Abstract
Aphids are worldwide pests, and in South America, they harm many crops including winter cereals. In the 1970s, the rapid expansion of the wheat crop area in the subtropical region of South America led to growth of aphid populations. The wide availability of food, associated with the low effectiveness of natural biological control, put the aphid populations out of balance, requiring intensive use of insecticides. At the end of the decade, biological control programs of aphids were initiated in Argentina, Brazil, and Chile, including the importation of natural enemies (mainly parasitoids), followed by their laboratory rearing and field release. With decreased use of highly hazardous pesticides, biological control by introduced and already-present parasitoid species was enhanced. The program was very successful and aphid populations have been kept at low levels. This review article explores the history of this program and its current status. In modern day agriculture, with intense multiple cropping systems, adoption of several conservation practices, and increased cultivation of wheat in tropical regions, we discuss ways to keep this program effective to maintain aphid populations on cereal crops at low acceptable levels through employing biological control agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological control program of aphids on winter cereals in South America through importation and introduction of aphid parasitoids (Hymenoptera: Braconidae) can be considered one of the most successful cases of biological control in the world. Control programs for aphids on winter cereals were launched in Argentina, Brazil, and Chile. These programs generally followed the scheme described by Van den Bosch and Messenger (1973), with introduction, mass rearing, and release of natural enemies, featuring the so-called classic method of biological control. The hypothesis was that the disequilibrium of aphids on wheat could be at least partially reversed by natural enemies that co-evolved with invasive aphid species. The introduction of natural enemy species would allow some to adapt to the ecological conditions in the humid subtropical region in South America. They would establish and multiply freely in wheat aphids, helping to control populations. The first targets of the program were Metopolophium dirhodum (Walker) and Sitobion avenae (F.) species with the greatest economic impact at that time (Zúñiga-Salinas 1982; Starý et al. 1993a). The goal was to reach parasitism levels that cause 10–15% mortality of aphids (Zúñiga-Salinas 1982). A second phase of this program began in 1992 in Chile, aiming to control Diuraphis noxia (Kurdjumov) (Starý et al. 1993b; Starý 1995).
This review presents the history of aphids as pests of winter cereals, the situation of natural biological control before the biological control program, and the effects of the introduction of exotic species of aphid parasitoids in Argentina, Brazil, and Chile. The events in the subtropical region of Brazil are described in more detail in this review, comparing scenarios before and after the beginning of the biological control program. Finally, the perspectives for the future of biological control of winter cereal aphids in the region are addressed, considering the changes that have occurred with the modernization of agriculture.
Aphids as pests in winter cereals in South America
The economic relevance of aphids (Hemiptera: Aphididae) as pests of cultivated plants is because of their high prolificity and reproduction rate, associated with their ability to disperse, migrate, and transmit phytopathogens (Ng and Perry 2004; Fingu-Mabola and Francis 2021). Cereal aphids have different preferences for plant organs, from the roots to the reproductive structures including stems and leaves. They cause distinct types of damage, such as necrosis, chlorosis, and tissue morphological changes by saliva action. Furthermore, aphids are efficient vectors of barley/cereal yellow dwarf virus (B/CYDV), the causal agents of yellow dwarf disease, which is widely distributed in the world and have major economic impact on cereal yields (Halbert and Voegtlin 1995; Lau et al. 2021).
Several species of aphids use grasses (Poaceae) as host plants, which include cultivated and non-cultivated pastures, ground cover species, and cereals such as wheat (Triticum aestivum L.), oats (Avena spp.), barley (Hordeum vulgare L.), rice (Oryza spp.), and corn (Zea mays L.) (Singh et al. 2015). In agricultural systems, grasses are cultivated in different seasons and together with native grasses, often perennial, forming a complex landscape in which aphids complete their life cycle (Stell et al. 2022). Numerous Old World plants (European and Asian) were successfully introduced and became established in New World (America) ecosystems (Malmstrom et al. 2007), including wheat, black oat, Avena strigosa (Schreb.), white oat (Avena sativa L.), barley, rye (Secale cereale L.), and triticale (× Triticosecale Wittmack).
In the same way, cereal aphid species from the Palearctic have been reported in the Neotropical Region and their relationship with host plants and natural enemies investigated (Bertels 1956, 1970, 1974; Corseuil 1958, 1959; Costa 1944; Fehn 1970, 1974; Pimenta and Smith 1976; Zúñiga and Suzuki 1976). The first species established in South America was the greenbug aphid, Schizaphis graminum (Rondani), first recorded in 1914 in Argentina, and later in Brazil, Chile, Colombia, Paraguay, Peru, Uruguay, and Venezuela (Reiniger 1941; Pimenta and Smith 1976; Zúñiga and Suzuki 1976). Another early species recorded from 1930 to 1970 was the corn aphid, Rhopalosiphum maidis (Fitch), with records in Argentina, Brazil, Colombia, Uruguay, and Venezuela, but described as uncommon on wheat (Pimenta and Smith 1976; Zúñiga and Suzuki 1976).
Beginning in the 1960s, the English grain aphid, S. (= Macrosiphum) avenae; the rose-grass aphid, M. (= Acyrthosiphum) dirhodum; and the bird cherry oat aphid, Rhopalosiphum padi (L.) were first recorded in the region. S. avenae and M. dirhodum became the two most important species in South American wheat crops. S. avenae and M. dirhodum were first detected in Chile in 1966, and then in Argentina, Brazil, Peru, and Venezuela (Zúñiga and Suzuki 1976). Although R. padi was frequently found on wheat, oat, and barley, it was considered less relevant at that time. Also in this genus, the rice root aphid, Rhopalosiphum rufiabdominale (Sasaki) in Brazil, Chile, Colombia, and Venezuela, occurred less frequently on wheat, but used to cause problems on rice crops. Another species, the yellow sugarcane aphid, Sipha flava (Forbes), was also reported in Brazil, Peru, and Venezuela (Pimenta and Smith 1976; Zúñiga and Suzuki 1976).
Subsequently, the Russian wheat aphid, D. noxia, was reported in Chile in 1987 and later in Argentina in 1991. It has high damage capacity, mainly to cereals such as barley and wheat (Reed and Kindler 1994). The hedgehog grain aphid, Sipha maydis (Passerini), was first reported in Argentina (Ortego et al. 2004; Corrales et al. 2007) and rapidly expanded to Brazil, Argentina, Chile, Paraguay, Bolivia, Peru, Ecuador, and Colombia (CABI 2022; Pereira et al. 2008).
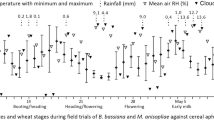
Among the winter cereal food sources for aphids, initially the area under wheat cultivation increased significantly in southern South America, from 354,680 to 4,104,144 ha in Brazil from 1965 to 1979 (OCEPAR 1990; Salvadori and Salles 2002). Between 1970 and 1980, the population densities of M. dirhodum and S. avenae were very high (Fig. 1), reaching up to 150 individuals of S. avenae per spike and 187 individuals of M. dirhodum per plant (Kober 1972; Pimenta and Smith 1976). Considering all aphid species, levels close to 250–300 aphids/plant were reached (Eichler et al. 1976; Netto et al. 1975; Pimenta and Smith 1976). In general, the estimated damage caused by aphids on wheat production in southern Brazil was greater than 20% in the period 1967–1972 (Caetano 1973). Damage to grain yield associated with these high aphid populations became frequent, and plots without aphid control suffered wheat yield losses of up to 88% and 56%, respectively, in 1974 and 1976 (Salvadori and Salles 2002).
The parasitoid fauna before the biological control program
The aphid parasitoid (Hymenoptera) fauna in Latin America is characterized by the prevalence of Aphidius platensis (Brèthes), Diaeretiella rapae (McIntosh), and Lysiphlebus testaceipes (Cresson). These were the only parasitoids reported in Brazil and Chile before the introduction of other species by the biological control programs described in this review (Starý 1995; Starý et al. 2007). Aphidius platensis and D. rapae are of Palearctic origin and were probably accidentally introduced into South America along with their aphid hosts. L. testaceipes, which has a Nearctic origin, may have been accidentally introduced or naturally expanded from North America to South America (Starý 1995; Starý et al. 2007). In Argentina, the aphid parasitoid species Aphelinus asychis Walker, A. abdominalis Dalman, and Praon volucre (Haliday) have also been reported in wheat aphids (Botto 1980; Botto et al. 1995; Starý and Delfino 1986). These species may also have been accidentally introduced into that country along with their aphid hosts.
