Abstract
The bronze bug, Thaumastocoris peregrinus Carpintero & Dellape (Heteroptera: Thaumastocoridae), is an exotic emerging pest in Eucalyptus commercial forests in South America, Africa and southern Europe. Information on the chemical communication system and reproductive ecology of this insect is scant, and it may be relevant for designing management strategies for eucalypt plantations. Adults and nymphs usually aggregate in the field, possibly by means of chemical signals. Males emit large amounts of 3-methyl-2-butenyl butyrate, which attracts conspecific adult males but not females. The ecological role of this putative male aggregation pheromone remains unknown. Here, we report olfactometer bioassays showing that late-instar male nymphs are also attracted to synthetic 3-methyl-2-butenyl butyrate and to adult male volatile extracts, which contain this compound as the major component. As previously shown for adult females, nymphs that moulted into females were not attracted to either volatile stimulus. The intra-gender attraction of nymphs and adults may be related to the exploitation of food resources, or as a reproductive strategy for newly emerged males. Further studies on the reproductive behaviour and mating system of T. peregrinus will contribute to understanding the ecological significance of male-male, adult-nymph attraction, as well as the practical applications that may result from these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insects are the most diverse group of eukaryotic organisms, with nearly one million species described, of which approximately half are plant feeders (Grimald & Engel 2005, Speight et al 2008). Many herbivorous insects are regarded as pests, since they feed on plants that humans grow as a source of food or raw materials, causing significant losses to production systems. Insect pests benefit from monoculture agricultural practices, and more so when their food plants are readily available in a new habitat as an exotic crop. When the insects become established in this new environment, usually as a result of human accidental introduction, none or few natural enemies will be available to regulate their populations (Strong et al 1984, Jolivet 1992, Schoonhoven et al 2005). Forest plantations are a classic example of long-term monocultures over extensive areas, and among these, the genus Eucalyptus represents about 20% of the world’s planted commercial forests, with several cases of introduction and establishment of insect pests from its native range (Ciesla 2011, Paine et al 2011, Branco et al 2015).
A recent example of a eucalypt-specialist formerly restricted to Australia and now widely distributed is the bronze bug, Thaumastocoris peregrinus Carpintero & Dellapé (Heteroptera: Thaumastocoridae). This species belongs to a small Cimicomorpha family that comprises six genera and about 20 species, including several sap-feeders of mono- and dicotyledonous plants (Slater 1973, Carpintero & Dellapé 2006). Over the last decade, T. peregrinus has been reported in several eucalypt-producing countries, namely South Africa, Zimbabwe, Malawi, Kenya, Argentina, Brazil, Uruguay, Chile, Paraguay, Italy, Spain and Portugal (Nadel & Noack 2012, Garcia et al 2013, Suma et al 2014), and has become a major emerging pest of eucalypt production in the Southern hemisphere (Jacobs & Neser 2005, Carpintero & Dellapé 2006, Noack et al 2011). It employs a lacerate-and-flush feeding strategy, causing the loss of photosynthetic surface area, defoliation, and even tree death (Jacobs & Neser 2005).
While the actual economic impact of T. peregrinus is yet unclear, elucidating its chemical communication system will certainly be important to monitor and/or prevent population outbreaks. However, most aspects concerning the basic biology of the insect remain unknown, in particular its mating behaviour and other intraspecific interactions. In recent years, the chemistry and ecological role of putative pheromones in T. peregrinus have been investigated. Volatile extracts and whole-body extracts from virgin adults showed remarkable differences between males and females. Male extracts contained large amounts of the hemiterpene ester, 3-methyl-2-butenyl butyrate (González et al 2012, Martins et al 2012), apparently produced in an eversible glandular-like rectal organ similar to those described in the family Miridae (Wheeler Jr. 1980). Smaller amounts of the same compound were also found in whole-body extracts of females (Martins et al 2012), but not in their volatile emissions (González et al 2012). Behavioural bioassays showed that adult males were attracted to both, conspecific male volatile extracts and synthetic 3-methyl-2-butenyl butyrate, while females showed no response (González et al 2012). Hence, this compound does not seem to be involved in sexual attraction or recognition, but rather to mediate interactions among males, possibly acting as a male aggregation pheromone. Since T. peregrinus usually occurs as aggregations of adults and nymphs, it is relevant to test if these chemically mediated interactions also involve juveniles. In this study, we evaluated if late-instar nymphs of T. peregrinus are attracted to adult male volatiles. Specifically, we performed Y-tube olfactometer bioassays to evaluate the response of male and female nymphs toward male volatile extracts and synthetic 3-methyl-2-butenyl butyrate.
