Abstract
Responses in taxonomic and functional composition of communities were analysed in small Amazonian streams at the small and large scale (habitat patches, river segment scale, and catchment scale). We hypothesised that similar responses in community structure to local environmental factors were a correlation between taxonomic and functional composition. To evaluate the response of taxonomic composition to environmental variables, redundancy analysis (RDA) and RLQ analysis were performed to investigate the response of community abundance (L) as a function of the environment (R) and traits (Q). The fourth-corner analysis was applied to summarize specific interactions between environmental variables and traits. Then, community taxonomic composition was associated with models at multiple scales of habitat (i.e. riparian/channel, substrates, and water variables). Likewise, the fourth-corner tests and RLQ axes showed associations between trait composition and environmental variables related to variables, such as riparian cover and channel morphology followed by variation in substrate size and composition. Unexpectedly, these results did not show specific associations between unique environmental variables and traits. At last, results showed that local conditions of stream habitat regulated community structure and functional composition of aquatic insects. Thus, these findings indicate that the local environmental filtering appears to be strongly associated with selected species traits adapted to occur in a range of habitat conditions. Despite the low number of analysed streams, these results provide important information for understanding the simultaneous variation in functional trait composition and community composition of aquatic insect assemblages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
How stream habitats support different species compositions and how species coexist within and among communities are recurring issues in riverine ecology (Vinson & Hawkins 1998). To address these questions, niche-based approaches have extensively been applied to explain and predict species distributions based on spatiotemporal variation in environmental conditions (Poff 1997). Additionally, studies have emphasized the influence of ecological processes on functional and evolutionary patterns in riverine assemblages (Usseglio-Polatera et al 2000). Often, species distribution is related to differences in their life-history traits, the availability of resources, and ecological interactions, mainly considering the contribution of differences in species traits and their categories as a proxy for responses to environmental filters (Menezes et al 2010, Schmera et al 2015).
When predicting species distribution, a common method is to model the environmental influence on community structure, but applying only this approach often fails to support specific responses to ecosystem processes (Frainer et al 2014). Because aquatic ecosystems have complex ecological dynamics (e.g. connectivity, dendritic networks, and dispersal limitations), it can be evaluated at many spatial scales: regional contexts (whole basins and drainages), mesoscale habitats (riparian structure and pool-riffle sequences), and microhabitats (substrate composition) (Brown 2003, Swan & Brown 2011). Thus, studies have been proposed wherein species classified into groups with similar biological and ecological traits are expected to respond similarly along specific environmental gradients (Usseglio-Polatera et al 2000, Tomanova & Usseglio-Polatera 2007). Therefore, functional classification of stream insect communities (i.e. behaviour, physiology, and morphology) has contributed to define how assemblages respond to the environment and key aspects of the environment that influence species distribution at multiple spatial scales (Colzani et al 2013).
Relationships between functional traits and environmental factors is considered a good indicator for understanding human effects on stream insect communities (Townsend et al 1997, Díaz et al 2007). Considering particular groups, weak relationships between taxonomical identity and functional composition may support minor implications for ecosystem function when species loss is common in communities (Flynn et al 2009). According to theoretical references in the habitat templet, initially proposed by Southwood (1977), trait composition is affected by a set of environmental conditions that determine species traits in particular habitats and shape local species composition (Townsend & Hildrew 1994). If similar physical conditions tend to promote equivalent biological trait responses between communities, then taxonomic and functional composition should exhibit dependent responses along environmental gradients (Vinson & Hawkins 1998, Heino et al 2007). Regard to this, previous finds confirmed that patterns for functional redundancy (mostly conducted on plants and vertebrates) is linked to variation in species richness. Then, if there is high species richness with low categories of functional traits, the equivalence between communities tends to be high. In contrast, if species richness is high with variation in unique species in community composition, the functional equivalence in trait composition may be low (Fonseca & Ganade 2001, Hubbell 2005, Luck et al 2013).
These patterns are quite well understood for many aquatic systems in temperate zones, while few patterns related to aquatic insect assemblages in tropical streams are well known especially in species-rich communities (Tomanova & Usseglio-Polatera 2007, Reynaga & Santos 2013). For tropical streams, few studies have compared patterns in taxonomic and functional trait structure in response to anthropogenic degradation of ecosystem conditions (Reynaga & Santos 2013, Tupinambás et al 2014). Therefore, Amazonian streams are considered good models to address these issues. The region is known for high species-rich ecosystems with aquatic biodiversity bearing more niches, enabling habitat specialization and longer food chains than correspondent environments in temperate regions (Tomanova et al 2006, Albert et al 2011).
