, Rio Grande do Sul, Brazil), in air-conditioned rooms with a temperature of 25 ± 2ºC, relative humidity of 60 ± 10% and 14-h photophase.
Rearing of P. forficifera
Rearing was established by collecting adults in olive orchards using a model 515 light trap (ISCA Tecnologias, Ijuí, RS, Brazil) equipped with ultraviolet light. The insects were transported to the laboratory and placed in a transparent plastic cage (22 cm high × 16 cm in diameter) made from 5-L PET bottles. To make the cage for rearing the adults, the top of the PET bottle was removed and tulle-like fabric was placed in the opening and secured with a rubber tie. The insects were fed a 10% aqueous honey solution and water was also provided by capillarity in a container (50 mL). To obtain eggs, filter paper (12.5 cm in diameter) was placed on the tulle, and a moistened vegetable sponge cloth (Spontex, PaneSponja, Ilhéus, BA, Brazil) was placed on top. After 24 h, the filter paper containing the spawn was removed and placed in a Petri dish (15 cm in diameter × 3 cm high) on top of another moistened filter paper. Twenty-four hours before hatching, the eggs were placed in a plastic box (30 cm wide × 30 cm high × 45 cm long) containing olive shoots of the Koroneiki variety, which served as food for larval development and also as a pupation site. After emergence, the insects were kept in rearing cages, with approximately 50 pairs, to obtain the eggs and carry out the experiments.
Determining the Pheromone-Emitting Sex for Field Mating
The experiment was conducted in a completely randomized experimental design with 15 replicates (traps) installed in the olive orchard (1.3 ha) located at Embrapa Clima Temperado (31º40′47"S, 52º26′24"W and altitude 57 m). Traps (Delta model) containing adhesive cardboard (20 × 18 cm) on the inside base were used. Three one-day-old virgin males or females were kept in a plastic cage (6 × 8 cm) wrapped in tulle and containing a piece of absorbent cotton moistened with an aqueous solution of honey (10%). The traps were fixed to the plants at a height of 1.5 m, arranged randomly in the orchard and interspersed according to the sex of insects. The insects were captured daily in the morning until the insects (males or females) died in the plastic cages. If P. forficifera was captured, the insects were counted and removed from the sticky card, after which the sex was identified based on the morphology of the last abdominal segments and the genital opening of the adults (Fig. 1A and B).
Detail at the end of the abdomen of Palpita forficifera adults, characterizing sexual dimorphism. A Males; B Female; C Delta trap used to determine the sex of the mating pheromone emitter and females trapped in the cages. The circles indicate the males captured; D Male adhered to the adhesive floor of Palpita forficifera
Emergence rate of Males and Females
Larvae up to 12 h old were individualized in glass tubes (2.5 × 8.5 cm) containing a shoot (apical bud and a leaf) of olive tree cv. Koroneiki fixed in 25 mL of gelled solution (3%) in order to maintain the turgidity of the food. The tubes were plugged with hydrophobic absorbent cotton and the food was replaced as necessary during the larval stage. Forty-eight hours after pupation, the insects were separated by sex according to Butt and Cantu (1962) and then placed in a plastic container (40 mL) with a perforated lid to allow aeration, containing a piece of moistened absorbent cotton. The pupae were observed daily and the date of emergence was recorded. The experimental design was entirely randomized with 80 repetitions (larvae) and the treatment factor tested was sex (female and male). The results were expressed as the percentage of females and males that emerged over time.
Mating Behavior
To obtain virgin insects, the pupae from the maintenance rearing were separated by sex and individualized in a plastic container (80 mL). Moistened absorbent cotton was placed inside to maintain humidity, after which they were kept in an air-conditioned chamber with a temperature of 25 °C and an inverted photoperiod of 14:10 h (Light:Dark). After emergence, pairs of P. forficifera of the same age were individualized in transparent plastic cages (10 cm in diameter × 10 cm high), with the top and bottom closed with tulle fabric fixed with a rubber tie. The adults were fed as described for maintenance rearing.
The experimental design was completely randomized with 30 replicates, each replicate being represented by a couple. The couples were kept in an air-conditioned room with lighting from 6 pm to 8am. Observations were made during the 10 h of scotophase between the hours of 08:00 and 18:00, for 24 consecutive days, using red light (Delisle and McNeil 1986). The independent variables were age at first copulation, mating time and number of copulations; and the dependent variables were mating percentage, mating duration and copulation percentage and duration.
