Abstract
The peanut thrips, Enneothrips enigmaticus (Thysanoptera: Thrypidae), is an important pest of the peanut (Arachis hypogaea) in South America. Due to concerns about the environment and human health induced by the extensive use of pesticides in the management control of pests, environmentally and friendlier tactics must be targeted. Thus, this study investigates, for the first time, the behavior of Xylocoris sordidus (Hemiptera: Anthocoridae) as a biological control agent for E. enigmaticus. The methodology included no-choice tests to assess whether the predation rate varies according to the developmental stage of the prey, as well as the predator’s developmental stage with the highest predation capacity. Additionally, an analysis of the functional response of adult and 5th instar nymphs of X. sordidus exposed to different densities of E. enigmaticus nymphs (1, 2, 4, 8, 16, and 32) was conducted. The results confirm the predation of peanut thrips by X. sordidus, with a higher predation rate in the nymphal stages of the prey. There was no difference in predation capacity between predator nymphs and adults, and exhibiting a type II functional response. Therefore, the potential of X. sordidus as a biological control agent for E. enigmaticus is confirmed, showing the importance of adopting measures to preserve this predator in peanut crops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peanut, Arachis hypogaea L. (Fabales: Fabaceae), is a globally significant oilseed crop. In Brazil, the 11th largest producer worldwide, the state of São Paulo is the leading producer (Farinelli et al. 2018; Santos et al. 2019), where a significant portion of the production is obtained in areas used for sugarcane crop (Fernandes and Michelotto 2022). In these areas, where peanut crops are usually secondary, it is mainly aimed at the recovery of the soil exploited by sugarcane crops; pest control is often neglected, which potentially reduces its production.
The peanut thrips are one of the most significant pests in South America’s peanut production, which was formerly known as Enneothrips flavens Moulton (Thysanoptera: Thrypidae). However, detailed studies involving morphological, biological, and molecular analyses revealed that this species has, in fact, a new denomination: Enneothrips enigmaticus Lima, Alencar, Nanini, Michelotto and Correa (Thysanoptera: Thrypidae) (Lima et al. 2022). These thrips exhibit a behavior of scraping the inner part of the closed peanut leaflets, causing abnormal leaf development with silver lesions on the adaxial leaves surface. This impairs the plant’s photosynthetic area and the development of new shoots (Almeida and Arruda 1962; Fernandes and Michelotto 2022). Consequently, damage to the crop can result in yield reductions ranging from 19.5 to 62.7% (Moraes et al. 2005).
In Brazil, the most common method for the management of peanut thrips is the use of chemical control (Calore et al. 2015; Michelotto et al. 2017). However, due to the adverse effects of chemical pesticides on the environment, and human health, also in addition to the efficiency of control, more sustainable pest management options are required (Barzman et al. 2015; Baker et al. 2020; Deguine et al. 2021). In this regard, biological control is a promising tactic that needs to be prioritized (Naranjo et al. 2015; van Lenteren et al. 2018).
Among arthropods, predators and parasitoids can act as biological control agents, in which specialist individuals are normally prioritized. This situation occurs because generalist natural enemies often attack a wide diversity of prey species, including pests and non-target organisms, which can generate uncertainty about their effectiveness in pest management (Koss and Snyder 2005; Krey et al. 2017; Kheirodin et al. 2020). Still, there are studies that support the use of generalist natural enemies or even indicate that biocontrol effectiveness tends to be greater by generalist agents (Stiling and Cornelissen 2005; Doğramaci et al. 2011; Messelink and Janssen 2014). The advantage of generalist natural enemies is their persistence even when the target pest is reduced. When using alternative food sources, the presence of these arthropods in the crop before the target pest becomes abundant makes it possible to suppress the pest before it can cause economic damage (Clercq 2002; Pijnakker et al. 2020; Andow et al. 2021). However, it is important to take into account that each species is different, and that their responses to various conditions will differ according to their specific characteristics, so each situation must be evaluated based on adapted criteria and parameters (Pilkington et al. 2010; Diehl et al. 2013; Loomans 2021).
Bugs from the Anthocoridae family are considered promising biological control agents, as they are generalist predators that feed on small arthropods such as aphids, psyllids, scale insects, and thrips (Saulich and Musolin 2009; Perdikis et al. 2011). The action of these insects against economically important pests has been reported, including the predation of Orius niger (Wolff) (Hemiptera: Anthocoridae) on Frankliniella spp. (Thysanoptera: Thripidae), Orius strigicollis (Poppius) (Hemiptera: Anthocoridae) on Tetranychus urticae (Koch) (Acarina: Tetranychidae), and the predation of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) by Xylocoris sp. (Heteroptera: Anthocoridae) (Miranda et al. 1998; Atakan 2006; Tuan et al. 2016). Additionally, anthocorids are considered thrips control agents, already used for this purpose in greenhouses (Yang et al. 2014; van Lenterem et al. 2018).