Responses of macroinvertebrate communities along environmental gradients at reach and catchment scales are expected to exhibit similar patterns for taxonomic and functional composition because they are strongly influenced by species richness that may control functional richness in the regional pools (Dolédec et al 2000, Mouchet et al 2010). Thus, it is expected that in high species-rich tropical streams, the taxonomic and functional trait composition produces contrary responses to habitat filtering, considering habitats with unique species and low functional redundancy (Dolédec et al 2000, Mouchet et al 2010). In view of this, the main goal of this study was to evaluate taxonomic and functional trait composition of aquatic insect assemblages in response to local inter-habitat variation of habitats among small streams. More importantly, we tested two hypotheses: (i) response of taxonomic and functional composition is redundant for environmental gradients in habitats at larger-scales (river segment scale, and catchment scale); (ii) functional composition in habitats from small scales (habitat patches) is influenced by specific environmental variables (i.e. links between specific variables and traits). To achieve this, we tested responses in taxonomic and functional composition of communities in streams from small- and large-scale (habitat patches, river segment scale, and catchment scale).
Materials and Methods
Study area
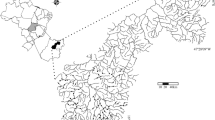
This study was performed at eight streams located at Floresta Nacional do Tapajós, an important protected area from the Tapajós River in the Amazon basin (Fig. 1). The area is covered by dense rain forest and located in the watershed of the Tapajós River, located in the south-west region of Pará State, Brazil. The forests are characterized as “terra firme,” or upper-level forest (80%), and have a small floodplain area with several “igapó” (flooded forest) areas (20%). The climate of the region, according to the Köppen classification, is a tropical monsoon climate (Am) with a short dry season from June to September.
Field sampling and sample processing
Aquatic insects were collected in the habitats at headwaters in the streams. The environmental variables were measured at the same sampling time during the dry season in June 2015. Specimens were collected using a circular dip net (190 mm in diameter, 0.25 mm mesh size) during a survey of benthic macroinvertebrates. A screening was performed in each riffle and pool zone and collected 20 substrate subsamples from each stream as replicates. Benthic subsamples were collected systematically from all available in-stream habitats (e.g. cobble, wood debris, vegetated banks, submerged leaves, sand, and other fine sediment) by kicking the substrate into the circular dip net. The same effort sampling were performed for all streams covering 150 m of each stream site from the downstream end of the reach moving upstream following an approach currently applied to assess aquatic habitat in Amazonian streams (Juen et al 2016). Specimens were sorted in the field and stored in alcohol at the Zoological Collection at Universidade Federal do Pará, Belém, Brazil. Then, insects were identified at the genus level or assigned them to the lowest taxonomic level using keys available in the literature (Hamada et al 2019).
Environmental data
In each stream, samples were collected on a 150 m stretch, which was subdivided into 10 continuous sections, 15 m in length, resulting in 11 cross-sectional transects. Habitat variables included measurement of stream channel morphology, in-stream habitat, and riparian structure. Finally, to test the influence of inter-habitat variation on insect assemblages in further analysis, we considered groups of habitat variables representing a hierarchical organization of streams as subsystems at three spatial scales (i.e. reach the system, pool/rifle system, and microhabitat system). We applied a selection process by removing variables from the environmental component; specifically, variables were removed if they (a) had values of zero for more than 90% of their data, (b) were highly correlated with other variables (Pearson correlations r > 0.7) or (c) were redundant with other variables.
The environmental component contained 12 variables grouped as riparian/channel variables: (1) canopy density mid-stream (%); (2) large woody debris (LWD) in and above the active channel (pieces/100 m); (3) thalweg mean depth (cm); and (4) mean ratio of wetted width to thalweg depth; substrate variables: (5) percentage of sand; (6) percentage of fine substrates (silt, clay, and muck); (7) percentage of roots (mostly Euterpe oleracea Mart., Arecaceae); and (8) percentage of wood and organic detritus; and water variables: (9) pH, (10) electrical conductivity (μS/cm), (11) temperature (°C), (12) dissolved oxygen (mg/L) (Table 1). The selected variables are based on ecological relevance and their past use in studies of aquatic insects in Amazonian streams (Couceiro et al 2012, Datry et al 2016, Juen et al 2016).