After the death of the female and/or at the end of the twenty-fourth day, the presence of spermatophores in the bursa copulatrix (copulatory pouch) of the females was checked. To do this, females were individualized in glass tubes (2.5 cm in diameter × 8.5 cm high), closed with plastic wrap and taken to the freezer (-10 °C), where they remained until the bursa copulatrix was dissected. Using a pair of histological scissors and a scalpel, the abdomen was detached from the thorax and placed in a glass tube containing 20 mL of potassium hydroxide solution (KOH 10%) for 48 h to remove the remains of tissue and fat adhered to the copulatory pouch. Subsequently, with the aid of tweezers, the abdomen was placed under a watch glass (Syracuse®) and dissected under a stereoscopic microscope (Leica, M80). Two histological needles were used to open the abdomen, one of which was used to trap the abdomen and the other to open and/or cut the integument. Once the abdomen was open, the fatty tissues that were still wrapped around the copulatory pouch were removed using a brush and water. The copulatory pouch was then opened and the spermatophores counted. This procedure was photographed under a stereoscopic microscope at 3.2 times magnification (Leica, M80).
Oviposition Time
The experiment was carried out using the same methodology as that described for the mating experiment. The experimental design was completely randomized, with 11 replicates, each represented by a couple. The treatment factor tested was the oviposition period. Six two-hour intervals were established during the scotophase period (18:00–20:00, 20:00–22:00, 22:00–24:00, 00:00–02:00, 02:00–04:00 and 04:00–06:00) and one 12-h interval (06:00–18:00) during the photophase for the evaluation and counting of eggs by changing the laying substrate. The experiment was carried out for seven consecutive days. The eggs were counted using a stereoscopic microscope (ZEISS, Germany, Stemi SV11).
Statistical Analysis
Data on the rate of emergence was analyzed using the Chi-square test (χ2) (p ≤ 0.05). The mating and oviposition data were subjected to analysis of variance using the Kruskal–Wallis test (p ≤ 0.05), followed by multiple comparison of means using the Nemenyi test (p ≤ 0.05) (Elliot and Hynan 2011). The number of copulations was analyzed using a second-order inverse polynomial regression model (p ≤ 0.05). The model was selected on the basis of low residuals, low p-value and high R2 and R2 adj. The ages (days), as there was no equation fit, were compared with 95% confidence intervals, these intervals were plotted on the graph and the differences were considered significant when there was no overlap between the vertical bars.
Results
In the field, it was observed that during the evaluation period only males were captured in traps containing virgin females (Fig. 1C and D). Males were captured in the traps from the third day of age, totaling 60% of the traps made available in the orchard.
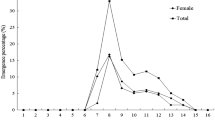
From laboratory studies, females and males of P. forficifera had the same emergence period and no significant difference was observed between the sexes (χ2 = 0.91, df = 1, P = 0.3421) (Fig. 2). For females, emergence occurred between 22 and 30 days after the larvae hatched and for males between 24 and 30 days. However, for both sexes emergence peaked at 25 days. With regard to mating behavior, only 66.67% of the couples evaluated copulated (Fig. 3A). In addition, mating occurred between the seventh and 23rd day of age of the adults, with the highest frequencies of first copulation occurring at seven, ten and sixteen days after emergence (χ2 = 68.71, gl = 23, P < 0.0001) (Fig. 3B). With regard to the mating of P. forficifera, it was found that the most suitable time was between 20:00 and 05:00. However, the majority (66.7%) of the insects copulated between 22:00 and 00:00 (Fig. 3C). The duration of the first copulation differed significantly with the age of the couples (χ2 = 38.6818, df = 23, P = 0.0215), ranging from 121 to 221 min, with an average of 174 min, i.e. the older the adults, the shorter the duration of copulation (Fig. 3D). However, only 22% of the couples recopulated (22%). These results were confirmed by dissecting the bursa copulatrix and, consequently, determining the presence and number of spermatophores (Fig. 4). In addition, it was evident that as more than one copulation occurred, the average duration of mating decreased significantly (F = 51.74, df = 4, P < 0.0001), with the data being fitted to the second-order inverse polynomial model (F = 14.17, df = 2, P = 0.01) (Fig. 5).
With regard to the time of oviposition, it was observed that the oviposition of P. forficifera differed significantly according to the periods evaluated (χ2 = 20.35, df = 5, P = 0.0011) (Fig. 6). However, 99% of egg-laying occurred from 20:00 to 06:00, indicating that this activity takes place during the night, as was the case for mating.