Xylocoris sordidus (Reuter) (Hemiptera: Anthocoridae) is a potential control agent for pests such as Plutella xylostella (L.) (Lepidoptera: Plutellidae), Corcyra cephalonica (Stainton) (Lepidoptera: Pyralidae), and Diatraea saccharalis (Fabr.) (Lepidoptera: Crambidae) (Santos et al. 2020; Santos and Bortoli 2018). The occurrence of this species has already been reported in peanut crops (Arbogast et al. 1983), although its role in the agroecosystem remains unknown. Hence, it is necessary to determine whether this species can act as a potential biological control agent for E. enigmaticus.
Information about the interactions between predator and prey, as well as the behavioral characteristics of the predator, is essential to advance the appropriate management of the target pest. One of the most used tests that allows us to understand the predator’s feeding behavior is the functional response test, which evaluates the consumption rate by a predator according to the variation in the density of the prey (Holling 1959, 1966; Dawes and Souza 2013). There are three types of functional responses: type I, with increase foraging linearly according to the prey densities; type II, where the predators must pay a cost in time for each resource used individually and the result is an asymptotic response; and type III, with a sigmoidal response according to the prey densities. Being to predator insects, the response types II and III are the most common, and several factors can influence the response example the stage of the prey or others (Albashir et al. 2004; Uiterwaal and DeLong 2018). The parameters of the functional response estimated are attack rate and handling time, describing some predatory behavior (Kalinoski and DeLong 2016). The functional response of some anthochorids has already been investigated in previous studies, such as Amphiareus constrictus (Stal), Blaptostethus pallescens Poppius, and Orius tristicolor (White) (Hemiptera: Anthocoridae) when preying on T. absoluta (Queiroz et al. 2015), Orius sauteri (Poppius) (Hemiptera: Anthocoridae) preying on Dendrothrips minowai Priesner (Thysanoptera: Thripidae) (Zhang et al. 2021), and Orius similis Zheng (Hemiptera: Anthocoridae) preying on Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) (Zeng et al. 2021). Furthermore, other studies have demonstrated that anthochorid predators have different preferences regarding the stage of development of their prey, which reinforces the need to know the behavior of the control agent according to the target pest and thus make management more efficient (Baez et al. 2004; Tavares et al. 2013; Rashedi et al. 2020).
Therefore, to evaluate the potential of X. sordidus in the biological control of peanut thrips, this study investigates, for the first time, the predatory capacity of X. sordidus through predation rate and functional response at different densities of nymphs and adults of E. enigmaticus sp. Therefore, this study is based on the following hypotheses: (1) X. sordidus preys on peanut thrips and is able to find them in the peanut leaflet; (2) the developmental stage of X. sordidus affects predation and functional response when feeding on E. enigmaticus sp.; and (3) the developmental stage of E. enigmaticus sp. affects predation by X. sordidus.
Materials and methods
The experiments were conducted in the Laboratory of Applied Ecology (ApEcoLab) and the Laboratory of Insect Biology and Rearing (LBCI) of the Department of Agricultural Production Sciences, Faculty of Agricultural and Veterinary Sciences, São Paulo State University (UNESP), Jaboticabal, SP, Brazil.
Obtaining the prey Enneothrips enigmaticus
Closed peanut (A. hypogaea L.) leaflets were collected from experimental fields at FCAV/Unesp (Jaboticabal, São Paulo, Brazil), at coordinates − 21.238286 latitude and − 48.284388 longitudes. Subsequently, the leaflets were examined using a stereoscopic magnifying glass to locate individuals of E. enigmaticus. The thrips found were counted, and the leaflets were separated for later use in the tests. Thrips were differentiated into nymphs or adults, with adults identified by the complete development of wings.
Obtaining the predator Xylocoris sordidus
The insects used were obtained from a rearing maintained at the Laboratory of Biology and Insect Rearing (LBIR) in Jaboticabal, SP (Brazil). The rearing follows the methodology described by Santos et al. (2020), with adaptations.
To establish a suitable environment for X. sordidus, oviposition small cotton rolls (approximately 3 cm in diameter × 10 cm in length), premoistened with deionized water, were placed in 25-ml glass vials containing 20 ml of deionized water. These vials were placed inside glass containers (11 cm in diameter, 17 cm in height), referred to as cages, containing folded paper towels shaped like “W,” which acted as shelters to prevent cannibalism. The cages were adapted with a lid opening (diameter of 5 cm) covered with voile fabric to improve internal air circulation.
After oviposition, the eggs present in the cotton were transferred to glass Petri dishes (14 cm in diameter, 2 cm in height) lined with moistened paper towels and cotton, preventing desiccation and the death of X. sordidus embryos and nymphs. Two-day-old eggs of D. saccharalis (obtained from the São Martinho Biofactory, Pradópolis, São Paulo, Brazil) were provided every 2 days for predator feeding. The plates were sealed with polyvinyl chloride film (PVC) film, and the nymphs were regularly observed until they reached adulthood. Subsequently, the insects were removed using fine-tipped brushes and transferred to cages for mating and oviposition. Container cleaning and maintenance of the rearing were performed every 2 days.