Functional trait composition
A trait categorical database were computed comprising six biological traits and 30 trait categories (Table S1, S2 in Supplementary Material). Trait groups and their modalities for each taxa were computed from available studies considering the limited knowledge on functional traits available for Neotropical species (e.g. Cummins et al 2005, Tomanova et al 2006, Tomanova & Usseglio-Polatera 2007, Colzani et al 2013, de Castro et al 2017). In addition, the categorical matrix of species traits were compared with prior studies that evaluated relationships between biological attributes of aquatic insects linked with the environment from temperate streams (Cummins 1973, Finn & Poff 2005, Poff et al 2006, Merritt & Cummins 2007, Merritt et al 2008). Trait variables recognized for aquatic insects and their habitat included the following modalities: two trophic trait groups (i.e. “food” and “guilds”), respiration mode, two morphological adaptations (i.e. body shape and specific adaptations to flow) and mobility mode (see Table S2 in Supplementary Material).
Data analysis
Prior to ordination methods, Hellinger transformation was applied to the species abundance matrix (L: insect composition) to best fit the variation in community composition. To test our first hypothesis and evaluate environmental influence on taxonomic community composition, a distance-based redundancy analysis (dbRDA) was performed on the abundance matrix (based on the Bray–Curtis distance). We tested the null hypothesis (i.e. no relationship) using an ANOVA with 999 permutations. (Legendre & Anderson 1999).
To test our second and third hypotheses, RLQ analysis and the fourth-corner method were performed to evaluate patterns in community composition based simultaneously on the influence of environmental variables and functional trait composition (Dolédec et al 1996, Legendre et al 1997). Relationships were analysed among the matrices of environmental variables (R), abundance (L: 135 taxa), and traits (Q). RLQ is an extension of co-inertia analysis that searches simultaneously for linear combinations of variables in Q and linear combinations of variables in R while maximizing covariance and using abundance-weighting in the L matrix. For this step, the R and Q tables were first submitted to principal component analysis (PCA) (the Q table using the Hill and Smith ordination method for mixing quantitative variables and factors) and the L table submitted to correspondence analysis.
The fourth-corner method was performed to test specific environment–trait relationships (i.e. relationships between Q and R) with two suitable models (hereafter referred to as models 2 and 4 according to Dray et al (2014). The first model tests the hypothesis that species assemblages are dependent upon the environmental characteristics of the sites where they are found (i.e. environmental control over species assemblages). The second model tests that the distributions of the species among the sites, which are related to their preferences for site conditions, depend on the adaptations (traits) of the species (i.e. non-random species attributes). This step consists of bivariate tests to analyse associations between one trait and one environmental variable at a time. Permutation methods were applied using adjusted p values (Bonferroni procedure for multiple tests of significance) for multiple comparisons using a significant level of α = 0.05. To visualize fourth-corner modelling results, standardized coefficients were applied for all environment–trait interaction correlations. We performed all statistical analyses with functions from the packages vegan (dbrda), ade4 (rlq), and mvabund (fourth-corner modelling) in R version 3.3.0.
Results
Insect Diversity
A total of 5469 aquatic insects were collected (Coleoptera, Diptera, Ephemeroptera, Hemiptera, Lepidoptera, Megaloptera, Odonata, Plecoptera, and Trichoptera) and classified into 135 taxa and categorized them into six groups based on functional traits (see Tables S2 and S3 in Supplementary Material). An average of 74 genera and 685 individuals were collected per stream. Diptera and Coleoptera were the orders with the highest richness, with 48 and 19 genera, respectively. Ephemeroptera and Diptera were the most abundant orders, with 1564 and 1467 individuals, respectively (Fig. 2). Among the most common genera, the following 20 represented 67% of the total relative abundance: Miroculis, Leptonema, Macrogynoplax, Farrodes, Anacroneuria, Campylocia, Gyretes, Macronema, Parapoynx, Riethia, Phaenopsectra, Limnophila, Hagenulopsis, Zonophora, Chimarra, Endotribelos, Macrostemum, Helicopsyche, Paratanytarsus, and Simulium (see Tables S3 in Supplementary Material).
