Abstract
The American grapevine moth (AGVM), Lasiothyris luminosa (Razowski & Becker) (Lepidoptera: Tortricidae), was recently registered as a new pest for table grapes in the Northeast region of Brazil. In the present study, two approaches were made aiming to aid information to support management strategies for the new pest control: (i) study AGVM biology in the laboratory and its population dynamics in the field and (ii) evaluate Trichogramma pretiosum Riley as a potential biological control method against L. luminosa. The AGVM population dynamics showed a similar trend in the three grapevine monitored vineyards. The larvae started occurring at 30 days after pruning (DAP), pre-bloom stage, with a peak population between 54 and 78 DAP, following a decrease until harvest. The AGVM females larva, pupa, and egg-adult period were longer than males. The egg-adult period was 42.1 and 45.2 days for male and females, respectively. Trichogramma pretiosum was able to parasitize L. luminosa eggs. The T. pretiosum egg-adult period was 10.2 days with a 98.5% pupa survivorship at 25 °C. The release of T. pretiosum in the vineyards resulted in 65.5 to 73.2% AGVM egg parasitism. Our findings bring the first biological and population dynamics information for L. luminosa, which can help developing efficient approaches to monitor and control the pest in grapevines. The release of T. pretiosum in the vineyard is a potential biological control option to AGVM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The American grapevine moth (AGVM), Lasiothyris luminosa (Razowski & Becker) (Lepidoptera: Tortricidae), was recently registered as a new pest for table grapes in the Northeast region of Brazil (Costa-Lima et al. 2016). The species status was confirmed, and the damage caused by the larval stage has been well characterized in grapevines from the flower bud formation to the ripening berry stage close to harvest. The study has also described the difficulty to control the pest, considering the larva endophagous feeding habit, penetrating in the plant structures and, consequently, resulting in low exposure to insecticides. This study reported for the first time L. luminosa as a pest. A previous taxonomic study reported the microlepidoptera occurrence in Santa Catarina State, Southern region of Brazil, which was based on an adult male of the species Saphenista luminosa (Razowski and Becker 1983). Later, a taxonomic revision transferred the species to the genus Lasiothyris (Razowski and Becker 1993). Other Tortricidae species with similar feeding habit to the AGVM have also been reported as key grapevine pests in other countries, such as Lobesia botrana (Den. & Schiff.), Eupoecilia ambiguella (Hubn.), and Paralobesia viteana (Loeb et al. 2011; Ioriatti et al. 2012; Thiéry et al. 2018).
In 2017, L. luminosa was included in the European and Mediterranean Plant Protection Organization alert list (EPPO 2017). In 2019, Argentina, Uruguay, and Paraguay imposed phytosanitary restrictions to imported grapes from Brazil due to L. luminosa (MERCOSUL 2019). In Brazil, the São Francisco Valley is responsible for about 99% of the exported table grapes (Bustamante 2009). In this region, the damage caused by the AGVM achieved U$5150/ha (Costa-Lima et al. 2016). Accordingly, Daane et al. (2018) have shown that viticulture sustainability has been affected by many arthropods pests’ invasions and, generally, the chemical control is initially adopted for short-term response. However, the authors highlight the importance to develop more effective and sustainable control strategies, which can be achieved by obtaining information for the pest species in the region.
In integrated pest management (IPM) programs, biological control is an underlying pillar (Naranjo et al. 2015). In the last decades, this control strategy has increased due to the development of biocontrol companies (Le Hesran et al. 2019), which has also happened in Brazil. Within the commercial biocontrol agents in Brazil, the egg parasitoids Trichogramma galloi Zucchi and Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae) are currently an option for different Lepidoptera pest control (Parra and Coelho 2019). In other countries, Trichogramma evanescens (Westwood), Trichogramma cacaeciae Marchal, and Trichogramma minutum Riley showed positive results on controlling Lepidoptera pests in grapevines (Hommay et al. 2002; Nagarkatti et al. 2003; El-Wakeil et al. 2009).
Considering the economic importance of table grape production and the potential losses caused by L. luminosa, studies are required to obtain biological and population dynamics information that can be used to develop efficient pest management strategies. The objectives of this study were to (i) characterize the pest biology in the laboratory and its population dynamics in the field and (ii) evaluate T. pretiosum as a potential biological control method against L. luminosa.