Older reports contain some synonymy problems, especially in the case of A. platensis, because this species was described several times and in different South American countries by Brèthes, in the genera Aphidius Nees and Diaeretus Förster (Starý 1995). Controversy remains about the synonymy with Aphidius brasiliensis (Brèthes) (Starý et al. 2007). As A. platensis is part of a cryptic species group called A. colemani species group, it has also been commonly reported as A. colemani (lato sensu) (Tomanović et al. 2014).
Before the reports of M. dirhodum and S. avenae in Brazil, records on natural enemies of wheat aphids were restricted to S. graminum without quantification of their suppression of aphid populations (Reiniger 1941; Costa 1944; Bertels 1956; Corseuil 1958). As the former two species were rampant, Kober (1972) considered the biological control of aphids on wheat to be insignificant, requiring chemical control to avoid losses, which could reach 100%. In south-central Paraná, in 1974, Pimenta and Smith (1976) reported high populations of M. dirhodum and S. avenae and a very low level of parasitism. These authors also found a significant, albeit late, incidence of the entomopathogen Entomophthora sp. and the abundant presence of aphid predator syrphids before the population peak, but which did not prevent the damage caused by aphids to grain yield.
At that time, the use of chemical insecticides was widespread. In Argentina, before 1979, the aphids M. dirhodum, S. graminum, and S. avenae could only be controlled by insecticides (Botto et al. 1995). In Brazil, in 1977, 98.6% of wheat crops in Rio Grande do Sul and Paraná states were sprayed once or twice, and, in many cases, three to four sprays were required for effective aphid control (Ambrosi 1987; Salvadori and Salles 2002). This intensive use of insecticides struck the already few endemic natural enemies, creating a vicious circle that made wheat production totally dependent on the use of insecticides.
Import and introduction of parasitoids to control aphids
The first intentional introductions of cereal aphid parasitoids in South America occurred in Chile in 1961 (Table 1), with Aphidius matricariae Haliday (Starý et al. 1993a), and in Argentina in the 1970s (Table 2), with the introduction of Aphidius ervi Haliday and Ephedrus plagiator (Nees) (Greco et al. 2020). These introductions were aimed at controlling aphids in crops such as alfalfa, but their effect also extended to wheat aphids, such as A. ervi against M. dirhodum and S. avenae in Argentina (Starý and Delfino 1986).
Biological control programs specifically for wheat aphids introduced six species of aphid parasitoids in Argentina (Table 1), eight in Chile (Table 2), and 12 species in Brazil (Table 3). These parasitoids originate from the USA and countries in Europe and in the Middle East. The introductions were carried out in a coordinated manner, involving the three countries in the late 1970s and early 1980s. In 1992, in Chile, three more species of aphid parasitoids were introduced to control D. noxia (Table 2).
In Brazil, the program was conducted by Embrapa Trigo (National Wheat Research Center) with support from FAO and the University of California. Parasitoids were the key insects in this program, and so they were used to make the program logo of the biological control program in Brazil (Fig. 2A). In this country, after the introduction and quarantine process, large-scale rearing of parasitoids was conducted at the insectary of Embrapa Trigo (Fig. 2B), and they were released in the field on wheat crops (Salles 1979). This facility, built in 1978, was named after Robert Van den Bosch, in honor of his contribution to the program and pioneer work on biological control of insect pests in general (Fig. 2C).
Initial releases, conducted by Embrapa’s staff in Brazil in all wheat-growing regions of Rio Grande do Sul state, were systematic and frequent. Sporadic releases were also done in the states of Santa Catarina, Paraná, and Mato Grosso do Sul. After the releases, sites were monitored to assess species establishment and parasitism levels on wheat aphids. At the same time, studies on biology, population dynamics, aphid damage, and insecticide selectivity were carried out. This aimed to develop complementary knowledge and technologies for the pest management program that would help to preserve biological control agents.
From 1982 to 1992, with some species already established, releases continued with less intensity and were directly done by farmers using parasitoids supplied by Embrapa. The objective was to consolidate the technology and maintain the motivation for the rational use of insecticides. During the 1978–1992 period, about 20 million parasitoids were produced and released (Salvadori and Salles 2002).
Results of aphid biocontrol
Some introduced species of parasitoids became established and aphid parasitism on wheat crops exceeded the target of the program. In Argentina, the populations of M. dirhodum, S. graminum, and S. avenae have strongly reduced (Botto et al. 1995). The parasitoids A. ervi and P. volucre were established before the wheat aphid biological control program (Starý and Delfino 1986; Botto et al. 1995) and Aphidius rhopalosiphi De Stefani-Perez, Aphidius uzbekistanicus Luzhetzki, and E. plagiator were considered established after the program (Greco et al. 2020). The control of the target aphid species, M. dirhodum and S. avenae, was carried out mainly by A. rhopalosiphi, A. uzbekistanicus, and A. ervi (Botto et al. 1995).
In Chile, in addition to the five species established in Argentina, introductions in the 1980s to control S. avenae and M. dirhodum also resulted in the establishment of Praon gallicum Starý (Starý 1995). The introductions carried out in 1991–1992 to control D. noxia resulted in the establishment of Aphidius avenae Haliday (Peñalver-Cruz et al. 2017). For species of the genus Ephedrus Haliday, information about their initial establishment is based on collections carried out a few months after the releases (Starý et al. 1993b; Starý 1995). The reduction of S. avenae and M. dirhodum populations by parasitoids was noticed in Chile soon after the introductions (Starý et al. 1993a). There, A. ervi was most abundantly parasitizing S. avenae, followed by P. volucre and A. uzbekistanicus; the parasitoids A. avenae, P. gallicum, A. colemani, A. matricariae, A. rhopalosiphi, and L. testaceipes were found in lower percentages in this aphid (Peñalver-Cruz et al. 2017). According Peñalver-Cruz et al. (2017), these parasitoids were also reported on R. padi, except for P. volucre, however, with a greater abundance of L. testaceipes, A. colemani, and A. uzbekistanicus. The geographic distribution of parasitoid species in the seven administrative regions of Chile was summarized by Starý (1993, b, 1995).
In Brazil, in the early 1980s, A. rhopalosiphi, A. uzbekistanicus, and P. volucre were considered established (Zúñiga-Salinas 1982) and, later, A. ervi (Starý et al. 2007). Established parasitoids have developed adaptive mechanisms to successfully survive and parasitize grass aphid species, maintaining themselves during the off-season of wheat on wild grasses and other crops (Zúñiga-Salinas 1982). After the introductions, the parasitism in S. avenae and M. dirhodum gradually increased. In different evaluated sites (e.g., Espumoso, RS), parasitism in S. avenae ranged from 46 to 62% in 1980 and 30 to 64% in 1981 (Zúñiga-Salinas 1982). High and increasing parasitism levels continued to be registered, while hyperparasitism was low and did not significantly affect the action of parasitoids (Zúñiga-Salinas 1982). Another significant result was the synchrony in the parasitoid-host relationship, with parasitism beginning early on the first aphids in autumn and winter (Zúñiga-Salinas 1982; Tambasco 1984). Population levels of and damage from S. avenae and M. dirhodum, which were extremely high in the 1970s, were drastically reduced after the biological control program. In 1979–1981, the maximum density of aphids/tiller ranged from 6.4 to 15.0 for S. avenae and 4.7 to 9.0 for M. dirhodum. Parasitism in 1980 and 1981 kept the density of these species below the economic threshold level (Zúñiga-Salinas 1982).
Even under favorable environmental conditions and lack of other natural enemies (predators and pathogens), no aphid outbreaks occurred in the years following the introduction of parasitoids in Brazil (Zúñiga-Salinas 1982; Tambasco 1984; Salvadori and Salles 2002). Aphid control using insecticides no longer increased grain yield in the wheat crop in subsequent years (Silva 1984; Silva and Ruedell 1984). Insecticide use to control aphids drastically dropped to less than 5% of the area cultivated with wheat (Ambrosi 1987). In addition, the biological control of aphids was naturally extended to other winter cereals such as barley, oats, and triticale.
