Abstract
Huanglongbing (HLB), the most destructive citrus disease worldwide, was first recorded in Brazil in 2004, and since then, more than 50 million trees identified with this disease have been eliminated. The disease is managed mainly by controlling the psyllid vector Diaphorina citri Kuwayama, 1908 (Hemiptera: Liviidae). Although the presence of the insect in commercial citrus groves is low, HLB infection rates increase in areas bordering the groves. The disease is transmitted by psyllids from host citrus plants in areas outside the managed groves, such as abandoned or organic groves and residential trees, and from orange jasmine plants in urban settings. In order to provide information to support HLB control, this study evaluated the biotic and abiotic variables that affect the dynamics of D. citri populations after releases of the parasitoid wasp Tamarixia radiata (Waterston, 1922) (Hymenoptera: Eulophidae) in external sources of HLB inocula. The study was divided into two parts. After releasing the parasitoids in non-commercial areas, we determined the following: (a) the variables that significantly affected the number of nymphs collected on shoots in the same non-commercial area; (b) the variables that significantly affected the number of adult psyllids collected in a neighboring commercial citrus area. Our results indicated that the number of nymphs in external areas was affected only by the host plant and rainfall. However, periodic parasitoid releases significantly reduced the number of adult psyllids collected in the commercial area. The results indicate that the release of parasitoids in external sources of inocula has the potential to maximize actions for D. citri control, contributing to the reduction of psyllid populations in commercial areas. Consequently, this strategy may help to manage the disease infection without an increase in insecticide use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since huanglongbing (HLB) or citrus-greening disease was first recorded in Brazil in 2004 (Teixeira et al2005), more than 50 million plants identified with the disease have been eliminated (CDA 2019). The disease is present in almost all citrus-producing regions worldwide (Bové 2006, Hall et al2013). Although HLB was recently detected in Brazil only, the vector, Asian citrus psyllid (ACP) Diaphorina citri Kuwayama, 1908 (Hemiptera: Liviidae), has been present in the country since 1938 (Costa Lima 1942). After the disease was detected, several control strategies were applied, including exclusive use of healthy citrus seedlings, elimination of symptomatic plants, and chemical control of the vector. Because of the intensive use of insecticide sprays to control D. citri, classical, augmentative, or conservation biological control (BC) could not be used in commercial areas, since the citrus growers rarely use selective insecticides.
Examining the HLB epidemiology, Bergamin-Filho et al (2016) found that the primary inocula occur in areas surrounding the groves, where the psyllids can develop and migrate, transmitting the bacterial infection. Previously, Tiwari et al (2010) found that large D. citri populations migrated from abandoned areas to commercial groves. These areas include abandoned groves (with no psyllid control), organically managed groves, residential backyards with citrus trees, and urban areas with ornamental orange jasmine (Murraya paniculata, a psyllid host) (Sétamou & Bartels 2015).
The ectoparasitoid Tamarixia radiata Waterston, 1922 (Hymenoptera: Eulophidae) was first recorded in 2004 in Brazil, associated with high rates of natural parasitism on D. citri nymphs (Gómez-Torres et al2006). Although T. radiata releases have been studied in order to control D. citri (Skelley & Hoy 2004, Flores & Ciomperlik 2017), studies focused on releasing the parasitoid in commercial areas indicated a low effectiveness of biological control, due to the intense use of chemical control (Beloti et al2015). An alternative strategy would be to release parasitoids in non-commercial areas, controlling the source of adult psyllids and their influx into commercial areas (Parra et al2016; Milosavljević et al2018).
Given the importance of managing D. citri in external sources of HLB inocula to control the spread of citrus-greening disease, the present study was composed of two different and independent studies, both of them focusing on parasitoid releases in non-commercial areas to control the psyllid vector: (1) releasing T. radiata in non-commercial areas to investigate if the parasitism affected these D. citri populations outside commercial areas; (2) releasing T. radiata in non-commercial areas (neighboring commercial area) to investigate if parasitoid releases in external areas affect the dynamics of adult psyllid populations within the commercial grove.