Relationships among Environmental Variables and Community Structure
Overall, our first hypothesis was corroborated by the response of community structure to the multiple scales of habitat characteristics. Results of distance-based redundancy analysis (dbRDA) indicated that the inter-habitat variation affected community composition of aquatic insect assemblages (and their abundances) (Table 2). All models indicating multiple scales of habitat, such as riparian/channel, substrate, and water variables influenced community composition and species distribution. The main predictors in each model were mean canopy density mid-stream (XCDENMID), mean wetted width (XWD_RAT) percentage of sand (PCT_SA), percentage of fine substrates (PCT_FN), temperature, and pH.
Summary of the Response of Community Structure to Environmental Variables and Trait Composition
Our results from RLQ and fourth-corner analysis corroborated the second and third hypothesis that assemblages are structured by environmental variables and traits for each group at multiple scales of habitat (Tables 3 and 4). For all models of habitat scale, the first two axes of RLQ analysis explained more than 60% of the total variance. Permutations tests on fourth-corner models (pseudo-F and Pearson r for one quantitative variable and one qualitative variable) showed that the overall functional trait composition was significantly correlated with the environmental variables (Table 3). However, we did not find support for our third hypothesis. No significant bivariate associations between specific traits and environmental variables were detected in the Fourth-corner test for specific environment–trait relationships (i.e. specific associations between a trait and an environmental variable).
The response of overall functional composition to environmental gradients can be summarized as a group of traits. Predator species (e.g. Polyplectropus, Cernotina, Aeschnosoma, and most Dipteran predators) and collector-gatherers (e.g. Americabaetis, Cryptonympha, Farrodes, Miroculis, and Waltzoyphius) exhibited the higher interactions. The association for environment and traits for shredder species (e.g. Anacaena and Hydrodessus) and collector-filterers (e.g. Chimarra, Leptonema, Macrostemum, and Simulium) was weakly significant. For scraper species (e.g. Askola, Hydrosmilodon, and Pheneps), only water variables were weakly associated with traits. Moreover, piercer taxa (e.g. Paratrephes, Tenagobia) were weakly associated with all groups of environmental variables. Then, as expected, traits for the type of foods were correlated to feeding habits, such as macroinvertebrates (MaIn) and coarse (CPOM) and fine (FPOM) particulate matter.
Discussion
We found that the distribution of aquatic insect species in the streams evaluated was regulated by their association with environmental conditions dependent on species traits that occur at multiple habitat scales. Our results showed that the variation in functional traits and taxonomical community composition had the same response to environmental variables. As expected, patterns of functional and taxonomical composition represented a response to environmental gradients reflected by different aspects of macro- and microhabitat conditions. Moreover, trait response to inter-habitat variation highlighted the key response of feeding functional groups to the environment, which could be associated with traits mostly related to species interactions. This pattern corroborated the hypothesis that community composition and species traits exhibited strong relationships under local environmental conditions; also, it is regulated by species interactions that drive local assembly rules in insect communities.
When considering the contribution of variation in species traits and their categories in response to environmental filtering, the variation in functional community composition can be mostly summarized as associations of functional feeding groups (FFG) to habitat structure. Our results revealed some patterns in the studied taxa and could be summarized according to patterns in assemblage structure, such as species richness and abundance (Merritt et al 2008). First, shredders (e.g. Miroculis) were the most abundant feeding group found; this group is common in autotrophic/heterotrophic aquatic systems because these organisms are strongly linked to variation in the riparian zone (Cummins et al 2005, Poff et al 2006). Our results corroborated this fact, as we found environmental variability in the studied streams, including a high percentage of woody debris and vegetal substrates ranging from coarse particulate organic matter (CPOM) to fine particulate organic matter (FPOM). The second most abundant group was the collector-gatherer group (e.g. Campylocia, Riethia, Hagenulopsis, Endotribelos, and Helicopsyche). In natural communities, these taxa are associated with environments that have heterogeneous substrates, channel stability, and habitats composed of cobbles, boulders, large woody debris, and rooted vascular plants (Cummins et al 2005). Thus, we found a relationship between the presence of collector-gatherer taxa and the variation in woody debris and substrate size. Rooted vascular plants were frequently found in riparian zones of most streams, contributing to canopy cover and channel stability (e.g. Euterpe oleracea Mart., Arecaceae).