The introduction of parasitoids to control wheat aphids certainly allowed the expansion of the species to neighboring countries, where there were no introductions, such as Uruguay and Paraguay. For example, in wheat-growing areas of Paraguay, the species L. testaceipes, E. plagiator, P. gallicum, P. volucre, A. avenae (= picipes), A. colemani, A. ervi, A. urbekistanikus, and A. rhopalosiphi have been reported (Gonzáles-Torres et al. 2018).
Brazilian case—current parasitoid guild and parasitism levels
Of the 12 exotic parasitoid species introduced in biocontrol program during the 1970s, three (A. uzbekistanicus, A. rhopalosiphi, and A. ervi) are still present in southern Brazil (Engel et al. 2022; Santos et al. 2022). A. colemani and L. testaceipes already occurred in the region; thus, it is impossible to know if the current population is composed of descendants of preexisting populations, introduced populations, or a mixture of both. In the case of A. colemani, Santos et al. (2019) found that the specimens collected during the biological control program (1978 to 1982), previously identified as Aphidius colemani (Viereck) (Zúñiga-Salinas 1982), are really A. platensis. Thus, the introduction of A. colemani (senso stricto) remains questionable, and it is not possible to determine exactly which species of this group were introduced (Santos et al. 2019). The establishment of only a few species of parasitoids in the subtropical region corroborates the theory of environmental filtering, which implies that a species must overcome several environmental barriers (filters) over time to become established in a new region (Outreman et al. 2018; Hajek et al. 2016).
Currently, in the winter cereal aphid parasitoid guild, the dominant species has been A. platensis (Santos et al. 2019; Engel et al. 2022). Surveys carried out in a recent 8-year time series (2011–2018) in Coxilha found A. platensis (61.4%), A. uzbekistanicus (7.3%), A. ervi (1.6%), A. rhopalosiphi (6.8%), D. rapae (18.6%), and L. testaceipes (1.3%) (Engel et al. 2022). The most abundant species, A. platensis, is considered one of the main biological control agents of cereal pest aphids in the world (Santos et al. 2019; Alvarez-Baca et al. 2020); this generalist parasitizes aphids belonging to tribes Aphidini and Macrosiphini in a wide host plant range (Starý et al. 2007). A. uzbekistanicus and A. rhopalosiphi are specialized parasitoids on winter cereal aphids (Starý et al. 2007; Santos et al. 2022). The rarity of A. ervi and L. testaceipes may be related to the collection method used and/or host species preference.
From its distribution center in the subtropical region, parasitoids have spread to tropical regions, becoming important not only in the biological control of cereal aphids but also in other crops (Starý et al. 2007; Pezzini et al. 2019). The adaptation of introduced parasitoid species to Brazilian conditions has been confirmed in field surveys with the recovery of the following: A. colemani, A. uzbekistanicus, A. ervi, A. rhopalosiphi, D. rapae, and L. testaceipes (Alves et al. 2005; Zanini et al. 2006; Starý et al. 2007; Bortolotto et al. 2012; Machado and Santos 2013).
Studies between 2009 and 2010 indicated that the level of parasitism in the species of aphids most commonly found in southern Brazil still remained high. For example, in Coxilha, RS, parasitism was found by the species M. dirhodum (67–33%), S. avenae (32–30%), S. graminum (31–25%), and R. padi (23–26%) (Rebonatto 2011).
Population oscillation of aphids and parasitoids
Aphids and parasitoids constitute one of the main networks of interactions between species associated with cultivated cereals throughout the world (Tomanović et al. 2008; Gagic et al. 2011, 2012; Alhmedi et al. 2018; Andrade et al. 2016). Air temperature is a regulatory factor in the patterns of population oscillation of aphids and parasitoids. Therefore, it determines the compatibilities of these interactions and may affect the final efficiency of biological control. In general, higher air temperature combined with low rainfall results in an increase in winged aphid populations. Parasitoid species have a lower thermal amplitude than aphids (Engel et al. 2022). Therefore, the efficiency of biological control by parasitoids may decrease in the hottest seasons of the year, in regions with warmer climates, and in climate change scenarios that predict an increase in temperature. In addition to the effect on the host, the direct effect of temperature on the parasitoid also influences biological control (Souza et al. 2017) as the thermal tolerance of each species determines its seasonality (Le Lann et al. 2011) and geographic distribution.
The biological control program and changes in the cropping system, such as no-tillage, which has become widely adopted in southern Brazil, might have helped to modify the composition of winter cereal aphid populations. For example, M. dirhodum, the predominant species in the past and one of the main BYDV vectors, now has a secondary role, being replaced by R. padi and S. avenae (Alves et al. 2005; Lau et al. 2008; 2009; Rebonatto 2011; Stoetzer et al. 2014; Rebonatto et al. 2015; Engel et al. 2022). The predominant species became aphids such as R. padi, which can colonize different hosts and adapt to a wide temperature range in southern Brazil. In addition to wheat in winter, other abundant hosts are available, such as oats in autumn and corn in summer, as well as extensive areas of ryegrass pastures. Studies with R. padi in the subtropical region, with suitable host plants available year-round, demonstrated the highest population growth in summer, decreasing in autumn and reaching the lowest levels in winter, increasing again in spring (Wiest et al. 2021) (Fig. 3A).
The population dynamics of parasitoids is seasonal with a peak in winter and another in winter-spring transition. For example, A. platensis and D. rapae peak in mid-winter, while A. uzbekistanicus and A. rhopalosiphi peak in late winter and early spring (Fig. 4a, d, g, j) (Engel et al. 2022). Based on winged cereal aphids (R. padi, R. rufiabdominalis, R. maidis, S. graminum, S. avenae, M. dirhodum, S. maydis, and S. flava) captured in traps, there are two peaks in populations in southern Brazil: the first during summer-fall transition and the second during winter-spring transition (Engel et al. 2022). The high parasitism levels at the beginning of winter may explain the drastic reduction of aphid populations in spring. Moreover, surveys in the summer indicated lower levels of parasitism (< 20%) (Rebonatto 2011).
Smoothed splines estimated by the GAMM model for the linear components of air temperature, rainfall, and time on Aphidius platensis (a, b, c), Diaeretiella rapae (d, e, f), Aphidius uzbekistanicus (g, h, i), and Aphidius rhopalosiphi (j, k, l). Red dashed line indicates the central boundary between negative and positive effects of linear components on parasitoid abundance. Shaded area indicates the 95% confidence interval. Splines estimated by the authors based on data obtained from Engel et al. (2022)
In addition to the effects of meteorological variables, the population fluctuation of aphids is affected by the presence of hosts and natural enemies (Bell et al. 2015; Tougeron et al. 2018). Changes in this population trend may be caused by variation in availability of host plants (bottom-up effect) or from the action of natural enemies (top-down effect) (Engel et al. 2022) (Fig. 3B). Thus, considering the adaptability of R. padi to different seasons of the year, it is possible that their populations prosper in unfavorable times for natural enemies (Engel et al. 2022). Furthermore, during autumn–winter, R. padi can act as a multiplying host of parasitoids that will parasitize other species of aphids whose growth is restricted to only a specific time of the year (Santos et al. 2022), such as M. dirhodum, which is more restricted to spring (Zúñiga-Salinas 1982; Rebonatto et al. 2015).
Geographic distribution
The wide climatic variation between the regions of Brazil makes it possible to verify the relationship between tolerance to high and low temperatures and the geographical distribution of parasitoids (Fig. 5). Three zones and 12 types of climates are classified throughout Brazil using Köppen’s criteria: A—tropical zone (climate types Af, Am, Aw, and As); B—dry zone (climate type Bsh); C—humid tropical zone (climate types Cfa, Cfb, Cwa, Cwb, Cwc, Csa, and Csb) (Alvares et al. 2013).