Discussion
From the field study, it was clear that the females of P. forficifera are responsible for emitting the pheromone for copulation, since there were only males of P. forficifera in the traps with the presence of females. This observation is in line with McNeil (1991) who mentions that in most cases in moths, the females are responsible for emitting the sex pheromone to attract the males for mating. For example, Ecdytolopha aurantiana (Lima, 1927) (Lepidoptera: Tortricidae) captured 8 to 13 males per trap, when six females were placed inside the cage in the capture trap (Bento et al. 2001). In the laboratory, according to the emergence rate of males and females, it was observed that there was no protandry (males emerge before females) or protogyny (females emerge before males), since males and females had the same emergence period. The same emergence behavior was recorded for Palpita indica (Saunders, 1851) (Lepidoptera: Crambidae) and Heliothis virescens (Fabricius, 1777) (Lepidoptera: Noctuidae) (Henneberry and Clayton 1984; Choi et al. 2009). For some lepidopterans, the emergence of males before females can confer an adaptive advantage for successful mating, especially when males compete for females (Zonneveld 1997; Carvalho et al. 1998). In the case of P. forficifera, the females are responsible for sexual selection, since they make the call to mate with the release of the sex pheromone, as observed in the field.
When analyzing mating behavior, it occurred between the seventh and 23rd day of age, with the highest frequencies of first copulation. Studies carried out with E. aurantiana show that the highest frequencies of mating occurred on the third and fourth day of age, corresponding to 44.7 and 36.8%, respectively (Bento et al. 2001). While for Atheloca subrufella (Hulst, 1887) (Lepidoptera: Phycitidae), the highest percentage of copulations (90%) occurred on the first day of life (Bento et al. 2006). In the case of P. forficifera, the delay in first mating and the observation that copulation was not concentrated in one period compared to other species, such as E. aurantiana, may be related to the physiological characteristics of the species (ovarian maturation) and also to a process of natural selection (McNeil 1991). In addition, P. forficifera mated between 20:00 and 05:00, with 66.7% of copulations occurring between 22:00 and 00:00. Studies with P. indica revealed that mating occurred throughout the entire period of scotophase, with a higher frequency between the second and fourth hours of scotophase (Choi et al. 2009). Copulation calling behavior was also observed in the first five hours of scotophase for Diaphania angustalis Snellen (Lepidoptera: Crambidae) (Shi et al. 2018). On the other hand, Bento et al. (2001) evaluated the mating of E. aurantiana and observed that the insects copulated from 5 to 9 pm, with 60% of the mating occurring between 7 and 8 pm. This difference in mating between P. forficifera and E. aurantiana is probably due to the latter's crepuscular habits, since P. forficifera seems to have nocturnal habits. Our results corroborate the indication that most moths are active during scotophase, and that this behavior is regulated by the circadian rhythm of each species. The fact that species emit pheromones at specific intervals is considered to be an important mechanism for ensuring reproductive isolation (Cardé and Roelofs 1973).
A study carried out with Crocidosema aporema (Walsingham, 1914) (Lepidoptera: Tortricidae) revealed that 73% of matings occurred between the fourth and sixth hour of scotophase, close to what we found for P. forficifera (Altesor et al. 2010). However, only 22% of P. forficifera pairs recopulated. A study carried out with Grapholita molesta (Busck) (Lepidoptera, Tortricidae) and Epiphyas postvittana (Walker, 1863) (Lepidoptera: Tortricidae), reported that females that copulated with a virgin male mated only once in their lifetime. This behavior may be related to the size of the spermatophore transferred, making the female less receptive to copulations (Foster and Howard 1999, Morais et al. 2012).
However, for other species, such as Cydia pomonella (Linnaeus, 1758) (Lepidoptera: Tortricidae), four to seven copulations have been observed in laboratory conditions and a maximum of two copulations in field conditions (Gehring and Madsen 1963). The number of copulations recorded during the monitoring of P. forficifera couples was verified by dissecting the bursa copulatrix and, consequently determining the presence and number of spermatophores. This result indicates that it is quicker and more practical to dissect the copulatory pouch in order to assess the number of matings of P. forficifera.