The functional feeding habit of a taxon is considered a good indicator that can be used to group taxa based on their functional traits, as we did in our study; this method revealed patterns previously known to describe community structure and stream habitats (Cummins et al 2005). When highlighting many trait states that co-occur and are tightly linked, the categories had strong phylogenetic or taxonomic affinities (Poff et al 2006). Shredders, collector-gatherers, and predators composed convergent assemblages, which were mostly associated with other traits, such as food resources, body form, specific adaptation to flow, mobility, and attachment to substrata. Considering pure environmental effects, RLQ analysis showed convergent trait assemblages (shredders and collector-gatherers) in streams with high dissolved oxygen concentrations. These assemblages often occur in shallow, high-flow habitats where individuals expend more energy to resist flow constraints (Tomanova et al 2006). In contrast, epi- and endobenthic burrowers (most of the predators and Diptera taxa) were found mainly in deep, low-flow stream reaches where habitats commonly have mineral substrata (e.g. sand and gravels) that are easier to penetrate (Moya et al 2007).
In riverine landscapes, local habitat attributes act as the main filter, and the variables evaluated here related to stream channel morphology, riparian structure, substrate size, and large wood debris play considerable roles in species composition and traits of aquatic insect assemblages (Heino et al 2005, Juen et al 2016). At local-scale, environmental filtering may act to control assemblages by allowing the distribution of similar coexisting species, which should lead to high functional redundancy of traits and similar response to habitat structure by both functional and taxonomical composition (Mouchet et al 2010). At that point, our results are in line with previous studies, highlighting that local physical habitat variables that appear to be more highly related to functional composition than to patterns of the taxonomic structure because the environment should select attributes regardless of taxonomic variation (Poff & Ward 1990, Finn & Poff 2005). Trait variables have been shown to effectively describe community patterns because joint taxonomical composition often summarize biological interactions (e.g. predation and competition) at the small to large-scale (Jonsson et al 2001). Patterns in community structure should also be evaluated from different scales, to detect effects of environmental filters acting on traits related to dispersal and life history (e.g. locomotion modes, resistance forms, and dispersal) (Heino, 2005).
Although small streams within the same regional context are often physically and chemically similar, they can also differ markedly because of habitat heterogeneity (LeCraw & Mackereth 2010). Our sampling sites were naturally acidic streams with discrete gradients of other limnological variables. These conditions provide evidence that streams with lower pH values are accompanied by a number of other chemical changes, and the response of an organism is caused by various physiological and behavioural strategies (Lewis 2008). Moreover, we found that specific local conditions (physical habitat and water variables) are the mechanisms driving species diversity and abundance. Additionally, a similar set of conditions had a strong influence on the functional composition. This phenomenon may be caused by ecosystem processes that are relatively unaffected by species substitutions if the substituted species has similar traits (Dangles et al 2004).
The observed patterns in community composition and functional traits may be related to local conditions that are often found in Amazonian streams, including black acidic waters, low values of electrical conductivity, and high variation in substratum characteristics. Moreover, for most tropical stream communities, these in-stream conditions are considered key factors for explaining the variance in community structure and ecosystem function at different scales (Jacobsen et al 2008).
In summary, our trait-based approach showed that both functional and taxonomical composition are dependent on local conditions; in this way, the habitat conditions associated with species interactions shape most of the functional composition of the aquatic insects. At the local scale, environmental gradients can produce similar effects influencing both traits and taxonomic patterns in aquatic insect communities. Despite the low number of analysed streams, our analyses provide important information for understanding the simultaneous variation in functional trait composition and community composition found in Amazonian streams. In addition, the present study seems to support habitat templet models for the aquatic insect communities in Amazonian streams. Thus, we can highlight that variation in stream riparian/channel, substrate, and water variables primarily predict trait composition at the stream scale. Therefore, we recommend that future research would address these issues by applying quantitative measures of traits related to biotic interactions to account unique features of Neotropical aquatic diversity.