Current distribution of parasitoid species of aphids associated with wheat in Brazil. Diaeretiella rapae and Lysiphlebus testaceipes (A). Aphidius colemani and Aphidius platensis (B). Praon volucre and Aphidius ervi (C). Aphidius uzbekistanicus and Aphidius rhopalosiphi (D). Parasitoid species established after their introduction in the biological control program (C, D). Source: Peronti et al. (2007), Starý et al. (2007), Macedo et al. (2010), Bortolotto et al. (2012), Pezzini et al. (2019), Santos et al. (2019), Souza et al. (2019), Venâncio et al. (2020), Engel et al. (2022), and Santos et al. (2022). New records: data of A. platensis from Goias; D. rapae from Pernambuco and Roraima; and L. testaceipes from Ceara, Goias, and Roraima. Climate classification using Köppen’s criteria according to Alvares et al. (2013): A tropical zone, climates types Af (without dry season), Am (monsoon), Aw (with dry winter), and As (with dry summer); B dry zone, climates type Bsh (semi-arid); and C humid tropical zone, climate types Cfa (oceanic climate, without dry season and with hot summer), Cfb (oceanic climate, without dry season, and with temperate summer), Cwa (with dry winter and hot summer), Cwb (with dry winter and temperate summer). The climate types Cwc, Csa, and Csb are not in the figure due to their small representativity in the Brazil territory
The climatic variation between the regions of Brazil influences the abundance and geographical distribution of aphids, which affects the distribution of parasitoids. Currently, in southern Brazil (the main wheat growing region and with the predominance of the climate types Cfa and Cfb), the most abundant winter cereal aphid species are R. padi and S. avenae; the species S. graminum, M. dirhodum, R. maidis, and R. rufiabdominalis can be classified as secondary; and S. flava and S. maydis as rare (Engel et al. 2022). This composition is different in regions of expansion of the culture, as in the Cerrado of Minas Gerais (Aw and Cwa), where S. avenae, R. padi, and S. graminum were found on wheat plants; however, R. padi represented only around 1% of the aphids in the samples, and S. avenae was the dominant species (97% of the total aphids) (Rezende et al. 2020).
In Brazil, the greater record of parasitoid species occurrence in regions with lower annual average temperatures (Cwa, Cwb, Cfa, and Cfb), in the South and Southeast of the country (Fig. 5), suggests that the low tolerance of most parasitoid species to high temperatures in Northern (Am, As, and Aw), Northeastern (Bsh), and Central-Western Brazil (Aw and As) is a determining factor in their geographic distribution. Laboratory tests evaluating the mortality of aphid parasitoids at different constant temperatures showed parasitoid species with lower mortality at high temperatures (30 °C), such as D. rapae (Souza et al. 2017) and L. testaceipes (Rodrigues et al. 2004), which were the only ones recorded in the Northern and Northeastern Brazil (Fig. 5A). Aphidius colemani, a parasitoid with high mortality at 30 °C, but with good fitness at 28 °C in the laboratory (Sampaio et al. 2005, 2007), was recorded in the Central-West and warmer regions of the Southeast, such as the Triângulo Mineiro and Northern Minas Gerais (Aw), the northernmost point of its occurrence (Fig. 5B). The known distribution of A. platensis (Fig. 5B) is similar to that of A. colemani, however, restricted to records after the redescription of this species (Tomanović et al. 2014), when it was possible to discriminate it from A. colemani. Praon volucre (Fig. 5C) exhibits high mortality at 28 °C (De Conti et al. 2011) and Southern Minas Gerais (Cwa and Cwb) is the northernmost point where it was recorded. It was not found in the Triângulo Mineiro (Aw and Cwa), a region with an average temperature higher than Southern Minas Gerais. While A. ervi (Fig. 5C) and A. uzbekistanicus (Fig. 5D) have been recorded in Southern and Southeastern Brazil (including regions with Aw climate), with a more restricted distribution, A. rhopalosiphi (Fig. 5D) is highly prevalent in Rio Grande do Sul (Cfa and Cfb) and cooler regions of Paraná (Cfb) (Santos et al. 2022), which contrasts with its sporadic occurrence in latitudes farther north, as in Paraná (Cfa) (Bortolotto et al. 2012), and the complete absence of records of this species in the Southeastern region of the country (Aw, Cwa, and Cwb) (Starý et al. 2007). In this case, both the greater adaptation of A. rhopalosiphi to low temperatures (Le Lann et al. 2011) and the restricted occurrence of its preferential host (M. dirhodum) may play a preponderant role in its geographic distribution (Santos et al. 2022).
Food webs: two structures in a 40-year interval
Aphid population fluctuation data obtained on yellow traps are supported by data from parasitoids emerged from aphids in the field, allowing the establishment of trophic networks. Data on the interactions between the aphid species R. padi, S. graminum, M. dirhodum, and S. avenae with the parasitoids extracted from Zúñiga-Salinas (1982) and from Santos et al. (2022) were compared by food webs (Fig. 6). Zúñiga-Salinas (1982) collected mummies directly on winter cereals, while Santos et al. (2022) used the parasitoid recruitment method (i.e., field exposure of aphid, on wheat plants), and both authors recorded the parasitoid-host specificity. Considering the 40-year gap between the food webs, there are notable differences in the proportion of organism abundance in the two trophic levels in the ecological networks (Zúñiga-Salinas 1982; Fig. 6A; Santos et al. 2022; Fig. 6B).
Quantitative food webs between aphids and parasitoids. Species are represented by black bars: Braconid parasitoids (up), host aphid (bottom); *proportion of 1.000. Insect units of top and bottom bars. Food webs built from data obtained by Zúñiga-Salinas (1982) March to November (a), and by Santos et al. (2022) April to November (b)
The ecological network between parasitoids and aphids has 17 links (Zúñiga-Salinas 1982) and 19 links (Santos et al. 2022). The group A. colemani < A. platensis > has the most important role in the biological control of aphid species in winter cereals, with ca. 40% of the total parasitism for both food webs. In the data of Zúñiga-Salinas (1982), A. uzbekistanicus was the second highest abundant (30%, n = 1747), with almost total host specificity to S. avenae. For Santos et al. (2022), however, A. uzbekistanicus was one of the species with the lowest abundance (< 2%, n = 186) of the total parasitoids collected. This suggests that the high populations of S. avenae in the late 1970s and early 1980s reflected the high abundance of the parasitoid (Santos et al. 2022).
The abundance of A. rhopalosiphi, comparing the data on the percentages of parasitoids that emerged from the same aphid host in relation to the two periods, was variable. For example, in the first years of the biological control program, A. rhopalosiphi parasitized 81% of M. dirhodum and 18% of S. avenae (Zúñiga-Salinas 1982). Currently, this species seems to be more of a generalist affecting 43% of M. dirhodum, 24% of S. graminum, 19% of S. avenae, and 13% of R. padi.
A. ervi was not considered established by Zúñiga-Salinas (1982) but was collected by Santos et al. (2022) on winter cereals. These authors also recorded L. testaceipes parasitizing R. padi and S. graminum until early autumn, while Zúñiga-Salinas (1982) did not record this parasitoid even in March, when temperatures were still favorable.
Although D. rapae parasitizes Macrosipini aphids on brassicaceous plants (Bradburne and Mithen 2000; Starý et al. 2007) and on winter cereals, it was found only on aphids of the Aphidini tribe, and with different rates of parasitism between the periods compared.
Perspectives on the biological control of winter cereal aphids
The role of aphid parasitoids on winter cereals in the agricultural landscape of the subtropical region of the neotropics has been demonstrated. With the expansion of winter cereals to the tropical region, it would be important to assess what contribution these established species could make to the balance of agricultural systems. Some species of parasitoids are established in the tropical region, but higher temperatures in the tropics may limit the ecological services of parasitoids. At the same time, more information on the population dynamics of pest aphids and their effect on the epidemiology of transmitted viruses in tropical conditions are necessary.
In addition, other aphid species that have been expanding their geographic distribution may represent new future challenges. For example, the occurrence of Melanaphis sorghi (Theobald) in Brazil was recently confirmed, and this species has been damaging sorghum in the Americans (USA, Mexico, and Argentina) (Nibouche et al. 2021). New expanding species can start to colonize new hosts, such as winter cereals, and act as vectors for new viruses. Melanaphis sacchari has successfully transmitted sugarcane yellow leaf virus, a phloem-restricted virus, to wheat, oat, and barley, indicating that it does feed on these hosts (Schenck and Lehrer 2000).