Predation rate on nymphs and adults of the prey
A no-choice test was conducted to evaluate whether the predation rate of X. sordidus differs when feeding on nymphs or adults of E. enigmaticus. Peanut leaflets containing 15 preys were placed in disposable polystyrene Petri dishes (6 cm in diameter), and the predators were transferred to these plates using a fine brush. Each plate contained a filter paper at the base along with a damp cotton ball, peanut leaflets with thrips, and only one unfed adult predator for 24 h. The insects were kept in a BOD (Biochemical Oxygen Demand) chamber at a temperature of 25 ± 2 °C and a 12-h photophase. After 24 h, the number of surviving thrips per plate was counted. The number of consumed preys was assessed by estimating the mean percentage of consumption 24 h after exposure to the prey. Twelve replications were performed for each treatment.
Predation rate of predator nymphs and adults
Another test was carried out to assess whether the predation rate of X. sordidus differs according to its developmental stage (adult or immature). Fifth instar nymphs and adults of X. sordidus were used in a no-choice test condition, being this test carried out under the same conditions as the previous predation rate test. Fifteen prey nymphs were offered to each predator on peanut leaflets placed in Petri dishes. Fifteen replications were conducted for different predator stages, and after 24 h, the number of surviving thrips per plate was counted. The insects were kept in a BOD (Biochemical Oxygen Demand) chamber at a temperature of 25 ± 2 °C and a 12-h photophase.
Functional response
For the analysis of the functional response, adults and 5th instar nymphs of X. sordidus were exposed to different densities of E. enigmaticus nymphs (1, 2, 4, 8, 16, and 32). Peanut leaflets with the predetermined number of each density were placed in disposable polystyrene Petri dishes (6 cm in diameter), and the predators were transferred to these plates using a fine brush. Each plate contained a filter paper at the base along with a damp cotton ball, peanut leaflets with thrips, and only one predator. The insects were kept in a BOD (Biochemical Oxygen Demand) chamber at a temperature of 25 ± 2 °C and a 12-h photophase. After 24 h of exposure, the number of E. enigmaticus nymphs consumed was counted. The predators were kept without food for a period of 24 h prior to tests starting, and 10 replications were conducted for each prey density.
Analysis of data
The frequency data from the choice tests were analyzed using Proc FREQ and interpreted using the chi-square test (χ2), where the null hypothesis assumed a 1:1 ratio, indicating that predation has no difference between prey types.
The survival rate of X. sordidus adults was compared between treatments using the log-rank test from the Kaplan–Meier method, utilizing Proc LIFETEST (SAS Institute 2022). Manly’s index was estimated using the following equation:
where β = represents the prey preference, j = refers to the number of treatments to which the prey was subjected, e = is the number of preys consumed during the exposure period (24 h), and A represents the total number of preys fed by the predator. This index produces values between 0 and 1. A value of 0.5 indicates that predation has no difference between prey types. Values above 0.5 indicate a difference in predation between types for prey. This method considers the decrease in prey density due to predation during the experimental evaluation (Sherratt and Harvey 1993). The difference between the number of preys consumed in predation tests under different choice conditions and those obtained by the Manly preference index was determined using the Student–Newman–Keuls and t-tests (P < 0.05) using Procs GLM or TTEST for comparison. The means of the percentage of prey consumed depending on the selection conditions were compared by the Student–Newman–Keuls test (P < 0.05) using Proc GLM (SAS Institute 2022). The type of functional response was determined by nonlinear logistic regression using Proc CATMOD (SAS Institute 2022). The polynomial function describing the relationship between Na/N0 and N0 was obtained using the equation:
Here, Na = represents the number of preys consumed, N0 = represents the number of preys provided, and P0, P1, P2, and P3, are the constant, linear, quadratic, and cubic coefficients, respectively, related to the curve’s slope. All parameters were estimated by maximum likelihood. When P1 > 0 and P2 < 0, the proportion of prey consumed is positively related to density, indicating a type III functional response. When P1 < 0, the proportion of prey consumed decreases monotonically with the initially offered number, describing a type II functional response (Juliano 2001). First, the cubic model was tested, but the equation terms were reduced until significance was achieved. The type of functional response was determined using nonlinear logistic regression based on a random equation proposed by Rogers (1972):
where Na = is the number of preys consumed, N0 = is the number of preys provided, a = is the attack rate, Th = is the handling time, and T = is the time the predator is exposed to the preys (24 h). The attack rate (a′) and handling time (Th) parameters were analyzed by nonlinear regression, as suggested by Rogers (1972), using Proc NLIN (SAS Institute 2022), and compared based on the generated confidence intervals (CI); if the CIs do not overlap, the difference between the means is significant (P < 0.05) (Di Stefano 2005). The maximum predation rate T/Th was also estimated.
Results
Predation rate on nymphs and adults of the prey
The no-choice test showed that X. sordidus adults consumed a significantly higher number of nymphs than adults of E. enigmaticus (χ2 = 27.4163; P < 0.0001), being 60.64% and 39.36% respectively (Fig. 1a).
Predation rate of predator nymphs and adults
The results demonstrate that both adults and nymphs of X. sordidus consume a statistically equivalent number of E. enigmaticus (χ2 = 2.2786; P = 0.1312), accounting for 52.99% and 47.01% of adults and nymphs, respectively (Fig. 1b). In other words, both nymphs and adults of X. sordidus can prey on E. enigmaticus nymphs.