Material and Methods
In the first part of this study, we monitored D. citri populations at 9 sites in non-commercial areas in the citrus belt of the state of São Paulo, to investigate the variables that may affect nymph dynamics including parasitism by T. radiata, by releasing parasitoids biweekly in these areas and observing the presence of eggs and larvae of T. radiata in D. citri nymphs (the “Releasing the parasitoid T. radiata to control D. citri populations in non-commercial areas” section). In the second part, we evaluated whether releasing T. radiata in a non-commercial area located in the municipality of Itapetininga (Rechã district), São Paulo, could affect the number of adult psyllids in a commercial grove (the “Releasing the parasitoid T. radiata to control D. citri populations inside commercial areas” section). The two studies are linked and complemented each other, since the first one studied the psyllid in its proliferation zone (non-commercial areas) and the second studied the adult vector psyllids that disperse to commercial areas from external sources of HLB inocula, carrying the greening disease.
Releasing the parasitoid T. radiata to control D. citri populations in non-commercial areas
For this experiment, nine non-commercial areas located in municipalities that compose the citrus belt of the state of São Paulo were chosen (Fig 1). These were divided into three groups: group (1) 3 areas with Citrus plants as hosts and with chemical control, in the municipalities of Leme (2.5 ha), Matão (2.5 ha), and Monte Azul Paulista (2.0 ha); group (2) 3 areas with citrus plants as hosts and without chemical control, in the municipalities of Anhembi (2.5 ha), Getulina (2.5 ha), and Itapetininga (2.5 ha), for both citrus areas the plants was 7 to 10 years old; and group (3) 3 areas with orange jasmine (Murraya paniculata) as host and without chemical control, in the municipalities of Cajobi (2.5 ha), Pirajuí (1.0 ha), and Rincão (1.5 ha) with unknown age but adult and vigorous plants.
Nymph population was monitored, biweekly, in each area, from February 2012 to January 2013. The first sampling (before start the parasitoid’s releasing) in the areas indicated that natural parasitism was present only in Rincão (22.2%), Itapetininga (3.5 %), and Pirajuí (0.96%). During the same period, releases were conducted at a rate of 400 parasitoids/ha at two different points, approximately at the center of the study areas, as part of a biological control program targeting D. citri. The individuals of T. radiata were reared according to Parra et al (2016). Tamarixia radiata adults (~ 24 h after emergence) were transported to the field in 2.5-cm glass tubes with a honey droplet for insect nourishment, packed in Styrofoam boxes.
In each area, 60 branches (≅ 10 cm) were collected at the upper third of the plant biweekly during the sampling period, from different trees selected randomly in different parts of the area in order to obtain a representative sample. The branches were individually bagged, placed in a Styrofoam box, and taken to the Laboratory of Insect Biology of the Entomology and Acarology Department, Luiz de Queiroz College of Agriculture, University of São Paulo (USP-ESALQ). At the laboratory, the branches were inspected for D. citri nymphs and T. radiata under a stereoscopic microscope. Each nymph was examined for the parasitoid, i.e., for the presence of T. radiata eggs, larvae or pupae attached, or mummified nymphs with an exit hole. The numbers of non-parasitized and parasitized nymphs were recorded. On each sampling date, air temperatures for each study site were obtained from the closest weather station. We also obtained data for monthly rainfall. Each study site was located within 5 km from the nearest meteorological station.
We analyzed whether D. citri populations were affected by the parasitism rate and/or other factors (temperature, monthly rainfall, host plant, and month) by using a generalized linear model (GLM) with a negative binomial distribution since over dispersion was observed in our count data (O’Hara & Kotze 2010). The dependent variable was the nymphs recorded at each site for each sample date. Host plant (citrus, orange jasmine), temperature, rainfall, parasitism rate, and sample month were used as explanatory variables. We studied a wide range of variables, not only the parasitism rate, in order to determine the biotic and abiotic factors that may affect psyllid populations, providing more information for use in management programs. We calculated P values of the selected model using the likelihood ratio test. Non-significant explanatory variables (P > 0.15) were removed to obtain the minimal adequate model, following the methodology used by Milosavljević et al (2018). Model selection with information criteria (Akaike’s information criterion [AIC]) was performed to identify the best model (lowest AIC value). All statistical analyses were performed in R® software (Development Core Team R 2018).