Starý et al. (1993a) state that the successful establishment and continuity of good efficiency levels to control aphids on wheat are favored by a diverse array of cultures and habitats commonly associated with the production of wheat in the different regions where it is cultivated in South America. Such cultures and habitats serve as a natural refuge and as sources of aphids that are alternative hosts to the parasitoids. However, considering the diversity and immensity of Brazilian wheat-growing regions, the information currently available on the establishment and distribution of parasitoid species is scarce and restricted to certain regions.
As the establishment of these parasitoids in South American conditions is confirmed, it is essential to develop, evaluate, and encourage the adoption of management practices to conserve and increase the populations of natural enemies. The conservation of natural enemies is critical in agricultural ecosystems, which are simplified and cannot maintain high populations of parasitoids and predators (Perović et al. 2010).
The structuring of the agro-ecosystem to benefit conservation biological control is an approach that requires knowledge and transformation of the agricultural landscape. Conservation biocontrol acts in the long term and is more stable and less expensive than chemical control (Boivin et al. 2012). Different studies on this subject show that the complexity of environments does not influence the diversity or abundance of aphid parasitoids in winter cereals, indicating that these insects can find the resources for their survival even in less complex environments (e.g., areas with a high percentage of agricultural use). However, parasitism levels tend to decrease with increased distance from the crop edges, indicating a strong dependence of these parasitoids on resources associated with the diversity of vegetation at the farm level (Tscharntke et al. 2005; Brewer et al. 2008; Holland et al. 2008; Vollhardt et al. 2008; Caballero-López et al. 2012).
Starý et al. (2007) reported several species parasitizing different aphid species not associated with winter cereals, which are responsible for the maintenance of these parasitoid populations in the summer.
In conjunction with practices that promote conservation biological control, techniques can be used to stimulate the action of parasitoids. Plants respond to insect herbivores by releasing volatile organic compounds that can attract predators and parasitoids or repel other herbivores and thus act as a defense mechanism against herbivory. Such compounds may also be perceived by surrounding plants, which adjust their defensive response in accordance with this risk of attack (Chamberlain et al. 2000; Heil and Silva-Bueno 2007). The cis-jasmone emitted in large quantities by plants after damage by insects can activate defense mechanisms against phytophagous insects and attract natural enemies (Birkett et al. 2000). Bruce et al. (2003) evaluated the cis-jasmone response when applied to wheat plants and noted a significant increase in the resistance of young plants to S. avenae in laboratory conditions and low populations of this insect in field experiments. Thus, the development of products to be applied in the field could enhance plant resistance to insects, by both attracting natural enemies and increasing plant resistance capabilities.
The application of silicon in the soil enhances the defenses activated by cis-jasmone acid, promoting resistance in wheat plants against S. avenae (Dias et al. 2014) and R. padi (Oliveira et al. 2020). In addition, silicon alters the composition of volatiles emitted by wheat plants, causing them to produce the compound geranyl acetone, responsible for repelling R. padi and attracting its parasitoid L. testaceipes (Oliveira et al. 2020). Hence, the use of silicon can provide direct benefits in the control of wheat aphids, as well as indirect benefits, by attracting their parasitoids.
A better understanding of the population dynamics of aphid pests of winter cereals is another important aspect when considering biological control within an integrated pest management approach. Therefore, continuous monitoring of the populations of winter cereal aphids is an important tool whose implementation should be a goal. Understanding the population dynamics of aphids enables early detection of possible risks. Continuous monitoring of their abundance, the percentage of viruliferous individuals in the population, and its resistance status to insecticides allow a rational approach to the adoption of different control tactics (NJF 2013; Harrington 2014).
Concluding remarks
After the introduction of parasitoids to control wheat aphids in South America, there was a significant quantitative and qualitative change in population patterns of the different aphid species, showing that more than 40 years later, this biological control project continues to bring positive results. The absurdly large population levels of aphids causing significant direct damage to wheat have not been repeated and parasitism remains active. Considering that the insect pest management adopted by wheat producers is mostly based on chemical control, the use of control practices that reduce the negative effect of insecticides on the population of natural enemies is mandatory. The adoption of insect pest monitoring procedures supporting insect control decisions based on action thresholds should be encouraged. These practices associated with the use of selective insecticides can favor the current situation for the biological control of aphid pests of winter cereals in the subtropical region.
The major challenges include establishing a robust and comprehensive insect monitoring program to support forecasting and decision-making systems; obtaining knowledge about the population dynamics of aphids and their natural enemies; establishing management practices of the agricultural landscape; and developing cultivars with higher levels of resistance or products that enhance the action of biological control agents. Finally, the biggest challenge is to make these technologies available to farmers for adoption within integrated pest management.
References
Alhmedi A, Raymaekers S, Tomanović Ž, Bylemans D, Beliën T (2018) Food web structure of aphids and their parasitoids in Belgian fruit agroecosystems. Entomol Sci 21:279–291. https://doi.org/10.1111/ens.12303
Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G (2013) Koppen’s climate classification map for Brazil. Meteorol Z 22(6):711–728. https://doi.org/10.1127/0941-2948/2013/0507
Alvarez-Baca JK, Alfaro-Tapia A, Lavandero B, Le Lann C, Van Baaren J (2020) Suitability and profitability of a cereal aphid for the parasitoid Aphidius platensis in the context of conservation biological control of Myzus persicae in orchards. Insects 11:381. https://doi.org/10.3390/insects11060381
Alves LFA, Prestes TMV, Zanini A, Dalmolin MF, Menezes Junior ADO (2005) Controle biológico natural de pulgões (Hemiptera: Aphididae) em lavoura de trigo por parasitóides (Hymenoptera, Aphidiinae), no município de Medianeira, PR. Brasil Semina Ciênc Agrár 26:155. https://doi.org/10.5433/1679-0359.2005v26n2p155
Ambrosi I (1987) Avaliação dos impactos sociais e econômicos das tecnologias geradas pelo Centro Nacional de Pesquisa de Trigo
Andrade TO, Krespi L, Bonnardot V, van Baaren J, Outreman Y (2016) Impact of change in winter strategy of one parasitoid species on the diversity and function of a guild of parasitoids. Oecologia 180:877–888. https://doi.org/10.3390/insects11060381