Material and methods
Population dynamics
In order to understand the AGVM larvae occurrence during the vineyard phenology, three 5 ha Vitis vinifera L. table grapes fields were followed. The samplings were taken from January to April, February to May, and March to June, in 2016, in vineyards A, B, and C, respectively. The experiments were carried out in a commercial table grape farm, in Lagoa Grande, Pernambuco, Brazil. The farm pruned the plants in staggered dates to establish a continuous grape harvest. Thus, each 5-ha vineyard used in the studies had a different pruning date. Weekly sampling was performed starting at 23 until 114 days after pruning (DAP). At each sampling date, 12 reproductive structures (inflorescence or grape bunches) were detached from the plant and allocated in paper bags. The first phenological stage sampled was the initial inflorescence (pre-bloom) and continued until the formation of mature grape bunches. The samplings were randomly made within the vineyards. In the laboratory, the vegetal structures were examined under stereomicroscope (× 80) and number of L. luminosa larvae was counted. Each inflorescence or grape bunch was considered a repetition.
Biology
In order to study the L. luminosa biology, assays were accomplished to find the grape berry stage that could complete the larval stage without interfering in the survivorship. Grape berries with 50 DAP (var. Sweet Jubilee) were chosen for the study. The adults were kept in cylindrical cages (10 cm diameter and 12 cm high) with fine mesh covering the top and a Petri dish at the bottom. A grape bunch was cut with eight berries and put on the Petri dish inside the cage. A 10% honey bee solution was offered as diet for the adults. After 12 h, the berries were removed to observe the egg presence under stereomicroscope (× 80). A single berry with one egg was isolated in a plastic recipient (9 cm diameter and 6 cm high) containing a fine mesh cover. Considering that AGVM larvae can migrate to other berries, three undamaged berries were added to the recipient. When more than one egg was found per berry, an entomologic pin was used to remove the others. The insect stage and mortality were daily observed. Once a week, three new berries were added and the rotten ones discarded. The whole study was conducted in climatic chambers at 25 °C (±1 °C), with 60% (±15%) relative air humidity and 12 h photophase. Each recipient with one L. luminosa was considered a repetition (n = 32).
Biological control
Trichogramma pretiosum were acquired from Koppert Biological Systems (Brazil), which is commercialized as parasitized Anagasta kuehniella (Zeller) eggs inside cardboard cells. Five berries with approximately 25 L. luminosa eggs, with less than 24 h, were offered to 1-day-old T. pretiosum adults, in a 100-mL Becker covered with plastic film. After 6 h, the grapes with AGVM eggs were removed and isolated in plastic recipients (9 cm diameter and 6 cm high) covered with a fine mesh lid. The presence of emerged T. pretiosum was evaluated every day. To report the pupae survivorship, the eggs were examined under stereo microscope (× 100) to quantify the number of blackened eggs with and without exit holes. Each egg was considered a repetition (n = 95), in a completely randomized design experiment.
In order to determine if T. pretiosum would be able to parasitize L. luminosa eggs in the field, three releases of 200,000 wasps per hectare were made from February to March 2016. Each parasitoid release was made in a different 5-ha area, in the same commercial farm where the population dynamics studies were accomplished. Cardboard cells with T. pretiosum were distributed in 50 points per hectare. The cells were attached under the steel wire used to conduct the vineyards. The three fields used in the experiments had 30 to 35 DAP. One week after each release, 20 inflorescences were sampled in each vineyard. In the laboratory, under stereo microscope (× 100), the number of L. luminosa eggs was counted, which could be identified by the darker (parasitized) and yellowish-cream color (viable) on the flower buds (Fig. 1). The eggs were then collected and isolated to confirm the T. pretiosum emergence or AGVM larvae eclosion. This was the first time T. pretiosum was released in a grapevine vineyard in the São Francisco Valley. Previous to the release, weekly samples were collected from June 2015 to January 2016, and no AGVM parasitized eggs were observed. Each inflorescence or grape bunch was considered a repetition (n = 20).
Statistical analysis
The whole study followed a completely randomized design and the statistical analysis and graphs were made with R statistical software (R Core Team 2019). For the population dynamic study, a curve was plotted using the ggplot2 package (Wickham 2016). The geom_smooth function was applied, where the standard error bounds are computed with the loess method using a t-based approximation, with a 0.95 confidential interval. For the L. luminosa immature stages and egg-adult period, Kaplan-Meier curves (Kaplan and Meier 1958) were plotted using the survminer package, and the male and female curves compared using the log-rank test (p < 0.05).
Results
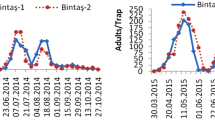
The L. luminosa population dynamics showed a similar trend in the three vineyards. The larvae started occurring from 30 DAP, on the pre-bloom stage. The number of larvae per inflorescence or grape bunch increased until 66 to 78 DAP (vineyards A and B) or until 54 to 66 DAP (vineyard C), following a decrease, but still present until harvest (Fig. 2).