Functional response of X. sordidus on E. enigmaticus nymphs
Xylocoris sordidus showed predatory ability in both nymph and adult stages. The results of the logistic regression analysis for the nymphs were significant (P < 0.05), with a negative linear coefficient (P1) of − 0.0428, indicating a type II functional response to the nymphs of the prey, E. enigmaticus (Table 1). Similarly, X. sordidus adults showed a type II functional response (P < 0.05; P1 − 0.0361) in relation to the prey.
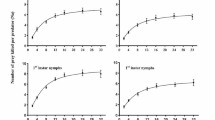
The functional response curves for nymphs and adults of X. sordidus on E. enigmaticus at different densities are shown in Fig. 2a and b. The number of thrips consumed by the nymphs and adults of X. sordidus increased with the increase in prey density but tended to stabilize from density 16. At the subsequent density 32, neither adults nor nymphs were able to prey on more than 20 thrips.
Nymphs and adults of X. sordidus showed a higher percentage of prey consumption when provided with four nymphs of E. enigmaticus. When exposed to four thrips nymphs, X. sordidus nymphs consumes on average three nymphs per day (representing 75% of the preys). However, when offered 32 nymphs, the consumption average was 14.2 nymphs (equivalent to 44.5% of the prey), suggesting that the predator nymphs are more efficient in locating the preys at lower prey densities. A similar trend was observed with X. sordidus adults, with consumption of 3.4 and 20 nymphs (86.6% and 62.7% of the preys) when exposed to 4 and 32 nymphs of E. enigmaticus, respectively.
The attack rate (a′) of X. sordidus preying nymphs of E. enigmaticus did not vary between the predator’s stages, with fifth instar nymphs and adults showing statistically equal (a′) values. The handling times (Th) were lower for adults (1.0650 h) than fifth instar nymphs (1.5878 h) and the maximum predation rate (T/Th) was higher for X. sordidus adults (Table 2).
Discussion
It was confirmed the potential of X. sordidus as a biological control agent to E. enigmaticus, being the predator able to locate the prey hidden in peanut leaflets. The predation capability of the bug increased as the number of thrips increased, and there was no difference in predation capability between nymphs and adult of the predators. However, X. sordidus adults showed a higher predation rate when feeding on prey nymphs.
The predation capability of X. sordidus was expected since several species from the Anthocoridae family are studied and commercialized to use in biological pest control. As an example, we can mention Orius insidiosus (Say) (Hemiptera: Anthocoridae) which is sold in several countries, including Brazil. The same happens with Xylocoris flavipes (Reuter) (Hemiptera: Anthocoridae) in North America where the major focus is the pest control of stored products. Furthermore, the fact that X. flavipes can locate and attack larvae that feed internally on stored grains, confirming the genus’ ability to prey in complex habitats (Donnelly and Phillips 2001).
Previous studies analyzing the action of anthocorids against thrips species also found functional response type II (Tavares et al. 2013; Liu et al. 2018). Predators that show functional response type II can be used in augmentative biological control programs. In this case, many predators should be released in the area to reduce the estimated prey population (van Lenteren et al. 2016).
Predators with a low handling time (Th) and a high attack rate (a′) are considered more effective as biological control agents (Salehi et al. 2016). In this study, X. sordidus adults demonstrated a lower handling time than fifth instar nymphs, indicating greater efficiency in capturing and consuming E. enigmaticus nymphs. This difference in handling time may be related to a variety of factors, such as voracity variation, satiation time, digestive capacity, and walking speed (Pervez and Omkar 2005; Seko and Miura 2008; Rahman et al. 2022). On the other hand, the attack rate remained constant between predator stages, suggesting that prey capture effectiveness is an intrinsic characteristic of the species, established in nymphs and maintained in adults.
Regarding the higher predation rate by nymphs compared to adult thrips, it may probably be due to adults being more mobile than nymphs and being able to escape predator attack more easily (Gitonga et al. 2002). Similar observations were made for Orius. insidiosus preying on thrips of the genus Frankliniella and Orius albidipennis Reuter (Hemiptera: Anthocoridae) preying on Aphis fabae Scopoli (Homoptera: Aphididae) (Baez et al. 2004; Rashedi et al. 2020). Furthermore, it is important to note that predation of immature nymphs is advantageous in reducing the pest population before individuals reach the reproductive stage, potentially reducing the next generation of the pest in the area.
Most anthocorids are predators in the nymphal and adult stages (Ballal and Yamada 2016). However, it is important to note that the prey consumption pattern can vary considerably among the different predator development stages. Sarker et al. (2016) found that the consumption of eggs of Cryptolestes pusillus (Schon.) (Coleoptera: Cucujidae) by X. flavipes increases with the nymphs’ age. Additionally, in other investigations, it was observed that predation by O. insidiosus on Caliothrips phaseoli (Hood) (Thysanoptera: Thripidae) was more effective in the adult stage compared to the immatures nymphs (Silva et al. 2023). This situation may occur because larger predatory arthropods usually move faster, detect prey at greater distances, and subdue prey easier (Uiterwaal and DeLong 2018).