Bell JR, Alderson L, Izera D, Kruger T, Parker S, Pickup J, Shortall CR, Taylor MS, Verrier P, Harrington R (2015) Long-term phenological trends, species accumulation rates, aphid traits and climate: five decades of change in migrating aphids. J Anim Ecol 84:21–34. https://doi.org/10.1111/1365-2656.12282
Bertels A (1956) Entomologia Agrícola do Sul do Brasil. Ministério da agricultura serviço de informação agrícola, Rio de Janeiro, Brazil
Bertels A (1970) Pragas do Trigo no campo e o seu combate. Pesqui Agropecuária Bras 5:81–89
Bertels A (1974) Observações sobre a dinâmica de populações de afídeos em trigo no Rio Grande do Sul em 1971. Pesqui Agropecuária Bras 9:71–72
Birkett MA, Campbell CAM, Chamberlain K, Guerrieri E, Hick AJ, Martin JL, Matthes M, Napier JA, Pettersson J, Pickett JA, Poppy GM, Pow EM, Pye BJ, Smart LE, Wadhams GH, Wadhams LJ, Woodcock CM (2000) New roles for cis-jasmone as an insect semiochemical and in plant defense. Proc Natl Acad Sci 97:9329–9334. https://doi.org/10.1073/pnas.160241697
Boivin G, Hance T, Brodeur J (2012) Aphid parasitoids in biological control. Can J Plant Sci 92:1–12. https://doi.org/10.4141/cjps2011-045
Bortolotto OC, De Oliveira Menezes Júnior A, Sampaio MV, Hoshino AT (2012) Parasitoides de pulgões-do-trigo que ocorrem no Norte do estado do Paraná e recaptura de Aphidius rhopalosiphi no Brasil. Semina Ciênc Agrár 33:3075–3080. https://doi.org/10.5433/1679-0359.2012v33n6Supl2p3075
Botto (1980) Aphelinus asychis Walker y Aphelinus abdominalis (Dalman), dos nuevos parasitos para los 'pulgones verde y amarillo de lo cereales’, en la Argentina. Rev Soc Ent Argentina 39:197–202
Botto EN, Monetti C, Ortego J, Dughetti A (1995) Natural enemies of cereal aphids and their potential impact on the Russian wheat aphid (Homoptera: Aphididae) in Argentina. (Scientific note). Vedalia Rev Int Control Biol Mex 2:39–40
Bradburne RP, Mithen R (2000) Glucosinolate genetics and the attraction of the aphid parasitoid Diaeretiella rapae to Brassica. Proc R Soc Lond B Biol Sci 267:89–95. https://doi.org/10.1098/rspb.2000.0971
Brewer MJ, Noma T, Elliott NC, Kravchenko AN, Hild AL (2008) A landscape view of cereal aphid parasitoid dynamics reveals sensitivity to farm- and region-scale vegetation structure. Eur J Entomol 105:503–511. https://doi.org/10.14411/eje.2008.066
Bruce TJ, Martin JL, Pickett JA, Pye BJ, Smart LE, Wadhams LJ (2003) cis-Jasmone treatment induces resistance in wheat plants against the grain aphid, Sitobion avenae (Fabricius) (Homoptera: Aphididae). Pest Manag Sci 59:1031–1036. https://doi.org/10.1002/ps.730
Caballero-López B, Bommarco R, Blanco-Moreno JM, Sans FX, Pujade-Villar J, Rundlöf M, Smith HG (2012) Aphids and their natural enemies are differently affected by habitat features at local and landscape scales. Biol Control 63:222–229. https://doi.org/10.1016/j.biocontrol.2012.03.012
CABI (2022) Sipha maydis. https://www.cabi.org/isc/datasheet/50172. Accessed 29 Mar 2022
Caetano VR (1973) Estudos sobre os afídeos vetores do vírus do nanismo amarelo da cevada, em especial de Acyrthosiphon dirhodum, em trigo, no sul do Brasil. Universidade Estadual de Campinas
Chamberlain K, Pickett JA, Woodcock CM (2000) Plant signalling and induced defence in insect attack. Mol Plant Pathol 1:67–72. https://doi.org/10.1046/j.1364-3703.2000.00009.x
Corrales CE, Castro AM, Ricci M, Dixon AFG (2007) Sipha maydis: distribution and host range of a new aphid pest of winter cereals in Argentina. J Econ Entomol 100:1781–1788. https://doi.org/10.1093/jee/100.6.1781
Corseuil E (1958) Pragas do trigo. Agrotecnica 51
Corseuil E (1959) Pragas que atacam os trigais. Bol. Campo
Costa RG (1944) Principais pragas do trigo. Bol. Agronômico 7
De Conti BF, Bueno VHP, Sampaio MV, van Lenteren JC (2011) Biological parameters and thermal requirements of the parasitoid Praon volucre (Hymenoptera: Braconidae) with Macrosiphum euphorbiae (Hemiptera: Aphididae) as host. Biocontrol Sci Technol 21:497–507. https://doi.org/10.1080/09583157.2011.560722
Dias PAS, Sampaio MV, Rodrigues MP, Korndörfer AP, Oliveira RS, Ferreira SE, Korndörfer GH (2014) Induction of resistance by silicon in wheat plants to alate and apterous morphs of Sitobion avenae (Hemiptera: Aphididae). Environ Entomol 43:949–956. https://doi.org/10.1603/EN13234
Eichler MR, Netto AP, Almeida A (1976) Controle de pulgões do trigo com o inseticida Afidrin. Divulg Agronômica 39
Engel E, Lau D, Godoy WAC, Pasini MPB, Malaquias JB, Santos CDR, Pivato J, Pereira PRV (2022) Oscillation, synchrony, and multi-factor patterns between cereal aphids and parasitoid populations in southern Brazil. Bull Entomol Res 112:143–150. https://doi.org/10.1017/S0007485321000729
Fehn LM (1970) Estudo da ação de inseticidas granulados, sistêmicos e de profundidade, no controle de pulgões, em trigo. Pesqui Agropecuária Bras 5:259–264
Fehn LM (1974) Espécies de pulgões observadas em trigo no Rio Grande do Sul em 1971, seu combate e suas diferentes influências sobre a produção. Pesqui Agropecuária Bras 9:73–80
Fingu-Mabola JC, Francis F (2021) Aphid–plant–phytovirus pathosystems: influencing factors from vector behaviour to virus spread. Agriculture 11:502. https://doi.org/10.3390/agriculture11060502
Gagic V, Tscharntke T, Dormann CF, Gruber B, Wilstermann A, Thies C (2011) Food web structure and biocontrol in a four-trophic level system across a landscape complexity gradient. Proc R Soc B Biol Sci 278:2946–2953. https://doi.org/10.1098/rspb.2010.2645
Gagic V, Hänke S, Thies C, Scherber C, Tomanović Z, Tscharntke T (2012) Agricultural intensification and cereal aphid-parasitoid-hyperparasitoid food webs: network complexity, temporal variability and parasitism rates. Oecologia 170:1099–1109. https://doi.org/10.1007/s00442-012-2366-0
Gassen DN, Tambasco FJ (1983) Controle biológico dos pulgões do trigo no Brasil. Inf Agropecuário 9:49–51
Gonzáles-Torres R, Segnana LRG, Arias OR, de López MBR (2018) Enemigos naturales de áfidos (Hemíptera: Aphididae) presentes en zonas productoras de trigo en Paraguay. Investig Agrar 20:78–83
Greco NM, Walsh GC, Luna MG (2020) Biological control in Argentina. In: Biological control in Latin America and the Caribbean: its rich history and bright future. CABI, Wallingford, UK, https://doi.org/10.1079/9781789242430.0021, pp 21–42
Hajek AE, Hurley BP, Kenis M, Garnas JR, Bush SJ, Wingfield MJ, van Lenteren JC, Cock MJW (2016) Exotic biological control agents: a solution or contribution to arthropod invasions? Biol Invasions 18:953–969. https://doi.org/10.1007/s10530-016-1075-8
Halbert S, Voegtlin D (1995) Biology and taxonomy of vectors of barley yellow dwarf viruses. In: D’arcy CJ, Burnett PA (eds) Barley yellow dwarf: 40 years of progress. The American Phytopathological Society, Minnesota, pp 217–258
Harrington R (2014) The Rothamsted Insect Survey strikes gold. Antenna 38:158–166
Heil M, Silva Bueno JC (2007) Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci 104:5467–5472. https://doi.org/10.1073/pnas.0610266104
Holland JM, Oaten H, Southway S, Moreby S (2008) The effectiveness of field margin enhancement for cereal aphid control by different natural enemy guilds. Biol Control 47:71–76. https://doi.org/10.1016/j.biocontrol.2008.06.010
Kober EAM (1972) Combate aos pulgões que atacam o trigo. Secretaria da Agricultura do Estado do Rio Grande do Sul. Secretaria da Agricultura do Estado do Rio Grande do Sul, Supervisão da Produção Vegetal, Unidade de Defesa e Fomento, Equipe de Defesa Fitossanitária, Porto Alegre, RS, Brazil