Lasiothyris luminosa (Razowski & Becker) mean larvae per inflorescence or grape bunch during grapevine (Vitis vinifera L.) reproductive cycle, in three different vineyards monitored in 2016 from January to April (A), February to May (B), and March to June (C) in the São Francisco Valley, Pernambuco, Brazil. The shaded areas correspond to the standard error bounds computed by the loess method with a 0.95 confidence interval
The AGVM egg stage development time showed no difference between sexes (p = 0.7). However, for the larva (p < 0.001), pupa (p = 0.0011), and egg-adult period (p = 0.012), the females had longer stages (Fig. 3). The egg-adult period was 42.1 ± 0.75 and 45.2 ± 0.35 days for male and females, respectively. Approximately 70% of the egg-adult period was represented by the larval stage.
Trichogramma pretiosum females were able to parasitize L. luminosa eggs. The egg-adult period was 10.2 ± 0.03 days with a 98.5 ± 0.29% pupa survivorship at 25 °C. With the T. pretiosum release in the fields, it was possible to detect parasitized AGVM eggs in the three vineyards of the study. The parasitism incidence ranged from 65.5 to 75.7% (Table 1).
Discussion
The peak of L. luminosa occurrence was in the initial berry ripening stage (65 to 85 DAP), before the berries start softening. However, it was possible to start detecting larvae since the pre-bloom stage until harvest. As observed by Costa-Lima et al. (2016), the larvae always penetrated the grapevine reproductive structures, behavior that reduces the efficiency of possible control approaches in this stage. These findings suggest that growers should start monitoring the pest in the pre-bloom stage. These results were similar in all three monitored vineyards, showing that the pre-bloom stage should also be used to initiate control methods to inhibit pest proliferation. In addition, a more efficient spray coverage is expected on inflorescences and young berries. As observed in a study with fungicides to control Botrytis cinerea Pers., the spray coverage decreased linearly with the cluster size and compactness increase (Hed et al. 2011). For another grapevine Lepidoptera pest, Cryptoblabes gnidiella Millière, similar observation was made due to the fact that larvae find refuge inside the compact grape bunches (de Oliveira et al. 2014). Insights to cultural control methods can also be guided based on the larvae occurrence, as to remove the attacked berries and bury under the ground to interrupt the L. luminosa cycle, same approach adopted for fruit flies (Chueca et al. 2013).
The lengths of the immature stages and egg-adult period recorded for L. luminosa were similar to other tortricids species, such as Lobesia botrana (Denis and Schiffermüller) (Thiéry and Moreau 2005) and Gymnandrosoma aurantiana (Lima) (Garcia and Parra 1999). The AGVM females egg-adult period at 25 °C (45.2 days) helps in understanding the peak population observed in the population dynamics study. The first generation will infest the flower buds at 30 DAP; 40 days after, a second generation will emerge, coinciding with the peak population observed in Fig. 2.
The T. pretiosum egg-adult period over L. luminosa was similar to other hosts, such as Helicoverpa armigera (Hübner) (dos Santos Carvalho et al. 2017) and Neoleucinodes elegantalis (Guenée) (de Oliveira et al. 2017). Our study confirmed the T. pretiosum capacity to parasitize AGVM eggs in vineyards in the Northeast region of Brazil. The aim was not to test control efficiency, but first to determine if T. pretiosum could parasitize L. luminosa eggs under laboratory controlled conditions, as well as if the released parasitoids would be able to confirm this capacity in the field, at high sunlight incidence and air temperatures, which are characteristics of the semi-arid region. Further tests to evaluate the number of parasitoids per hectare and the release interval are needed. Based on our initial results, the table grape farms with L. luminosa occurrence are releasing 200,000 T. pretiosum per hectare per week, starting at 25 DAP. This is the first report of T. pretiosum use in vineyards. Other Trichogramma species released in vineyards has also been cited with positive results on controlling Tortricidae pests, i.e., L. botrana, P. viteana, E. ambiguella, and Epiphys potvittana Walker, such as the following: T. cacaeciae in Germany (Castaneda-Samayoa et al. 1993); Trichogramma carverae Oatman & Pinto in Australia (Glenn and Hoffmann 1997); T. evanescens in Egypt (El-Wakeil et al. 2009); T. evanescens and T. cacaeciae in France (Hommay et al. 2002); and T. minutum in the USA (Nagarkatti et al. 2003).