However, in this study, a different pattern from the one described above was observed. Predation of E. enigmaticus by fifth instar nymphs and adults showed no statistical difference. Supporting these results, LeCato (1976) identified a similar predation pattern in X. flavipes when consuming larvae of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae), and Arnó et al. (2008) in Orius laevigatus (Fieber) (Hemiptera: Anthocoridae) preying on immatures of F. occidentalis. These studies converge to emphasize the importance of understanding variations in predatory behavior among the different predator development stages and different prey species. Additionally, it is possible to note that predator behavior can vary according to the predator and prey species, even within the same family.
It is important to consider that while analyses of predator functional responses are useful, results in field conditions may differ from responses in laboratory conditions. This is because the foraging effectiveness of the predator can be affected by a combination of many factors, such as temperature and prey availability, among others (Ge et al. 2018; Hassanzadeh-Avval et al. 2019). Thus, the results of this study serve as a basis for the use of X. sordidus in biological control programs. However, field experiments are still necessary to elucidate the interactions between X. sordidus and E. enigmaticus (Ge et al. 2018).
Although the potential of X. sordidus as a biological control agent is confirmed, it is worth mentioning that better pest control results can be achieved by preserving populations of the natural enemies. For this purpose, some tactics can be used, such as reducing the use of insecticides or using selective insecticides (Torres and Bueno 2018; Lin et al. 2021). Additionally, planting polycultures and selecting non-agricultural host plants in the crop areas can also contribute to increasing and sustaining natural enemies in the region (Perdikis; Peñalver-Cruz et al. 2019; Atakan and Pehlivan 2020).
In conclusion, this study provides evidence that X. sordidus has the potential to be an effective predator against E. enigmaticus by contributing to the reduction of pests’ populations. Conservation tactics that favor the presence of natural enemies in crops should be prioritized to ensure the persistence of the predators in crop area, and the use of augmentative biological control should also be evaluated. The information reported here can help improve peanut pest management and contribute to the development of sustainable agriculture.
Data Availability
The data that support the findings of this study are available from the first author upon reasonable request.
References
Albashir A., Aljetlawi Erik, Sparrevik Kjell, Leonardsson (2004) Prey–predator size‐dependent functional response: derivation and rescaling to the real world. J Anim Ecol 73(2):239–252. https://doi.org/10.1111/jae.2004.73.issue-2https://doi.org/10.1111/j.0021-8790.2004.00800.x
Almeida PRD, Arruda HVD (1962) Controle do tripes causador do prateamento das fôlhas do amendoim, por meio de inseticidas. Bragantia 21:679–687. https://doi.org/10.1590/S0006-87051962000100039
Andow DA, Barratt BI, Pfannenstiel RS, Paula DP (2021) Exotic generalist arthropod biological control agents: need to improve environmental risk assessment to ensure safe use. Biol Control 66:1–8. https://doi.org/10.1007/s10526-020-10067-2
Arbogast RT, Flaherty BR, Press JW (1983) Demography of the predaceous bug Xylocoris sordidus (Reuter). Am Midl Nat 109:398–405. https://doi.org/10.2307/2425421
Arnó J, Roig J, Riudavets J (2008) Evaluation of Orius majusculus and O. laevigatus as predators of Bemisa tabaci and estimation of their prey preference. Biol Control 44:1–6. https://doi.org/10.1016/j.biocontrol.2007.10.009
Atakan E (2006) Associations between Frankliniella spp. and Orius niger populations in cotton. Phytoparasitica 34:221–234. https://doi.org/10.1007/BF02980949
Atakan E, Pehlivan S (2020) Influence of weed management on the abundance of thrips species (Thysanoptera) and the predatory bug, Orius niger (Hemiptera: Anthocoridae) in citrus mandarin. Appl Entomol Zool 55:71–81. https://doi.org/10.1007/s13355-019-00655-9
Baez I, Reitz SR, Funderburk JE (2004) Predation by Orius insidiosus (Heteroptera: Anthocoridae) on life stages and species of Frankliniella flower thrips (Thysanoptera: Thripidae) in pepper flowers. Environ Entomol 33:662–670. https://doi.org/10.1603/0046-225X-33.3.662
Baker BP, Green TA, Loker AJ (2020) Biological control and integrated pest management in organic and conventional systems. Biol Control 140:104095. https://doi.org/10.1016/j.biocontrol.2019.104095