Releasing the parasitoid T. radiata to control D. citri populations inside commercial areas
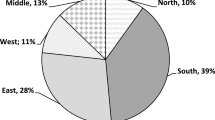
The study was carried out in a commercial grove (Itapetininga; 23°35′30″S, 48°03′11″W). Graminha Farm is a commercial citrus grower with a total area of 2700 ha. The farm has a strict pest-sampling program, with biweekly visual inspection of 1% of the citrus trees for psyllid nymphs. Adult psyllids were monitored with yellow sticky traps located every 150 m along the entire farm border, and other sticky traps within the groves, totaling to 735 traps. The farm uses chemical-control sprays for mature trees and soil-drench applications for new trees (bifentrin and dimethoate). However, the psyllids continue to be present because the insects move in from non-commercial areas outside the farm where no control is practiced. D. citri can disperse as far as 1.6 or 2.2 km from the breeding site (Ferreira 2014, Lewis-Rosenblum et al2015).
The farm is bordered by the Rechã district that has approximately 4500 residents, and with citrus trees in residential backyards, all of which can serve as hosts for the psyllid (considered as pest inocula). The district has approximately 200 host plants including all the types mentioned. This experiment was carried out in way to represent the real conditions of the infestation occurring in commercial areas once, as described by Bergamin-Filho et al (2016), psyllid movement onto that areas still occur despite of intensive insecticide sprays.
We obtained, from the farm, the number of adult psyllids per yellow sticky trap biweekly from August 2014 to August 2015. At the beginning of the study, we also started biweekly releases of T. radiata at 8 different points, inside the Rechã district, approximately 200 m from each other, at a rate of 400 parasitoids/point, and continued the releases for the entire sampling period. Each parasitoid-release location was located approximately 2 km from the farm border. Temperature and monthly rainfall were obtained from automatic meteorological station located inside the farm.
We analyzed whether the number of psyllids per sampling date (dependent variable) was affected by the cumulative number of parasitoid releases since the beginning of the study and/or by abiotic factors (temperature, month, and rainfall) (explanatory variables), using generalized linear models (GLM). We calculated P values of the selected model using the likelihood ratio test. Non-significant explanatory variables (P > 0.15) were removed to obtain the minimal adequate model, following the methodology used by Milosavljević et al (2018). Model selection with information criteria (Akaike’s information criterion) was performed to identify the best model (lowest AIC value). All statistical analyses were performed in R software (Development Core Team R 2018).
Results
Effects of the parasitism rate on D. citri nymphs in non-commercial areas
In Leme, Matão, and Monte Azul Paulista (Group 1, regular insecticide use), D. citri nymphs and the parasitoid were technically absent. Therefore, we did not use these three areas in our analysis. In the municipalities that composed groups 2 and 3, the incidence of nymphs per branch ranged from 1.92 to 2.8 and 0.88 to 2.57, respectively, throughout the study.
The number of D. citri nymphs was not significantly affected by month, T. radiata parasitism (Fig 2), temperatures, or interactions among these variables (P > 0.15). Nymphs were significantly affected only by the host plant (estimate = – 0.24026, SE = 0.13503, χ2 = 2.9089, Df = 1, P = 0.088) and rainfall (estimate = – 0.03161, SE = 0.02078, χ2 = 2.2295, Df = 1, P = 0.135). Regarding the host plant, the mean number of psyllids per sampling was equal to 117.06 and 93.58 in citrus and orange jasmine, respectively (Fig 3).