Currently, spinetoram is the only insecticide registered to control L. luminosa in grapevines in Brazil. This product is not selective to T. pretiosum (Khan et al. 2015). Field experiments with T. pretiosum have shown its capacity to parasitize and persist for at least 3 days (Kazmer and Luck 1995; Coelho et al. 2016). Similar studies are needed for the parasitoid in grapevines in the Northeast of Brazil, which will help determining the interval gap between releasing T. pretiosum and spraying spinetoram. While this data is not available, table grape growers in the São Francisco Valley are maintaining a 3-day interval before and after release, without spraying insecticides that are not compatible with the parasitoid.
Our findings bring for the first time biological and population dynamics information for L. luminosa in grapevines produced in semi-arid conditions. The obtained results will help in guiding effective approaches to monitor and control this new pest in the São Francisco Valley, Brazil. In addition, according to our results, the release of T. pretiosum in the vineyard showed potential as a biological control approach to the AGVM management.
References
Bustamante PMAC (2009) A Fruticultura no Brasil e no Vale do São Francisco: Vantagens e Desafios. Rev Econ Nordeste 40:153–171
Castaneda-Samayoa O, Holst H, Ohnesorge B (1993) Evaluation of some Trichogramma species with respect to biological control of Eupoecilia ambiguella Hb. and Lobesia botrana Schiff. (Lep., Tortricidae). Z Pflanzenk Pflanzen 100:599–610
Chueca P, Garcera C, Urbaneja A, Molto E (2013) A new mechanised cultural practice to reduce Ceratitis capitata Wied.populations in area-wide IPM. Span J Agric Res 11:1129–1136. https://doi.org/10.5424/sjar/2013114-4585
Coelho A, Rugman-Jones PF, Reigada C, Stouthamer R, Parra JRP (2016) Laboratory performance predicts the success of field releases in inbred lines of the egg parasitoid Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). PLoS One 11:e0146153. https://doi.org/10.1371/journal.pone.0146153
Costa-Lima TC, Moreira GRP, Gonçalves GL, Specht A (2016) Lasiothyris luminosa (Razowski and Becker) (Lepidoptera: Tortricidae): a new grapevine pest in Northeastern Brazil. Neotrop Entomol 45:336–339. https://doi.org/10.1007/s13744-016-0379-9
Daane KM, Vincent C, Isaacs R, Ioriatti C (2018) Entomological opportunities and challenges for sustainable viticulture in a global market. Annu Rev Entomol 63:193–214. https://doi.org/10.1146/annurev-ento-010715-023547
de Oliveira JEM, De Araújo Fernandes MH, De Castro Gama F et al (2014) Uso da técnica de confusão sexual no manejo populacional de Cryptoblabes gnidiella (Lepidoptera: Pyralidae) em videira. Pesq Agrop Bras 49:853–859. https://doi.org/10.1590/S0100-204X2014001100004
de Oliveira CM, de Oliveira JV, Barbosa DR e. S, et al. (2017) Biological parameters and thermal requirements of Trichogramma pretiosum for the management of the tomato fruit borer (Lepidoptera: Crambidae) in tomatoes. Crop Prot 99:39–44. https://doi.org/10.1016/j.cropro.2017.04.005
dos Santos Carvalho G, Silva LB, Reis SS et al (2017) Biological parameters and thermal requirements of Trichogramma pretiosum reared on Helicoverpa armigera eggs. Pesq Agrop Bras 52:961–968. https://doi.org/10.1590/S0100-204X2017001100001
El-Wakeil NE, Farghaly HT, Ragab ZA (2009) Efficacy of Trichogramma evanescens in controlling the grape berry moth Lobesia botrana in grape farms in Egypt. Arch Phytopathol Plant Protect 42:705–714. https://doi.org/10.1080/03235400701390422
EPPO (2017) New data on quarantine pests and pests of the EPPO Alert List. Reporting Service no. 08 - 2017
Garcia MS, Parra JRP (1999) Comparação de dietas artificiais, com fontes protéicas variáveis, para criação de Ecdytolopha aurantiana (Lima) (Lepidoptera: Tortricidae). An da Soc Entomológica do Bras 28:219–232. https://doi.org/10.1590/s0301-80591999000200004
Glenn DC, Hoffmann AA (1997) Developing a commercially viable system for biological control of light brown apple moth (Lepidoptera: Tortricidae) in grapes using endemic Trichogramma (Hymenoptera: Trichogrammatidae). J Econ Entomol 90:370–338. https://doi.org/10.1093/jee/90.2.370