Ballal CR, Yamada K (2016) Anthocorid predators. In: Omkar (Ed) Ecofriendly pest management for food security, 1st edn. Elsevier, New York, pp 183–216
Barzman M, Bàrberi P, Birch ANE, Boonekamp P, Dachbrodt-Saaydeh S, Graf B, Sattin M (2015) Eight principles of integrated pest management. Agron Sustain Dev 35:1199–1215. https://doi.org/10.1007/s13593-015-0327-9
Calore RA, Ferreira MC, Galli JC (2015) Efeitos de adjuvantes no controle de Enneothrips flavens Moulton, 1941 (Thysanoptera: Thripidae) na cultura do amendoim. Bras Cienc Agrar 10:74–81. https://doi.org/10.5039/agraria.v10i1a5043
Clercq PD (2002) Dark clouds and their silver linings: exotic generalist predators in augmentative biological control. Neotrop Entomol 31:169–176. https://doi.org/10.1590/S1519-566X2002000200001
Dawes JHP, Souza M (2013) A derivation of Holling’s type I, II and III functional responses in predator–prey systems. J Theor Biol 327:11–22. https://doi.org/10.1016/j.jtbi.2013.02.017
Deguine JP, Aubertot JN, Flor RJ, Lescourret F, Wyckhuys KA, Ratnadass A (2021) Integrated pest management: good intentions, hard realities. A Review Agron Sustain Dev 41:38. https://doi.org/10.1007/s13593-021-00689-w
Di Stefano J (2005) Effect size estimates and confidence intervals: an alternative focus for the presentation and interpretation of ecological data. In: Burk AR (ed) New Trends in ecology research. Nova Science, New York, pp 71–102
Diehl E, Sereda E, Wolters V, Birkhofer K (2013) Effects of predator specialization, host plant and climate on biological control of aphids by natural enemies: a meta-analysis. J Appl Ecol 50:262–270. https://doi.org/10.1111/1365-2664.12032
Doğramaci M, Arthurs SP, Chen J, McKenzie C, Irrizary F, Osborne L (2011) Management of chilli thrips Scirtothrips dorsalis (Thysanoptera: Thripidae) on peppers by Amblyseius swirskii (Acari: Phytoseiidae) and Orius insidiosus (Hemiptera: Anthocoridae). Biol Control 59:340–347. https://doi.org/10.1016/j.biocontrol.2011.09.008
Donnelly BE, Phillips TW (2001) Functional response of Xylocoris flavipes (Hemiptera: Anthocoridae)-effects of prey species and habitat. Environ Entomol 30:617–624. https://doi.org/10.1603/0046-225X-30.3.617
Farinelli JBM, Horita K, Santos DFL (2018) Analysis of the economic viability of the peanut crop in the region of Jaboticabal, São Paulo. Científica 46:215–220. https://doi.org/10.15361/1984-5529.2018v46n3p215-220
Fernandes OA, Michelotto MD (2022) Pragas do amendoim. In: Rada A et al (eds) Manual de entomologia: pragas das culturas, 1st edn. Ouro fino, MG, Agronômica Ceres, pp 41–56
Ge Y, Camara I, Wang Y, Liu P, Zhang L, Xing Y, Shi W (2018) Predation of Aphis craccivora (Hemiptera: Aphididae) by Orius sauteri (Hemiptera: Anthocoridae) under different temperatures. J Econ Entomol 111:2599–2604. https://doi.org/10.1093/jee/toy255
Gitonga LM, Overholt WA, Löhr B, Magambo JK, Mueke JM (2002) Functional response of Orius albidipennis (Hemiptera: Anthocoridae) to Megalurothrips sjostedti (Thysanoptera: Thripidae). Biol Control 24:1–6. https://doi.org/10.1016/S1049-9644(02)00001-4
Hassanzadeh-Avval M, Sadeghi-Namaghi H, Fekrat L (2019) Factors influencing functional response, handling time and searching efficiency of Anthocoris minki Dohrn (Hem.: Anthocoridae) as predator of Psyllopsis repens Loginova (Hem.: Psyllidae). Phytoparasitica 47:341–350. https://doi.org/10.1007/s12600-019-00739-w
Holling CS (1959) Some characteristics of simple types of predation and parasitism. Can Entomol 91:385–398. https://doi.org/10.4039/Ent91385-7
Holling CS (1966) The functional response of invertebrate predators to prey density. Mem Entomol Soc Can 98:5–86. https://doi.org/10.4039/entm9848fv
Juliano SA (2001) Non-linear curve fitting: predation and functional response curves. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments, 2nd edn. Chapman & Hall, New York, pp 178–196
Kheirodin A, Simmons AM, Legaspi JC, Grabarczyk EE, Toews MD, Roberts PM, Schmidt JM (2020) Can generalist predators control Bemisia tabaci? Insects 11:823. https://doi.org/10.3390/insects11110823
Koss AM, Snyder WE (2005) Alternative prey disrupt biocontrol by a guild of generalist predators. Biol Control 32(2):243–251. https://doi.org/10.1016/j.biocontrol.2004.10.002
Krey KL, Blubaugh CK, Chapman EG, Lynch CA, Snyder GB, Jensen AS, Snyder WE (2017) Generalist predators consume spider mites despite the presence of alternative prey. Biol Control 115:157–164. https://doi.org/10.1007/BF02371908
LeCato GL (1976) Predation by Xylocoris flavipes [Hem.: Anthocoridae]: Influence of stage, species and density of prey and of starvation and density of predator. Entomophaga 21:217–221. https://doi.org/10.1007/BF02371908