Effect of the number of parasitoid releases in an external area on the number of adult Asian citrus psyllids collected inside a commercial area
In the second part of our study, the cumulative number of parasitoid releases (estimate – 0.08874, SE = 0.0460310, χ2 = 90783, Df = 1, P = 0.0026) was the only variable found to be significant (Fig 4). The climate variables (temperature and rainfall) and the month did not significantly affect the D. citri population.
Discussion
Effects of the parasitism rate on D. citri nymphs in non-commercial areas
Between 2005 and 2010, the use of agrochemicals in Brazilian citriculture increased more than 600% (Neves et al2011). Beloti et al (2015) found that more than half of the 25 insecticides recommended for citrus pests were classified as harmful (IOBC class 4), and only 20% were considered harmless to T. radiata. This information could explain our results for the commercial groves with insecticide use, where no parasitism was recorded. This observation demonstrates the importance of environments without any control for D. citri reproduction and dispersal, mainly to commercial groves (Bergamin-Filho et al2016).
Rainfall was negatively correlated with the number of psyllid nymphs. Heavy rainfall reduces populations by washing 1st- and 2nd-instar nymphs from the trees. Only adults manage to survive because they hide on the lower surfaces of the leaves and twigs (Aubert 1987, Ahmed et al2004, Teck et al2011a). Hall et al (2008) also observed that high levels of monthly rainfall, above 150 mm, drastically reduced the density of nymphs, due to a flushing effect that leaves nymphs exposed to the rain impact. However, in our study, the temperature did not significantly affect the number of nymphs. Published sources report divergent effects of climate variables on the dynamics of D. citri nymphs. Chong et al (2010) found no correlation of natural factors (wind, rainfall, relative humidity, and temperature) with psyllid populations. Similarly, Michaud (2004) found no correlation between rainfall and psyllid populations. However, according to Tsai et al (2002), weather factors should in fact directly affect flushing, this will affect the D. citri populations.
Host plant also significantly affected the population of psyllid nymphs. A larger number of insects were associated with citrus plants than with orange jasmine. Although orange jasmine was reported as the most suitable host for psyllid development (Aubert 1987, Nava et al2007; Teck et al2011b), Stockton et al (2017) observed that nymphs were larger in size and developed more rapidly on citrus than on orange jasmine. Other studies found no significant difference in biological traits (oviposition, development time, longevity, viability) between psyllids on citrus and orange jasmine (Alves et al2014, 2018). However, all these studies were conducted under laboratory conditions, whereas we evaluated the number of nymphs in the field.
Surprisingly, the parasitism rate did not significantly affect the nymph population. Milosavljević et al (2018) found that only large nymphs (4th and 5th instars) are susceptible to the parasitoid. Gómez-Torres et al (2012) found the same under laboratory conditions. In our study, we did not separate nymphs by instar and the effects of the parasitoid on the nymph population were probably masked. Another potential reason was the size of the sampled area. Our study was conducted in the field, and therefore, we could not precisely determine the spatial dynamics of the parasitoid after its release, nor the variables that affected its spatial distribution. Additionally, recruitment of adult psyllids from other areas to the resident population may affect the dynamics of nymphs on shoots. For future studies, we recommend sampling nymphs over a larger area in order to cover more possible sites with psyllids to where the parasitoids can disperse.
We also observed that the parasitism rate fluctuated substantially from month to month across the study cities, with 1–3 peaks per year, but parasitism peaks were not concentrated in preferred months as reported in previous studies (Kistner et al2016, Milosavljević et al2018). Our results can be explained by the mild temperatures during most of the year in the tropical and subtropical climates of the study sites. For instance, the coldest municipality sampled in this study, Itapetininga, had winter mean temperatures from 15.03 to 17.01°C. According to Gómez-Torres et al (2012), the optimal temperature for parasitism is 26.3°C, but parasitism rates around 25% can be observed at 15°C. In a study in Taiwan, Chu & Chien (1991) also did not find that parasitism peaks were concentrated in certain months. Therefore, considering the mild year-round temperatures in São Paulo, high parasitism rates can be observed in different seasons, even during the winter (June to September in the southern hemisphere). The only exception was Anhembi, which had parasitism rates around 5% during most of the year, with only one accentuated peak in March and another smaller one in August. According to Santos (2013), low levels of parasitism in this region can be associated with insecticide drift from nearby commercial groves that affects the development of young parasitoids and the oviposition rate in females.