References
Albert JS, Carvalho TP, Petry P, Holder MA, Maxime EL, Espino J, Corahua I, Quispe R, Rengifo B, Ortega H, Reis RE (2011) Aquatic biodiversity in the Amazon: habitat specialization and geographic isolation promote species richness. Animals 1:205–241. https://doi.org/10.3390/ani1020205
Brown BL (2003) Spatial heterogeneity reduces temporal variability in stream insect communities. Ecol Lett 6:316–325. https://doi.org/10.1046/j.1461-0248.2003.00431.x
Colzani E, Siqueira T, Suriano MT, Roque FO (2013) Responses of aquatic insect functional diversity to landscape changes in Atlantic Forest. Biotropica 45:343–350. https://doi.org/10.1111/btp.12022
Couceiro SRM, Hamada N, Forsberg BR, Pimentel TP, Luz SLB (2012) A macroinvertebrate multimetric index to evaluate the biological condition of streams in the Central Amazon region of Brazil. Ecol Indic 18:118–125. https://doi.org/10.1016/j.ecolind.2011.11.001
Cummins KW (1973) Trophic relations of aquatic insects. Annu Rev Entomol 18:183–206
Cummins KW, Merritt RW, Andrade PC (2005) The use of invertebrate functional groups to characterize ecosystem attributes in selected streams and rivers in South Brazil. Stud Neotrop Fauna Environ 40:69–89. https://doi.org/10.1080/01650520400025720
Dangles O, Gessner MO, Guerold F, Chauvet E (2004) Impacts of stream acidification on litter breakdown: implications for assessing ecosystem functioning. J Appl Ecol 41:365–378. https://doi.org/10.1111/j.0021-8901.2004.00888.x
Datry T, Moya N, Zubieta J, Oberdorff T (2016) Determinants of local and regional communities in intermittent and perennial headwaters of the Bolivian Amazon. Freshw Biol 61:1335–1349. https://doi.org/10.1111/fwb.12706
de Castro DMP, Dolédec S, Callisto M (2017) Landscape variables influence taxonomic and trait composition of insect assemblages in Neotropical savanna streams. Freshw Biol 62:1472–1486. https://doi.org/10.1111/fwb.12961
Díaz AM, Alonso MLS, Gutiérrez MRV-A (2007) Biological traits of stream macroinvertebrates from a semi-arid catchment: patterns along complex environmental gradients. Freshw Biol 53:071204011451001-??? doi:https://doi.org/10.1111/j.1365-2427.2007.01854.x
Dolédec S, Chessel D, ter Braak CJF, Champely S (1996) Matching species traits to environmental variables: a new three-table ordination method. Environ Ecol Stat 3:143–166. https://doi.org/10.1007/BF02427859
Dolédec S, Olivier JM, Statzner B (2000) Accurate description of the abundance of taxa and their biological traits in stream invertebrate communities: effects of taxonomic and spatial resolution. Fundam Appl Limnol 148:25–43. https://doi.org/10.1127/archiv-hydrobiol/148/2000/25
Dray S, Choler P, Dolédec S, Peres-Neto PR, Thuiller W, Pavoine S, ter Braak CJF (2014) Combining the fourth-corner and the RLQ methods for assessing trait responses to environmental variation. Ecology 95:14–21. https://doi.org/10.1890/13-0196.1
Finn DS, Poff NL (2005) Variability and convergence in benthic communities along the longitudinal gradients of four physically similar Rocky Mountain streams. Freshw Biol 50:243–261. https://doi.org/10.1111/j.1365-2427.2004.01320.x
Flynn DFB, Gogol-Prokurat M, Nogeire T, Molinari N, Richers BT, Lin BB, Simpson N, Mayfield MM, DeClerck F (2009) Loss of functional diversity under land use intensification across multiple taxa. Ecol Lett 12:22–33. https://doi.org/10.1111/j.1461-0248.2008.01255.x
Fonseca CR, Ganade G (2001) Species functional redundancy, random extinctions and the stability of ecosystems. J Ecol 89:118–125. https://doi.org/10.1046/j.1365-2745.2001.00528.x
Frainer A, McKie BG, Malmqvist B (2014) When does diversity matter? Species functional diversity and ecosystem functioning across habitats and seasons in a field experiment. J Anim Ecol 83:460–469. https://doi.org/10.1111/1365-2656.12142
Hamada N, Nessimian JL, Querino RB (2019) Insetos aquáticos na Amazônia brasileira : taxonomia, biologia e ecologia, 1st edn. Editora do INPA, Manaus
Heino J (2005) Functional biodiversity of macroinvertebrate assemblages along major ecological gradients of boreal headwater streams. Freshw Biol 50:1578–1587. https://doi.org/10.1111/j.1365-2427.2005.01418.x
Heino J, Mykrä H, Kotanen J, Muotka T (2007) Ecological filters and variability in stream macroinvertebrate communities: do taxonomic and functional structure follow the same path? Ecography (Cop) 30:217–230. https://doi.org/10.1111/j.2007.0906-7590.04894.x