Lima ÉFB, Alencar ÁRSD, Nanini F, Michelotto MD, Corrêa AS (2022) “Unmasking the Villain”: integrative taxonomy reveals the real identity of the key pest (Thysanoptera: Thripidae) of peanuts (Arachis hypogaea L.) in South America. Insects 13:120. https://doi.org/10.3390/insects13020120
Lin T, Zeng Z, Chen Y, You Y, Hu J, Yang F, Wei H (2021) Compatibility of six reduced-risk insecticides with Orius strigicollis (Heteroptera: Anthocoridae) predators for controlling Thrips hawaiiensis (Thysanoptera: Thripidae) pests. Ecotoxicol Environ Saf 226:112812. https://doi.org/10.1016/j.ecoenv.2021.112812
Liu P, Jia W, Zheng X, Zhang L, Sangbaramou R, Tan S, Shi W (2018) Predation functional response and life table parameters of Orius sauteri (Hemiptera: Anthocoridae) feeding on Megalurothrips usitatus (Thysanoptera: Thripidae). Fla Entomol 101:254–259. https://doi.org/10.1653/024.101.0216
Loomans AJ (2021) Every generalist biological control agent requires a special risk assessment. Biocontrol 66:23–35. https://doi.org/10.1007/s10526-020-10022-1
Messelink GJ, Janssen A (2014) Increased control of thrips and aphids in greenhouses with two species of generalist predatory bugs involved in intraguild predation. Biol Control 79:1–7. https://doi.org/10.1016/j.biocontrol.2014.07.009
Michelotto MD, De Godoy IJ, Pirotta MZ, Santos JF, Finoto EL, Pereira Fávero A (2017) Resistance to thrips (Enneothrips flavens) in wild and amphidiploid Arachis species. PLoS ONE 12:e0176811. https://doi.org/10.1371/journal.pone.0176811
Miranda MMM, Picanço MC, Zanuncio JC, Guedes RNC (1998) Ecological life table of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Biocontrol Sci Technol 8:597–606. https://doi.org/10.1080/09583159830117
Moraes ARAD, Lourenção AL, Godoy IJD, Teixeira GDC (2005) Infestation by Enneothrips flavens Moulton and yield of peanut cultivars. Sci Agric 62:469–472. https://doi.org/10.1590/S0103-90162005000500010
Naranjo SE, Ellsworth PC, Frisvold GB (2015) Economic value of biological control in integrated pest management of managed plant systems. Annu Rev Entomol 60:621–645. https://doi.org/10.1146/annurev-ento-010814-021005
Peñalver-Cruz A, Alvarez-Baca JK, Alfaro-Tapia A, Gontijo L, Lavandero B (2019) Manipulation of agricultural habitats to improve conservation biological control in South America. Neotrop Entomol 48:875–898. https://doi.org/10.1007/s13744-019-00725-1
Perdikis D, Fantinou A, Lykouressis D (2011) Enhancing pest control in annual crops by conservation of predatory Heteroptera. Biol Control 59:13–21. https://doi.org/10.1016/j.biocontrol.2011.03.014
Pervez A, Omkar A (2005) Functional responses of coccinellid predators: an illustration of a logistic approach. J Insect Sci 5:1–6. https://doi.org/10.1093/jis/5.1.5
Pijnakker J, Vangansbeke D, Duarte M, Moerkens R, Wäckers FL (2020) Predators and parasitoids-in-first: From inundative releases to preventative biological control in greenhouse crops. Front Sustain Food Syst 4:595630. https://doi.org/10.3389/fsufs.2020.595630
Pilkington LJ, Messelink G, van Lenteren JC, Le Mottee K (2010) “Protected Biological Control”–biological pest management in the greenhouse industry. Biol Control 52:216–220. https://doi.org/10.1016/j.biocontrol.2009.05.022
Queiroz OS, Ramos RS, Gontijo LM, Picanço MC (2015) Functional response of three species of predatory pirate bugs attacking eggs of Tuta absoluta (Lepidoptera: Gelechiidae). Environ Entomol 44:246–251. https://doi.org/10.1093/ee/nvu026
Rahman MA, Sarker S, Ham E, Lee JS, Lim UT (2022) Prey preference of Orius minutus and its functional response in comparison that of O. laevigatus, on Tetranychus urticae. J Asia Pac Entomol 25:101912. https://doi.org/10.1016/j.aspen.2022.101912
Rashedi A, Rajabpour A, Sohani NZ, Rasekh A (2020) Prey stage preference and functional response of Orius albidipennis (Hetetroptera, Anthocoridae) to Aphis fabae (Homomoptera, Aphididae). Int J Trop Insect Sci 40:13–19. https://doi.org/10.1007/s42690-019-00045-2
Rogers DJ (1972) Random search and insect population models. J Animal Ecol 41:369–383. https://doi.org/10.2307/3474
Salehi Z, Yarahmadi F, Rasekh A, Sohani NZ (2016) Functional responses of Orius albidipennis Reuter (Hemiptera, Anthocoridae) to Tuta absoluta Meyrick (Lepidoptera, Gelechiidae) on two tomato cultivars with different leaf morphological characteristics. Entomol Gen 36:127–136
Santos NAD, Bortoli SAD (2018) Xylocoris sordidus (Reuter) (Hemiptera: Anthocoridae): possível agente de controle biológico aplicado. Universidade de São Paulo, Thesis
Santos DFL, Silva BL, MoraesFarinelli JB, Horita K, Souza CAF, Montoro SB (2019) Economic viability of peanut production on leased land in the Jaboticabal region of São Paulo state, Brazil. Revista Agro@mbiente On-line 13:142–154. https://doi.org/10.18227/1982-8470ragro.v13i0.5342