Effect of the number of parasitoid releases in an external area on the number of adult Asian citrus psyllids collected in a neighboring commercial area
The highest number of psyllids was collected in October 2014 and the lowest in April 2015. The primary factor affecting the number of adult psyllids captured was the number of parasitoid releases, which was negatively correlated with the response variable. That is, the number of psyllids showed a tendency to decrease as the number of parasitoid releases increased, indicating that biological control by releasing parasitoids in external areas achieved its main goal. Our study complements that of Hall & Rohrig (2015), who suggested that higher release rates are more effective in managing psyllids. Here, we found that the number of releases affected the success of biological control by T. radiata, but not the release rate as previously reported by Hall & Rohrig (2015).
This is the first report describing the release of the parasitoid T. radiata in areas outside commercial groves and significantly affecting the number of adult psyllids within the groves. Recently, Milosavljević et al (2018) did not find any effect of T. radiata activity on population trends of D. citri adults in non-commercial areas. They associated this result with the continuous recruitment of new psyllids to the resident population and with compensatory survivorship of large nymphs when competition is reduced by parasitism. However, in their study, the authors tested if the natural parasitism by T. radiata in non-commercial urban sites affects the number of adults in those same areas. Our study used a different approach, since we studied adult psyllids in a commercial area, where the level of chemical control was sufficiently high to prevent the presence of eggs and nymphs and consequently of parasitic wasps.
In our study, adult psyllids were not affected by any climate variable (temperature and rainfall). Aubert (1987) suggested that rainfall dislodges adult psyllids from plants and promotes the growth of entomopathogenic fungi, negatively affecting the number of insects. However, this was not confirmed by Milosavljević et al (2018), who found that the numbers of adult psyllids were affected by the year, temperature, and month, but not by rainfall. Tomaseto et al (2018) suggested that the temperature may indirectly affect the number of adult psyllids by interfering with their flight capacity. By contrast, Martini et al (2016) found that adult psyllids were affected by relative humidity, but not by temperature. In general, there is extensive controversy about the abiotic factors that affect the dynamics of D. citri, and more studies are needed to clarify and explore the possible abiotic factors affecting adult populations. One suggestion is a long-term study covering a wide range of climate conditions. Our study lasted only 1 year, which may not have been sufficient to indicate trends in the number of psyllids affected by abiotic factors.
In conclusion, this strategy could be termed “External Management,” since the focus of this approach aims to manage the vector psyllid outside commercial areas, to reduce the influx of psyllids, and could be included as a strategy in the integrated management of D. citri, combined with other measures to increase the parasitoid populations, including introduction, conservation, and multiplication (Parra et al2002).
This study clarified some important points regarding the release of parasitoids into external sources of HLB inocula and helped us to determine possible improvements in our experimental design for application in future studies.
The first part of our study agreed with previous studies that identified external areas as proliferation zones for psyllids. We found that nymph populations were affected only by the host plant and rainfall. For future studies, we recommend that nymphs be separated by size in order to determine the effect of the parasitism rate on psyllid dynamics. We also recommend sampling insects in a larger and more representative area.
In the second part of the study, we found that the number of parasitoid releases in non-commercial areas affected the number of psyllids inside the commercial area. This is the first study of the efficiency of this strategy, although further studies are needed to clarify some unsolved problems. For instance, what is the optimal number of parasitoids to be released and what is the periodicity of release? What are the long-term effects on psyllid populations? What is the spatial distribution of the parasitoid T. radiata after release? All these questions should be addressed in future studies in order to provide more information on this management strategy and improve its efficiency.