Lau D, Schons J, Yamazaki-Lau E, Pereira PS, Salvadori JR, Parizoto G, Mar TB (2008) Ocorrência do Barley/Cereal yellow dwarf virus e seus vetores em cereais de inverno no Rio Grande do Sul em 2007. Comun Téc INFOTECA-E 236:1–7
Lau D, Pereira PDS, Salvadori JR, Schons J, Parizoto G, Mar TB (2009) Ocorrência do Barley/Cereal yellow dwarf virus e seus vetores em cereais de inverno no Rio Grande do Sul, Santa Catarina, Paraná e Mato Grosso do Sul em 2008. Comun Téc INFOTECA-E 256:1–7
Lau D, Mar TB, Santos CDR, Engel E, Pereira PRV, d S, (2021) Advances in understanding the biology and epidemiology of barley yellow dwarf virus (BYDV). In: Oliver R (ed) Achieving durable disease resistance in cereals. Burleigh Dodds Science Publishing, Cambridge, England, pp 1–39
Le Lann C, Roux O, Serain N, Van Alphen JJM, Vernon P, Van Baaren J (2011) Thermal tolerance of sympatric hymenopteran parasitoid species: does it match seasonal activity? Physiol Entomol 36:21–28. https://doi.org/10.1111/j.1365-3032.2010.00758.x
Macedo LPM, Moura Filho ER, Carvalho AS, Bezerra CES, Silveira LCP (2010) Ocorrência de Lysiphlebus testaceipes parasitando Aphis gossypii em melancia, no Estado do Rio Grande do Norte, Brasil. Ciênc Rural 40:2030–2032. https://doi.org/10.1590/S0103-84782010005000148
Machado CD, Santos RSSD (2013) Pulgões do trigo e ação de parasitoides em Augusto Pestana, Noroeste do estado do Rio Grande do Sul, Brasil. Rev Bras Agroecol 8:179–186
Malmstrom CM, Shu R, Linton EW, Newton LA, Cook MA (2007) Barley yellow dwarf viruses (BYDVs) preserved in herbarium specimens illuminate historical disease ecology of invasive and native grasses. J Ecol 95:1153–1166. https://doi.org/10.1111/j.1365-2745.2007.01307.x
Netto, AP, Eichler MR, Almeida A (1975) Ensaio de campo com os inseticidas Saphicol C.E. e Pirimor LVC, visando o controle dos afídeos do trigo. Trigo E Soja 8–13
Ng JCK, Perry KL (2004) Transmission of plant viruses by aphid vectors. Mol Plant Pathol 5:505–511. https://doi.org/10.1111/j.1364-3703.2004.00240.x
Nibouche S, Costet L, Medina RF, Holt JR, Sadeyen J, Zoogones A-S, Brown P, Blackman RL (2021) Morphometric and molecular discrimination of the sugarcane aphid, Melanaphis sacchari, (Zehntner, 1897) and the sorghum aphid Melanaphis sorghi (Theobald, 1904). PLoS ONE 16:e0241881. https://doi.org/10.1371/journal.pone.0241881
NJF (2013) Suction traps in studying distribution and occurrence of insects and forecasting pests. (Nordic Association of Agricultural Scientists) Proceedings of NJF Seminar, Kristianstad
OCEPAR (1990) Trigo, produção, industrialização e comercialização. Sindicato e organização de cooperativas do estado do Paraná, Paraná
Oliveira RS, Peñaflor MFG, Gonçalves FG, Sampaio MV, Korndörfer AP, Silva WD, Bento JMS (2020) Silicon-induced changes in plant volatiles reduce attractiveness of wheat to the bird cherry-oat aphid Rhopalosiphum padi and attract the parasitoid Lysiphlebus testaceipes. PLoS ONE 15(4):e0231005. https://doi.org/10.1371/journal.pone.0231005
Ortego J, Difabio ME, Durante MPM (2004) New records and actualized check list of aphids (Hemiptera: Aphididae) from Argentina. Rev Soc Entomológica Argent 63
Outreman Y, Andrade TO, Louâpre P, Krespi L, Violle C, van Baaren J (2018) Multi-scale and antagonist selection on life-history traits in parasitoids: a community ecology perspective. Funct Ecol 32:736–751. https://doi.org/10.1111/1365-2435.13007
Peñalver-Cruz A, Ortiz-Martínez S, Villegas C, Tomanović Ž, Zepeda-Paulo F, Žikić V, Lavandero B (2017) Abundance and prevalence of Aphidius avenae; (Hymenoptera: Braconidae: Aphidiinae) in Chile. Cienc E Investig Agrar 44:207–214. https://doi.org/10.7764/rcia.v44i2.1719
Pereira PRV da S, Salvadori JR, Lau D (2008) Pulgão-preto-dos-cereais, Sipha maydis Passerini, 1860 (Hemiptera: Aphididae). Embrapa Trigo-FolderFolhetoCartilha INFOTECA-E
Peronti A, Fraga FB, Rosa K de CC, Teixeira MT, Silva ML (2007) Efeitos da fragmentação florestal e da expansão agrícola sobre a comunidade de insetos fitófagos e himenópteros parasitoides no Parque Nacional da Serra dos Órgãos e arredores. Ciênc E Conserv Na Serra Órgãos Brasília Ibama
Perović DJ, Gurr GM, Raman A, Nicol HI (2010) Effect of landscape composition and arrangement on biological control agents in a simplified agricultural system: a cost–distance approach. Biol Control 52:263–270. https://doi.org/10.1016/j.biocontrol.2009.09.014
Pezzini C, e Silva D, Köhler A (2019) Influence of temperature on the occurrence of Myzus persicae (Sulzer) (Hemiptera: Aphididae) parasitoids in tobacco crops in Rio Grande do Sul, Brazil. Rev Fac Nac Agron Medellin 72:8801–8808
Pimenta HR, Smith JG (1976) Afídeos, seus danos e inimigos naturais em plantações de trigo (Triticum sp.) no estado do Paraná. OCEPAR, Curitiba
Rebonatto A (2011) Flutuação sazonal de espécies de afídeos (Hemiptera: Aphididae) associadas ao trigo. Universidade de Passo Fundo. Master Degree Dissertation, Agronomy Post Graduate Program/ Plant Pathology, University of Passo Fundo
Rebonatto A, Salvadori J, Lau D (2015) Temporal changes in cereal aphids (Hemiptera: Aphididae) populations in Northern Rio Grande do Sul, Brazil. J Agric Sci 7:p71. https://doi.org/10.5539/jas.v7n10p71
Reed DK, Kindler SD (1994) Report of trip to Argentina and Chile. In: Proceedings 6th Russian Wheat Aphid Workshop. pp 163–168
Reiniger CH (1941) Uma ameaça para os trigais do Sul. Combate biológico dos pulgões (afídeos). Chác E Quintais 64:697–699
Rezende GF, Sampaio MV, Machado BQV, de Lima DT, Perdomo DN, Celoto FJ, Albuquerque CJB, Silva EJM, de Oliveira RS, Pereira HS (2020) Effect of silicon on aphid populations and wheat yield in Minas Gerais, Brazil. Semina Ciênc Agrár 41:2481–2494. https://doi.org/10.5433/1679-0359.2020v41n6p2481
Rodrigues SMM, Bueno VHP, Sampaio MV, Soglia MCM (2004) Influência da temperatura no desenvolvimento e parasitismo de Lysiphlebus testaceipes (Cresson) (Hymenoptera: Braconidae, Aphidiinae) em Aphis gossypii Glover (Hemiptera: Aphididae). Neotrop Entomol 33:341–346. https://doi.org/10.1590/S1519-566X2004000300011
Salles LAD (1979) Programa de controle biológico dos pulgões do trigo: relatório anual. Embrapa Trigo, Passo Fundo, RS, Brazil
Salvadori JR, Salles LAD (2002) Controle Biológico de Pulgões no Trigo. In: Parra JRP (ed) Controle Biológico no Brasil Parasitoides e Predadores, 1st edn. Manole Ltda, São Paulo, pp 427–443
Sampaio MV, Bueno VHP, Rodrigues SMM, Soglia MCM (2005) Resposta à temperatura de Aphidius colemani Viereck (Hymenoptera, Braconidae, Aphidiinae) originário de três regiões climáticas de Minas Gerais, Brasil. Rev Bras Entomol 49:141–147. https://doi.org/10.1590/S0085-56262005000100016
Sampaio MV, Bueno VHP, Rodrigues SMM, Soglia MCM, de Conti BF (2007) Desenvolvimento de Aphidius colemani Viereck (Hymenoptera: Braconidae, Aphidiinae) e alterações causadas pelo parasitismo no hospedeiro Aphis gossypii Glover (Hemiptera: Aphididae) em diferentes temperaturas. Neotrop Entomol 36:436–444. https://doi.org/10.1590/S1519-566X2007000300012
Santos CDRD, Sampaio MV, Lau D, Redaelli LR, Jahnke SM, Pivato J, Carvalho FJ (2019) Taxonomic status and population oscillations of Aphidius colemani species group (Hymenoptera: Braconidae) in Southern Brazil. Neotrop Entomol 48:983–991. https://doi.org/10.1007/s13744-019-00716-2