Heino J, Parviainen J, Paavola R, Jehle M, Louhi P, Muotka T (2005) Characterizing macroinvertebrate assemblage structure in relation to stream size and tributary position. Hydrobiologia 539:121–130. https://doi.org/10.1007/s10750-004-3914-3
Hubbell SP (2005) Neutral theory in community ecology and the hypothesis of functional equivalence. Funct Ecol 19:166–172. https://doi.org/10.1111/j.0269-8463.2005.00965.x
Jacobsen D, Cressa C, Mathooko JM, Dudgeon D (2008) Macroinvertebrates. Composition, life histories and production. Trop stream Ecol 65–105. https://doi.org/10.1016/B978-012088449-0.50006-6
Jonsson M, Malmqvist B, Hoffsten PO (2001) Leaf litter breakdown rates in boreal streams: does shredder species richness matter? Freshw Biol 46:161–171. https://doi.org/10.1046/j.1365-2427.2001.00655.x
Juen L, Cunha EJ, Carvalho FG, Ferreira MC, Begot TO, Andrade AL, Shimano Y, Leão H, Pompeu PS, Montag LFA (2016) Effects of oil palm plantations on the habitat structure and biota of streams in eastern Amazon. River Res Appl 32:2081–2094. https://doi.org/10.1002/rra.3050
LeCraw R, Mackereth R (2010) Sources of small-scale variation in the invertebrate communities of headwater streams. Freshw Biol 55:1219–1233. https://doi.org/10.1111/j.1365-2427.2009.02347.x
Legendre P, Anderson MJ (1999) DISTANCE-BASED REDUNDANCY ANALYSIS: TESTING MULTISPECIES RESPONSES IN MULTIFACTORIAL ECOLOGICAL EXPERIMENTS. Ecol Monogr 69:1–24. https://doi.org/10.1890/0012-9615(1999)069[0001:DBRATM]2.0.CO;2
Legendre P, Galzin R, Harmelin-Vivien ML (1997) Relating behavior to habitat: solutions to thefourth-corner problem. Ecology 78:547–562. https://doi.org/10.1890/0012-9658(1997)078[0547%3ARBTHST]2.0.CO%3B2
Lewis WM (2008) Physical and chemical features of tropical flowing waters. Trop Stream Ecol:1–21. https://doi.org/10.1016/B978-012088449-0.50003-0
Luck GW, Carter A, Smallbone L (2013) Changes in bird functional diversity across multiple land uses: interpretations of functional redundancy depend on functional group identity. PLoS One 8:e63671. https://doi.org/10.1371/journal.pone.0063671
Menezes S, Baird DJ, Soares AMVM (2010) Beyond taxonomy: a review of macroinvertebrate trait-based community descriptors as tools for freshwater biomonitoring. J Appl Ecol 47:711–719. https://doi.org/10.1111/j.1365-2664.2010.01819.x
Merritt RW, Cummins KW (2007) Trophic relationships of macroinvertebrates. Methods in Stream Ecology. Elsevier, In, pp 585–601
Merritt RW, Cummins KW, Berg MB (2008) An introduction to the aquatic insects of North America, 4th edn. Kendall Hunt Publishing, Dubuque
Mouchet MA, Villéger S, Mason NWH, Mouillot D (2010) Functional diversity measures: an overview of their redundancy and their ability to discriminate community assembly rules. Funct Ecol 24:867–876. https://doi.org/10.1111/j.1365-2435.2010.01695.x
Moya N, Tomanova S, Oberdorff T (2007) Initial development of a multi-metric index based on aquatic macroinvertebrates to assess streams condition in the upper Isiboro-Sécure Basin, Bolivian Amazon. Hydrobiologia 589:107–116. https://doi.org/10.1007/s10750-007-0725-3
Poff NL (1997) Landscape filters and species traits: towards mechanistic understanding and prediction in stream ecology. J North Am Benthol Soc 16:391–409. https://doi.org/10.2307/1468026
Poff NL, Olden JD, Vieira NKM et al (2006) Functional trait niches of North American lotic insects: traits-based ecological applications in light of phylogenetic relationships. J North Am Benthol Soc 25:730–755. https://doi.org/10.1899/0887-3593(2006)025[0730:FTNONA]2.0.CO;2
Poff NL, Ward JV (1990) Physical habitat template of lotic systems: recovery in the context of historical pattern of spatiotemporal heterogeneity. Environ Manag 14:629–645. https://doi.org/10.1007/BF02394714
Reynaga MC, Dos Santos DA (2013) Contrasting taxonomical and functional responses of stream invertebrates across space and time in a Neotropical basin. Fundam Appl Limnol / Arch für Hydrobiol 183:121–133. https://doi.org/10.1127/1863-9135/2013/0501
Schmera D, Podani J, Heino J, Ero T (2015) A proposed unifi ed terminology of species traits in stream ecology. Freshw Sci 34:823–830. https://doi.org/10.1086/681623
Southwood TRE (1977) Habitat , the Templet for Ecological Strategies ? J Anim Ecol 46:336–365, Habitat, the Templet for Ecological Strategies?