The strategy proposed in this study (External Management) can be combined with the current measures for HLB management (planting healthy seedlings, eradicating symptomatic plants, and applying insecticides), mainly in the borders to prevent the vector migration discussed here, and can help to reduce HLB disease in citrus crops. Establishing the parasitoid throughout an area over time can gradually reduce the occurrence of HLB and consequently the need for agrochemicals.
As described for HLB pathosystem, External Management could be applied to other pests, even those that are not disease vectors, enhancing the Integrated Pest Management. The release of natural enemies in sites outside commercial areas, where insect pests are capable of reproducing, can contribute to reducing pest migration especially in the early stages of plant growth.
References
Ahmed S, Ahmad N, Khan RR (2004) Studies on population dynamics and chemical control of citrus psylla, Diaphorina Citri. Int J Agric Biol 6:970–973
Alves GR, Diniz AJF, Parra JRP (2014) Biology of the huanglongbing vector Diaphorina citri (Hemiptera: Liviidae) on different host plants. J Econ Entomol 107:691–696. https://doi.org/10.1603/Ec13339
Alves GR, Beloti VH, Faggioni-Floriano KM, Carvalho SA, Moral RA, Demétrio CGB, Parra JRP, Yamamoto PT (2018) Does the scion or rootstock of Citrus sp. affect the feeding and biology of Diaphorina citri Kuwayama (Hemiptera: Liviidae)? Arthropod-Plant Interactions 12:77–84. https://doi.org/10.1007/s11829-017-9555-z
Aubert B (1987) Trioza erytreae Del Guercio and Diaphorina citri Kuwayama (Homoptera: Psylloidea), the two vectors of citrus greening disease: biological aspects and possible strategies. Fruits 42:149–162
Beloti VH, Alves GR, Araújo DFD et al (2015) Lethal and sublethal effects of insecticides used on citrus, on the ectoparasitoid Tamarixia radiata. PLoS One 10:1–14. https://doi.org/10.1371/journal.pone.0132128
Bergamin-Filho A, Inoue-Nagata AK, Bassanezi RB et al (2016) The importance of primary inoculum and area-wide disease management to crop health and food security. Food Secur 8:221–238. https://doi.org/10.1007/s12571-015-0544-8
Bové JM (2006) Huanglongbing a destructive newly emerging. J Plant Pathol 88:7–37
CDA Coordenadoria de Defesa Agropecuária (2019) Dados da Citricultura Paulista. In: Dados da Citric. Paul. http://www.defesaagropecuaria.sp.gov.br/www/gdsv/index?action=dadosCitriculturaPaulista. Accessed 21 fev 2019
Chong J-H, Roda AL, Mannion CM (2010) Density and natural enemies of the asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae), in the residential landscape of southern florida. J Agr Urban Entomol 27:33–49. https://doi.org/10.3954/11-05.1
Chu YI, Chien CC (1991) Utilization of natural enemies to control of psyllid vectors transmitting citrus greening. In: Proceedings integrated control of plant virus diseases. p 135–145
Costa Lima AM (1942) Psylloidea. In: ___ Insetos do Brasil. Escola Nacional de Agronomia, Rio de Janeiro, p 94–112
Development Core Team R (2018) R: a language and environment for statistical computing
Ferreira R V (2014) Influência do tipo de controle de Huanglongbing em áreas citrícolas na dispersão de Diaphorina citri e na disseminação da doença para pomares próximos. Dissertation. Fundecitrus: Araraquara SP. p 71
Flores D, Ciomperlik M (2017) Biological control using the ectoparasitoid, Tamarixia radiata, against the Asian Citrus Psyllid, Diaphorina citri, in the Lower Rio Grande Valley of Texas. Southwest Entomol 42:49–59. https://doi.org/10.3958/059.042.0105