However, as more than one copulation occurred, the average duration of mating decreased, from 170 to 85 min in insects that copulated once and five times, respectively. This is different in Cnephasia jactatana Walker, 1863 (Lepidoptera: Tortricidae), where the duration of mating is similar in all copulations (Jiménez-Pérez and Wang 2004). For P. forficifera, this behavior is strategically important because it can increase feeding and oviposition time for females and reduce predation risks for both sexes (Keller and Reeve 1995). In addition, approximately 99% of the females laid between 20:00 and 06:00, indicating that this activity takes place during the night. Within the scotophase period, the peak of oviposition was concentrated between 20:00 and 02:00 h, making the nocturnal habit of P. forficifera oviposition evident, with a predominance of this activity in the first six hours of scotophase. Although the study was carried out in laboratory conditions, where there was a drastic change from photophase to scotophase, it is believed that this behavior is similar to field conditions. For most lepidopterans, light has a great influence on reproductive and sexual behavior, and the decrease in light intensity at dusk stimulates these activities for many species. In P. forficifera, the presence of light clearly inhibited oviposition, demonstrating that the insect is sensitive to this stimulus.
The information obtained in this study is useful and relevant for the olive tree production sector, providing a basis for studies into the isolation and detection of the P. forficifera pheromone. This strategy is considered essential for olive tree producers in Brazil and Uruguay. Currently, control of the pest in orchards is carried out on a scheduled basis, without taking into account the presence or absence of the pest in the orchard.
References
Altesor P, Horas VR, Arcia MP, Rossini C, Zarbi PHG, González A (2010) Reproductive behaviour of Crocidosema (=Epinotia) aporema (Walsingham) (Lepidoptera: Tortricidae): temporal pattern of female calling and mating. Neotrop Entomol 39:324–329. https://doi.org/10.1590/S1519-566X2010000300003
Asolur (2019) Associación Olivícola Uruguaya. (http://www.extravirgenuruguay.com/po/uruguai-produtor) (accessed 07 Jan 2019)
Bento JMS, Parra JRP, Vilela EF, Walder JM, Leal WS (2001) Sexual behavior and diel activity of citrus fruit borer Ecdytolopha aurantiana. J Chem Ecol 27:2053–2065
Bento JMS, Nava DN, Chagas MCM, Costa HA, Libardi DJ, Parra RP (2006) Biology and mating behavior of the coconut moth Atheloca subrufella (Lepidoptera: Phycitidae). Fla Entomol 89:199–203
Butt BA, Cantu E (1962) Sex determination of lepidopterous pupae. USDA, Washington, p 7
Cardé RT, Roelofs WF (1973) Temperature modification of the male sex pheromone response and factores affecting female calling in Holomelina immaculata (Lepidoptera: Arctiidae). Can Entomol 105:1505–1512. https://doi.org/10.4039/Ent1051505-12
Carvalho MC, Queiroz PCD, Ruszczyk A (1998) Protandry and female size-fecundity variation in the tropical butterfly Brassolis sophorae. Oecologia 116:98–102. https://doi.org/10.1007/s004420050567
Choi KS, Lee JM, Park JH, Cho JR, Song JH, Kim DS, Boo KS (2009) Sex pheromone composition of the cotton caterpillar, Palpita indica (Lepidoptera: Pyralidae), in Korea. J Asia Pac Entomol 12:269–275. https://doi.org/10.1016/j.aspen.2009.07.003
Coutinho EF, Jorge RO, Haerter JA, Costa VB (2015) Oliveira: aspectos técnicos e cultivo no sul do Brasil. 1 ed. Embrapa, Brasília, 181p
Delisle J, McNeil JN (1986) The effect of photoperiod on calling behavior of virgin females of the true armyworm, Pseudaletia unipucta (Haw.) (Lepidoptera: Noctuidae). J Insect Physiol 32:199–206. https://doi.org/10.1016/0022-1910(86)90059-4
Elliot AC, Hynan LS (2011) A SAS® macro implementation of a multiple comparison post hoc test for a Kruskal-Wallis analysis. Comput Methods Programs Biomed 102:75–80
FAOSTAT (2019) Food and Agriculture Organization of the United Nation in (http://www.fao.org/faostat/en/#data) (accessed 07 Jan 2023)