Hed B, Ngugi HK, Travis JW (2011) Use of gibberellic acid for management of bunch rot on Chardonnay and Vignoles grape. Plant Dis 95:269–278. https://doi.org/10.1094/PDIS-05-10-0382
Hommay G, Gertz C, Kienlen JC, Pizzol J, Chavigny P (2002) Comparison between the control efficacy of Trichogramma evanescens Westwood (Hymenoptera: Trichogrammatidae) and two Trichogramma cacoeciae Marchal strains against grapevine moth (Lobesia botrana Den. and Schiff.), depending on their release density. Biocontrol Sci Tech 28:219–232. https://doi.org/10.1080/0958315021000016234
Ioriatti C, Lucchi A, Varela LG (2012) Grape berry moths in western european vineyards and their recent movement into the New World. In: Bostanian NJ, Vincent C, Isaacs R (eds) Arthropod management in vineyards: pests, approaches, and future directions. Springer Netherlands, Amsterdam, pp 339–359. https://doi.org/10.1007/978-94-007-4032-7_14
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481. https://doi.org/10.2307/2281868
Kazmer DJ, Luck RF (1995) Field tests of the size-fitness hypothesis in the egg parasitoid Trichogramma pretiosum. Ecology 76:412–425. https://doi.org/10.2307/1941200
Khan MA, Khan H, Ruberson JR (2015) Lethal and behavioral effects of selected novel pesticides on adults of Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). Pest Manag Sci 71:1640–1648. https://doi.org/10.1002/ps.3972
Le Hesran S, Ras E, Wajnberg E, Beukeboom LW (2019) Next generation biological control – an introduction. Entomol Exp Appl 167:eea.12805. https://doi.org/10.1111/eea.12805
Loeb G, Cha D, Hesler S, Linn C, Zhang A, Teal PE, Roelofs W (2011) Monitoring grape berry moth (Paralobesia vitianna: Lepidoptera) in commercial vineyards using a host plant based synthetic lure. Environ Entomol 4:1511–1522. https://doi.org/10.1603/EN10249
MERCOSUL (2019) Sub-standard 3. 7. 19 Requisitos fitossanitários para Vitis vinifera (videira) segundo país de destino e origem, para os estados partes. Buenos Aires
Nagarkatti S, Tobin PC, Saunders MC, Muza AJ (2003) Release of native Trichogramma minutum to control grape berry moth. Can Entomol 135:589–598. https://doi.org/10.4039/n02-099
Naranjo SE, Ellsworth PC, Frisvold GB (2015) Economic value of biological control in integrated pest management of managed plant systems. Annu Rev Entomol 60:621–645. https://doi.org/10.1146/annurev-ento-010814-021005
Parra JRP, Coelho A (2019) Applied biological control in Brazil: From laboratory assays to field application. J Insect Sci 19:1–6. https://doi.org/10.1093/jisesa/iey112
R Core Team (2019) R: a language and environment for statistical computing. Vienna, Austria
Razowski J, Becker VO (1983) Brazilian Cochylidii ( Lepidoptera, Tortricidae). Acta Zool Cracov 26:421–464
Razowski J, Becker VO (1993) Revision of the cochyline genus Lasiothyris Meyrick (Lepidoptera, Tortricidae), with a description of 8 new species. Acta Zool Cracov 36:121–136
Thiéry D, Moreau J (2005) Relative performance of European grapevine moth (Lobesia botrana) on grapes and other hosts. Oecologia 143:548–557. https://doi.org/10.1007/s00442-005-0022-7
Thiéry D, Louâpre P, Muneret L, Rusch A, Sentenac G, Vogelwith F, Iltis C, Moreau J (2018) Biological protection against grape berry moths. A review. Agron Sustain Dev 38:1–18. https://doi.org/10.1007/s13593-018-0493-7
Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York
Acknowledgements
We would like to thank the table grape grower for the vineyard areas used in the study, to LD Geremias for the manuscript review, and to ST Freitas for the manuscript English style review.
Author information
Authors and Affiliations
Contributions
TCCL conceived and designed the research. TCCL, AFT, and ATPA conducted the experiments. TCCL analyzed the data and wrote the manuscript. All authors read, reviewed, and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Edited by Anne-Nathalie Volkoff
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Costa-Lima, T.C., de Araújo, A.T.P. & Torris, A.F. Biology and Population Dynamics of the American Vine Moth and the Potential Biocontrol with Trichogramma pretiosum. Neotrop Entomol 50, 470–475 (2021). https://doi.org/10.1007/s13744-021-00850-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-021-00850-w