Gómez-Torres ML, Nava DE, Gravena S et al (2006) Registro de Tamarixia radiata (Waterston) (Hymenoptera: Eulophidae) em Diaphorina citri Kuwayama (Hemiptera: Psyllidae) em São Paulo, Brasil. Rev Agric 81:112–117
Gómez-Torres ML, Nava DE, Parra JRP (2012) Life table of Tamarixia radiata (Hymenoptera: Eulophidae) on Diaphorina citri (Hemiptera: Psyllidae) at different temperatures. J Econ Entomol 105:338–343. https://doi.org/10.1603/EC11280
Hall DG, Rohrig E (2015) Bionomics of Asian citrus psyllid (Hemiptera: Liviidae) associated with orange jasmine hedges in Southeast Central Florida, with special reference to biological control by Tamarixia radiata. J Econ Entomol 108:1198–1207. https://doi.org/10.1093/jee/tov052
Hall DG, Hentz MG, Adair RC (2008) Population ecology and phenology of Diaphorina citri (Hemiptera: Psyllidae) in two Florida citrus groves. Environ Entomol 37:914–924. https://doi.org/10.1603/0046-225X(2008)37[914:PEAPOD]2.0.CO;2
Hall DG, Richardson ML, Ammar ED, Halbert SE (2013) Asian citrus psyllid, Diaphorina citri, vector of citrus huanglongbing disease. Entomol Exp Appl 146:207–223. https://doi.org/10.1111/eea.12025
Kistner EJ, Amrich R, Castillo M, Strode V, Hoddle MS (2016) Phenology of Asian citrus psyllid (Hemiptera: Liviidae), with special reference to biological control by Tamarixia radiata, in the residential landscape of southern California. J Econ Entomol 109:1047–1057. https://doi.org/10.1093/jee/tow021
Lewis-Rosenblum H, Martini X, Tiwari S, Stelinski LL (2015) Seasonal movement patterns and long-range dispersal of Asian citrus psyllid in Florida citrus. J Econ Entomol 108:3–10. https://doi.org/10.1093/jee/tou008
Martini X, Pelz-Stelinski KS, Stelinski LL (2016) Factors affecting the overwintering abundance of the Asian citrus psyllid (Hemiptera: Liviidae) in Florida Citrus (Sapindales: Rutaceae) orchards. Fla Entomol 99:178–186. https://doi.org/10.1653/024.099.0204
Michaud JP (2004) Natural mortality of Asian citrus psyllid (Homoptera: Psyllidae) in central Florida. Biol Control 29:260–269. https://doi.org/10.1016/S1049-9644(03)00161-0
Milosavljević I, Amrich R, Strode V, Hoddle MS (2018) Modeling the phenology of Asian citrus psyllid (Hemiptera: Liviidae) in urban Southern California: effects of environment, habitat, and natural enemies. Environ Entomol 47:233–243. https://doi.org/10.1093/ee/nvx206
Nava DE, Torres MLG, Rodrigues MDL et al (2007) Biology of Diaphorina citri (Hem., Psyllidae) on different hosts and at different temperatures. J Appl Entomol 131:709–715. https://doi.org/10.1111/j.1439-0418.2007.01230.x
Neves MF, Trombin VG, Milan P et al (2011) O retrato da citricultura brasileira. Markestrat, Ribeirão Preto
O’Hara RB, Kotze DJ (2010) Do not log-transform count data. Methods Ecol Evol 1:118–122. https://doi.org/10.1111/j.2041-210X.2010.00021.x
Parra JRP, Botelho PSM, Correa-Ferreira BS, Bento JMS (2002) Controle biológico: Uma visão inter e multidisciplinar. In: JRP P, Botelho PSM, Correa-Ferreira BS, Bento JMS (eds) Controle Biológico no Brasil Parasitoides e Predadores. Manole, São Paulo, pp 125–137
Parra JRP, Alves GR, Diniz AJF, Vieira JM (2016) Tamarixia radiata (Hymenoptera: Eulophidae) × Diaphorina citri (Hemiptera: Liviidae): mass rearing and potential use of the parasitoid in Brazil. J Integr Pest Manag 7:1–11. https://doi.org/10.1093/jipm/pmw003