Foster SP, Howard AJ (1999) The effects of mating, age at mating, and plant stimuli, on the lifetime fecundity and fertility of the generalist herbivore Epiphyas postvittana. Entomol Exp Appl 91:287–295. https://doi.org/10.1046/j.1570-7458.1999.00495.x
Gehring RD, Madsen HF (1963) Some aspects of the mating and oviposition behavior of the Codling Moth, Carpocapsa pomonella. J Econ Entomol 56:140–143. https://doi.org/10.1093/jee/56.2.140
Hayden JE, Buss LJ (2013) Olive shootworm, Palpita persimilis Munroe (Insecta: Lepidoptera: Crambidae). EENY556/IN995. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://doi.org/10.32473/edis-in995-2013
Hegazi EM, Konstantopoulou MA, Milonas P, Herz A, Mazomenos BE, Khafagi WE, Zaitun A, Abdel-Rahman SM, Helal I, El-Kemny S (2007) Mating disruption of the jasmine moth Palpita unionalis (Lepidoptera: Pyralidae) using a two pheromone componente blend: A case study over three consecutive olive growing seasons in Egypt. Crop Prot 26:837–844. https://doi.org/10.1016/j.cropro.2006.08.003
Henneberry TJ, Clayton TE (1984) Time of emergence, mating, sperm movement, and transfer of ejaculatory duct secretory fluid by Heliothis virescens (F.) (Lepidoptera: Noctuidae) under reversed light-dark cycle laboratory conditions. Ann Entomol Soc Am 77:301–305. https://doi.org/10.1093/aesa/77.3.301
Jiménez-Pérez A, Wang Q (2004) Male remating behavior and its effect on female reproductive fitness in Cnephasia jactatana Walker (Lepidoptera: Tortricidae). J Insect Behav 17:685–694. https://doi.org/10.1023/B:JOIR.0000042549.59147.50
Keller L, Reeve HK (1995) Why do females mate with multiple males? The sexually selected sperm hypothesis. Adv Stud Behav 24:291–315. https://doi.org/10.1016/S0065-3454(08)60397-6
Mazomenos BE, Konstantopoulou M, Stefanou D, Skareas S, Tzeiranakis LC (2002) Female calling behaviour and male response to the synthetic sex pheromone components of Palpita unionalis (Lepidoptera: Pyralidae). IOBC Wprs Bulletin 25:1–10
McNeil JN (1991) Behavioral ecology of pheromone-mediated communication in moths and its importance in the use of pheromone traps. Ann Rev Entomol 36:407–430. https://doi.org/10.1146/annurev.en.36.010191.002203
Morais RMD, Redaelli LR, Sant’Ana J (2012) Age and multiple mating effects on reproductive success of Grapholita molesta (Busck) (Lepidoptera, Tortricidae). Rev Bras Entomol 56:319–324. https://doi.org/10.1590/S0085-56262012005000039
Paullier J (2013) Plagas del olivo. In M.A. Grompone and J. Villamil (eds.), Aceites de olive: de la planta al consumidor. INIA: Hemisferio Sur, Montevideo. (pp. 169–181)
Ricalde MP, Nava DE, Loeck AE, Coutinho EF, Bisognin A, Garcia FMR (2015) Insects related to Olive culture in Rio Grande do Sul State. Brazil Cienc Rural 45:2125–2130. https://doi.org/10.1590/0103-8478cr20141477
Ricalde MP, Nava DE, Loeck AE, Coutinho EF, Bisognin A, Garcia FRM (2014) Occurrence of caterpillar of the Olive Tree, Palpita forficifera (Lepidoptera: Pyralidae) in olive groves in the state of Rio Grande do Sul. Acta Hort. 1057. https://doi.org/10.17660/ActaHortic.2014.1057.45
Scheunemann T, Grützmacher AD, Nörnberg SD, Gonçalves RS, Nava DE (2017) Deu Traça Cultivar H F 105:14–16
Shi X, T. Ma S, Zhang Z, Sun X, Chen C, Wang C, Jia Y, Liang Y, Zhu, YH, Wen X (2018) Calling and mating behavior of Diaphania angustalis (Lepidoptera: Crambidae). J Econ Entomol 111(5): 2250-2254
Zonneveld C (1997) Being big or emerging early? Polyandry and the trade-off between size and emergence in male butterflies. Am Nat 149:946–965
Acknowledgements
This study was carried out with the support of the Coordination for the Improvement of Higher Education Personnel—Brazil (CAPES)—Funding Code 001.
Author information
Authors and Affiliations
Contributions
TS, RMB, SDN, RSG and DB conducted, analyzed and wrote the manuscript. DEN and DB revised the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Edited by André VL Freitas
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Scheunemann, T., Manica-Berto, R., Nörnberg, S.D. et al. Mating behavior and oviposition of Palpita forficifera (Lepidoptera: Crambidae). Neotrop Entomol 53, 738–745 (2024). https://doi.org/10.1007/s13744-024-01174-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-024-01174-1