Santos NA, Ramalho DG, Marques HM, Godoy JDS, De Bortoli CP, Magalhães GO, De Bortoli SA (2020) Interaction between the predator Xylocoris sordidus and Bacillus thuringiensis bioinsecticides. Entomol Exp Appl 168:371–380. https://doi.org/10.1111/eea.12896
Sarker AC, Islam W, Parween S (2016) Host-stage specific effects on the biological parameters of Xylocoris flavipes (reuter) preyed on Cryptolestes pussilus (SCHON.). Trop Agric Res 19:305–312
SAS Institute (2022) SAS/Graph Software: Reference, Vol. 1, Version 8. SAS Institute, Cary, NC, USA
Saulich AK, Musolin DL (2009) Seasonal development and ecology of anthocorids (Heteroptera, Anthocoridae). Entomol Rev 89:501–528. https://doi.org/10.1134/S0013873809050017
Seko T, Miura K (2008) Functional response of the lady beetle Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) on the aphid Myzus persicae (Sulzer) (Homoptera: Aphididae). Appl Entomol Zool 43:341–345. https://doi.org/10.1303/aez.2008.341
Sherratt TN, Harvey IF (1993) Frequency-dependent food selection by arthropods: a review. Biol J Linn Soc 48:167–186. https://doi.org/10.1111/j.1095-8312.1993.tb00885.x
Silva LP, Souza IL, Marucci RC, Guzman-Martinez M (2023) Doru luteipes (Dermaptera: Forficulidae) and Orius insidiosus (Hemiptera: Anthocoridae) as nocturnal and diurnal predators of thrips. Neotrop Entomol 52:263–272. https://doi.org/10.1007/s13744-022-00982-7
Stiling P, Cornelissen T (2005) What makes a successful biocontrol agent? A meta-analysis of biological control agent performance. Biol Control 34:236–246. https://doi.org/10.1016/j.biocontrol.2005.02.017
Tavares AM, Torres JB, Silva-Torres CS, Vacari AM (2013) Behavior of Montandoniola confusa Streito & Matocq (Hemiptera: Anthocoridae) preying upon gall-forming thrips Gynaikothrips ficorum Marchal (Thysanoptera: Phlaeothripidae). Biol Control 67:328–336. https://doi.org/10.1016/j.biocontrol.2013.09.004
Torres JB, Bueno ADF (2018) Conservation biological control using selective insecticides–a valuable tool for IPM. Biol Control 126:53–64. https://doi.org/10.1016/j.biocontrol.2018.07.012
Tuan SJ, Lin YH, Peng SC, Lai WH (2016) Predatory efficacy of Orius strigicollis (Hemiptera: Anthocoridae) against Tetranychus urticae (Acarina: Tetranychidae) on strawberry. J Asia Pac Entomol 19:109–114. https://doi.org/10.1016/j.aspen.2015.12.007
Uiterwaal SF, DeLong JP (2018) Multiple factors, including arena size, shape the functional responses of ladybird beetles. J Appl Ecol 55:2429–2438. https://doi.org/10.1111/1365-2664.13159
van Lenteren JC, Hemerik L, Lins JC Jr, Bueno VH (2016) Functional responses of three neotropical mirid predators to eggs of Tuta absoluta on tomato. Insects 7:34. https://doi.org/10.3390/insects7030034
van Lenteren JC, Bolckmans K, Köhl J, Ravensberg WJ, Urbaneja A (2018) Biological control using invertebrates and microorganisms: plenty of new opportunities. Biocontrol 63:39–59. https://doi.org/10.1007/s10526-017-9801-4
Yang NW, Zang LS, Wang S, Guo JY, Xu HX, Zhang F, Wan FH (2014) Biological pest management by predators and parasitoids in the greenhouse vegetables in China. Biol Control 68:92–102. https://doi.org/10.1016/j.biocontrol.2013.06.012
Zeng G, Zhi JR, Zhang CR, Zhang T, Ye JQ, Zhou L, Ye M (2021) Orius similis (Hemiptera: Anthocoridae): a promising candidate predator of Spodoptera frugiperda (Lepidoptera: Noctuidae). J Econ Entomol 114:582–589. https://doi.org/10.1093/jee/toaa318
Zhang Q, Zhang R, Zhang Q, Ji D, Zhou X, Jin L (2021) Functional response and control potential of Orius sauteri (Hemiptera: Anthocoridae) on tea thrips (Dendrothrips minowai Priesner). Insects 12:1132. https://doi.org/10.3390/insects12121132
Funding
CNPq (National Council for Scientific and Technological Development) and Capes (Coordination for the Improvement of Higher Education Personnel).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. All authors contributed to material preparation and data collection. Analysis was performed by D. G. R. All authors conducted an extensive literature review and contributed to the article's writing and result discussion. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Edited by Lessando Moreira Gontijo
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Oliveira, S.J., Nascimento, V.F., de Lacerda, L.B. et al. Predator–Prey Interaction Between Xylocoris sordidus (Hemiptera: Anthocoridae) and Enneothrips enigmaticus (Thysanoptera: Thripidae). Neotrop Entomol 53, 391–399 (2024). https://doi.org/10.1007/s13744-023-01126-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-023-01126-1