Santos TRG (2013) Volumes de calda e faixas de aplicação em pulverização aérea para o controle de Diaphorina citri Kuwayama (Hemiptera: Liviidae) em citros. Fundecitrus
Sétamou M, Bartels DW (2015) Living on the edges: spatial niche occupation of asian citrus psyllid, Diaphorina citri kuwayama (Hemiptera: Liviidae), in citrus groves. PLoS One 10. https://doi.org/10.1371/journal.pone.0131917
Skelley LH, Hoy MA (2004) A synchronous rearing method for the Asian citrus psyllid and its parasitoids in quarantine. Biol Control 29:14–23. https://doi.org/10.1016/S1049-9644(03)00129-4
Stockton DG, Pescitelli LE, Ebert TA, Martini X, Stelinski LL (2017) Induced preference improves offspring fitness in a phytopathogen vector. Environ Entomol 46:1090–1097. https://doi.org/10.1093/ee/nvx135
Teck SLC, Fatimah A, Beattie A et al (2011a) Seasonal population dynamics of the Asian citrus psyllid, Diaphorina citri Kuwayama in Sarawak. Am J Agric Biol Sci 6:527–535
Teck SLC, Fatimah A, Beattie A et al (2011b) Influence of host plant species and flush growth stage on the Asian citrus psyllid, Diaphorina citri Kuwayama. Am J Agric Biol Sci 6:536–543
Teixeira DC, Ayres AJ, Kitajima EW et al (2005) First report of a huanglongbing-like disease of citrus in São Paulo state, Brazil and association of a new liberibacter species, candidatus liberibacter americanus, with the disease. Plant Dis 89:107
Tiwari S, Lewis-rosenblum H, Pelz-stelinski K, Stelinski LL (2010) Incidence of candidatus liberibacter asiaticus infection in abandoned citrus occurring in proximity to commercially managed groves incidence of candidatus liberibacter asiaticus infection in abandoned citrus occurring in proximity to commercially managed. J Econ Entomol 103:1972–1978. https://doi.org/10.1603/EC10149
Tomaseto AF, Miranda MP, Moral RA et al (2018) Environmental conditions for Diaphorina citri Kuwayama (Hemiptera: Liviidae) take-off. J Appl Entomol 142:104–113. https://doi.org/10.1111/jen.12418
Tsai JH, Wang JJ, Liu YH (2002) Seasonal abundance of the Asian citrus psyllid, Diaphorina citri (Homoptera: Psyllidae) in Southern Florida. Fla Entomol 85:446–451
Acknowledgments
We extend our thanks to Janet W. Reid (JWR Associates) for the English revision, and to all staff members of Fundecitrus and Citrosuco who helped in the execution of the study. AGG holds a fellowship awarded by FAPESP (2017/26657-7) and GRA holds a fellowship awarded by FAPESP (2013/04291-0).
Funding
Financial support for this study was provided by the Fundo de Defesa da Citricultura – Fundecitrus (Project Tamarixia 837), Citrosuco (Project Tamaradiwa 1044), and FAPESP (2017/26657-7, 2013/04291-0).
Author information
Authors and Affiliations
Contributions
AJFD and JRPP conceived the project, conducted the field experiments, and wrote the manuscript. AGG and CR performed the statistical analysis. AGG, CR, JMV, and GRA wrote the manuscript and participated in discussing the results, critiquing the scientific aspects, and proofreading the manuscript.
Corresponding author
Additional information
Edited by Lessando Moreira Gontijo – UFV
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Diniz, A.J.F., Garcia, A.G., Alves, G.R. et al. The Enemy is Outside: Releasing the Parasitoid Tamarixia radiata (Hymenoptera: Eulophidae) in External Sources of HLB Inocula to Control the Asian Citrus Psyllid Diaphorina citri (Hemiptera: Liviidae). Neotrop Entomol 49, 250–257 (2020). https://doi.org/10.1007/s13744-019-00736-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-019-00736-y