Swan CM, Brown BL (2011) Advancing theory of community assembly in spatially structured environments: local vs regional processes in river networks. J North Am Benthol Soc 30:232–234. https://doi.org/10.1899/10-150.1
Tomanova S, Goitia E, Helešic J (2006) Trophic levels and functional feeding groups of macroinvertebrates in Neotropical streams. Hydrobiologia 556:251–264. https://doi.org/10.1007/s10750-005-1255-5
Tomanova S, Usseglio-Polatera P (2007) Patterns of benthic community traits in neotropical streams: relationship to mesoscale spatial variability. Fundam Appl Limnol / Arch für Hydrobiol 170:243–255. https://doi.org/10.1127/1863-9135/2007/0170-0243
Townsend C, Doledec S, Scarsbrook M (1997) Species traits in relation to temporal and spatial heterogeneity in streams: a test of habitat templet theory. Freshw Biol 37:367–387. https://doi.org/10.1046/j.1365-2427.1997.00166.x
Townsend CR, Hildrew AG (1994) Species traits in relation to a habitat templet for river systems. Freshw Biol 31:265–275. https://doi.org/10.1111/j.1365-2427.1994.tb01740.x
Tupinambás TH, Cortes RMV, Varandas SG, Hughes SJ, França JS, Callisto M (2014) Taxonomy, metrics or traits? Assessing macroinvertebrate community responses to daily flow peaking in a highly regulated Brazilian river system. Ecohydrology 7:828–842. https://doi.org/10.1002/eco.1406
Usseglio-Polatera P, Bournaud M, Richoux P, Tachet H (2000) Biological and ecological traits of benthic freshwater macroinvertebrates: relationships and definition of groups with similar traits. Freshw Biol 43:175–205. https://doi.org/10.1046/j.1365-2427.2000.00535.x
Vinson MR, Hawkins CP (1998) BIODIVERSITY OF STREAM INSECTS: variation at local, basin, and regional scales 1. Annu Rev Entomol 43:271–293
Acknowledgements
We thank Dr. Luciano Fogaça de Assis Montag, for coordinating the project FAPESPA: ICAAF no. 85/2014 Edital 008/2013 - PRONEM. We are also grateful to Darlison Andrade and José Risonei Assis da Silva, administrators of Floresta Nacional do Tapajós, and the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) staff for assistance in field sampling and research permit to study the area.
Funding
This work was financially supported by Fundação Amazônia Paraense de Amparo à Pesquisa (Pará State Research Foundation - FAPESPA) through the project FAPESPA: ICAAF no. 85/2014 Edital 008/2013 - PRONEM for financial support. Scholarships were financially provided by Conselho Nacional de Desenvolvimento Científico e Tecnológico (Brazilian National Research Council - CNPq) to GN (process 141866/2013-6), EC (process 165908/2014-9), LJ (process 307597/2016-4), and NH (process 307849/2014-7).
Author information
Authors and Affiliations
Contributions
GN and LJ conceived the research. GN and EJC conducted the field sampling and analysed the data. NH contributed with laboratorial training and revised the taxonomic data. GN wrote the manuscript. All authors revised and approved the manuscript.
Corresponding author
Additional information
Edited by Yulin Gao – CAAS, China
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 363 kb)
Rights and permissions
About this article
Cite this article
Nicacio, G., Cunha, E.J., Hamada, N. et al. How Habitat Filtering Can Affect Taxonomic and Functional Composition of Aquatic Insect Communities in Small Amazonian Streams. Neotrop Entomol 49, 652–661 (2020). https://doi.org/10.1007/s13744-020-00780-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-020-00780-z