Santos CDRD, Lau D, Redaelli LR, Jahnke SM, Engel E, Sampaio MV (2022) Aphid-parasitoids trophic relationship in a cereal crop succession system: population oscillation and food webs. Agr for Entomol 24(4):516–530. https://doi.org/10.1111/afe.12513
Schenck S, Lehrer AT (2000) Factors affecting the transmission and spread of sugarcane yellow leaf virus. Plant Dis 84:1085–1088. https://doi.org/10.1094/PDIS.2000.84.10.1085
Silva MTB (1984) Ocorrência de pulgões e seus inimigos naturais e avaliação dos seus danos ao trigo. In: Contribuição do Centro de Experimentação e Pesquisa, XIII Reunião Nacional de Pesquisa de Trigo. Fecotrigo, Cruz Alta, RS, Brazil, p 142
Silva MTB, Ruedell J (1984) Efeito de seis níveis populacionais de pulgões na fase vegetativa do trigo. In: Contribuição do Centro de Experimentação e Pesquisa, XIII Reunião Nacional de Pesquisa de Trigo. Fecotrigo, Cruz Alta, RS, Brazil, p 113
Singh R, Singh G, Agrawal R, Tiwari A, Patel S, Sharma A, Singh B (2015) Host plant diversity of aphids (Homoptera: Aphididae) infesting cereals and grasses (Poaceae) in India. Int J Zool Investig 1:91–117
Souza MF, Veloso LFA, Sampaio MV, Davis JA (2017) Influence of host quality and temperature on the biology of Diaeretiella rapae (Hymenoptera: Braconidae, Aphidiinae). Environ Entomol 46:995–1004. https://doi.org/10.1093/ee/nvx108
Souza IL, Tomazella VB, Santos AJN, Moraes T, Silveira LCP (2019) Parasitoids diversity in organic Sweet Pepper (Capsicum annuum) associated with Basil (Ocimum basilicum) and Marigold (Tagetes erecta ). Braz J Biol 79:603–611
Starý P (1993) The fate of released parasitoids (Hymenoptera: Braconidae, Aphidiinae) for biological control of aphids in Chile. Bull Entomol Res 83:633–639. https://doi.org/10.1017/S0007485300040062
Starý P (1995) The Aphidiidae of Chile (Hymenoptera, Ichneumonoidea, Aphidiidae). Dtsch Entomol Z 42:113–138. https://doi.org/10.1002/mmnd.19950420112
Starý P, IngM G, IngH N, Remaudière G (1993a) Environmental research on aphid parasitoid biocontrol agents in Chile (Hym., Aphidiidae; Hom., Aphidoidea). J Appl Entomol 115:292–306. https://doi.org/10.1111/j.1439-0418.1993.tb00394.x
Starý P, Sampaio MV, Bueno VHP (2007) Aphid parasitoids (Hymenoptera, Braconidae, Aphidiinae) and their associations related to biological control in Brazil. Rev Bras Entomol 51:107–118. https://doi.org/10.1590/S0085-56262007000100018
Starý P, Delfino MA (1986) Parasitoids (Hym., Aphidiidae) of aphids (Hom., Aphididae) in Tucuman, Argentina. Boll Lab Entomol Agrar Filippo Silvestri Portici
Starý P, Rodríguez A. F, Gerding P. M (1993b) Liberaciones de enemigos naturales del pulgón ruso del trigo, Diuraphis noxia (Kurdjumov), en Chile. Agric Téc
Stell E, Meiss H, Lasserre-Joulin F, Therond O (2022) Towards predictions of interaction dynamics between cereal aphids and their natural enemies: a review. Insects 13:479. https://doi.org/10.3390/insects13050479
Stoetzer A, Kawakami J, Marsaro Júnior AL, Lau D, Pereira PRVS, Antoniazzi N (2014) Protective effect and economic impact of insecticide application methods on barley. Pesqui Agropecuária Bras 49:153–162. https://doi.org/10.1590/S0100-204X2014000300001
Tambasco FJ (1984) Determinação de níveis de dano de pulgões em trigo. Embrapa Trigo, Passo Fundo, RS, Brazil
Tomanović Ž, Kavallieratos NG, Starý P, Petrović-Obradović O, Athanassiou CG, Stanisavljević L (2008) Cereal aphids (Hemiptera: Aphidoidea) in Serbia: seasonal dynamics and natural enemies. Eur J Entomol 105:495–501. https://doi.org/10.14411/eje.2008.064
Tomanović Ž, Petrovic A, Mitrovic M, Kavallierats NG (2014) Molecular and morphological variability within the Aphidius colemani group with redescription of Aphidius platensis Brethes (Hymenoptera: Braconidae: Aphidiinae). Bull Entomol Res 104:552–565. https://doi.org/10.1017/s0007485314000327
Tougeron K, Damien M, Le Lann C, Brodeur J, van Baaren J (2018) Rapid responses of winter aphid-parasitoid communities to climate warming. Front Ecol Evol 6. https://doi.org/10.3389/fevo.2018.00173
Tscharntke T, Klein AM, Kruess A, Steffan-Dewenter I, Thies C (2005) Landscape perspectives on agricultural intensification and biodiversity – ecosystem service management. Ecol Lett 8:857–874. https://doi.org/10.1111/j.1461-0248.2005.00782.x
Van den Bosch R, Messenger PS (1973) Biological control. Intext Educational, New York, NY, USA
Venâncio H, Bianchi RA, Lobato TOS, Sampaio MV, Santos JC (2020) Tritrophic interaction between the Mexican sunflower, the aphid Aphis gossypii and natural enemies in a greenhouse experiment. Acta Sci Biol Sci 42:e47120. https://doi.org/10.4025/actascibiolsci.v42i1.47120
Vollhardt IMG, Tscharntke T, Wäckers FL, Bianchi FJJA, Thies C (2008) Diversity of cereal aphid parasitoids in simple and complex landscapes. Agric Ecosyst Environ 126:289–292. https://doi.org/10.1016/j.agee.2008.01.024
Wiest R, Salvadori JR, Fernandes JMC, Lau D, Pavan W, Zanini WR, Toebe J, Lazzaretti AT (2021) Population growth of Rhopalosiphum padi under different thermal regimes: an agent-based model approach. Agric for Entomol 23:59–69. https://doi.org/10.1111/afe.12404
Zanini A, Prestes TMV, Dalmolin MF, Alves LFA, Menezes Jr. A de O (2006) Ocorrência de Lysiphlebus testaceipes (Cresson) (Hymenoptera: Aphidiidae) parasitando pulgões (Hemiptera: Aphididae), em trigo em Medianeira, PR. Neotrop Entomol 35. https://doi.org/10.1590/S1519-566X2006000200020
Zúñiga E, Suzuki H (1976) Ecological and economic problems created by aphids in Latin America. Outlook Agric 8:311–319. https://doi.org/10.1177/003072707600800602
Zúñiga-Salinas E (1982) Controle biológico de afídeos do trigo (Homoptera: Aphididae) por meio de parasitóides no Planalto Médio do Rio Grande do Sul. PhD Thesis, Federal University of Paraná
Acknowledgements
We thank CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for masters and the CNPq for doctorate fellowships (CNPq 141011/2020-3) of the fifth author, and CAPES (88887371811/2019-00) for the fellowship of the sixth author.
Funding
This review was supported by the Embrapa project “Integrated platform for monitoring, simulation, and decision making in epidemic management caused by insect-borne viruses” Process: No. 13.16.05.006.00.00.
Author information
Authors and Affiliations
Contributions
All authors planned and proposed the structure of the text together. The literature search and data analysis were organized as follows: Douglas Lau—current populations of cereal aphids and their parasitoids in the subtropical region of Brazil; Marcus Vinicius Sampaio—historical aspects of biological control programs in Argentina, Chile, and Brazil, and geographic distribution of stablished parasitoids in Brazil; José Roberto Salvadori—historical aspects about biological control program in Brazil; Paulo Roberto Valle da Silva Pereira—perspectives on the biological control of cereal aphids; Carlos Diego Ribeiro dos Santos—food webs; Eduardo Engel—effect of abiotic factors on aphid and parasitoid populations. Antônio Ricardo Panizzi and Alberto Luiz Marsaro Júnior critically revised the work.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Edited by Yelitza Coromoto Colmenarez
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lau, D., Sampaio, M.V., Salvadori, J.R. et al. Historical and Contemporary Perspectives on the Biological Control of Aphids on Winter Cereals by Parasitoids in South America. Neotrop Entomol 52, 172–188 (2023). https://doi.org/10.1007/s13744-022-01013-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-022-01013-1