Material and Methods
Insects
Virgin adults and nymphs were obtained from a laboratory colony reared on Eucalyptus tereticornis (Martínez et al 2014). Adult males and females were kept together for mating and oviposition in mesh-screen cages (35 × 50 × 70 cm), and fed with fresh E. tereticornis branches kept in distilled water in 0.5-L Erlenmeyer flasks. The cages were kept under controlled laboratory conditions (20 ± 5°C, 55 ± 10% RH) and natural photoperiod. From these cages, egg clusters were harvested and incubated (25.0 ± 0.4°C, 55.0 ± 4.0% RH, 12:12 L:D) in Petri dishes containing E. tereticornis leaf discs floating on distilled water. Upon hatching, nymphs were transferred to a mesh-screen cage (35 × 50 × 70 cm) and kept under incubator conditions as described above, with access to E. tereticornis branches. Twice a week, IV–V instar nymphs were separated for behavioural studies. Adult virgin males for volatile extracts were obtained similarly, allowing the nymphs to moult into adults and separating males and females before cuticle sclerotization. The adults were distinguished by an abdominal asymmetry typical of the males (Carpintero & Dellapé 2006).
Volatile collection and analysis
Volatile organic compounds were obtained from virgin males enclosed in glass chambers (24 cm length, 4.6 cm diam.) with four E. tereticornis leaves. Volatiles from 50 males were adsorbed on 50 mg of Haysep-Q 80/100 mesh, with a current of charcoal-filtered humidified air (300 mL/min) during 72 h (24°C, 14:10 L:D photoperiod). Volatiles were eluted with 1 mL distilled hexane, and a fraction of this extract (200 μL) was concentrated to 100 μL under a stream of N2 for GC-MS analysis. The remaining extract was used for bioassays without concentration. GC-MS analyses were performed to determine the presence of 3-methyl-2-butenyl butyrate in the volatile extracts. These were done using a QP-2010 Shimadzu GC-MS equipped with an AT-5 MS column (Alltech, USA) (30 m × 0.25 mm i.d., 0.25 μm film thickness), operated with a constant carrier flow of 1 mL/min (He). The temperature of the GC oven was programmed from an initial temperature of 40°C (1 min), then heated to 300°C at 10°C/min, and held for 3 min. The injector temperature was 220°C and the interphase temperature was 250°C. Injection (1 μL) was in the splitless mode, and mass spectra were acquired from m/z 30 to 350 in the scan mode (70 eV).
Behavioural bioassays
We used a Y-tube olfactometer to test the behavioural response of IV–V instar T. peregrinus nymphs towards olfactory stimuli (Haynes & Millar, 2012). The stimuli were either the male volatile extracts or synthetic 3-methyl-2-butenyl butyrate. 3-Methyl-2-butenyl butyrate was synthesised from 3-methyl-2-buten-1-ol and vinyl butyrate, using a biocatalyzed transesterification as previously described (González et al 2012). Five microlitres of the extract in hexane (0.25 maleeq or 0.0035 maleeq/h) were applied onto a piece of filter paper (1 × 1 cm). The synthetic compound was also applied onto filter paper (1 × 1 cm) at a dose of 1 μg (10 μL of a 100 ppm hexane solution). In both cases, the control arms of the olfactometer contained a piece of filter paper of equal size, treated with the corresponding amount of hexane. In addition, one freshly cut leaf of E. tereticornis was added to both sides of the olfactometer.
The experiments were performed during daylight, using a glass olfactometer with round-section arms (20 cm length, 4 cm diam.). The stimuli and control were placed in separate glass tubes (10 cm length, 4 cm diam.) connected to the olfactometer arms by Teflon tubing, and they were changed with freshly treated filter paper in every replicate. Charcoal-filtered humidified air was pushed and pulled through the olfactometer at a total flow of 1.2 L/min. The tested insects were placed individually at the entrance of the central tube, and their behaviour was observed for 10 min under fluorescent light. The relative position of the tested stimulus and the control were alternated between replicates to prevent any positional bias in the behaviour of the insects. We visually registered the first arm choice as a way to determine orientation/attraction toward odour cues, and calculated the total residence time in the olfactometer arm, as an indication of arrestment or searching behaviour. Finally, we registered the number of entries to each arm during the experimental period, as further indication of preference by the insects. After the experiment, the nymphs were reared individually in 2-mL capped centrifuge tubes, with access to water and pieces of E. tereticornis leaves, and sexed after moulting into adults. We initially tested 130 individuals for each volatile stimulus, of which 96 and 110 responded (entered in at least one arm) in the experiments with male volatile extracts or synthetic compound, respectively. Out of these, 46 and 59 nymphs moulted into adults, respectively, and were hence sexed and used for the statistical analyses.
To analyse the orientation/attraction toward odour cues (first arm choice) and preference between olfactometer arms (number of entries to each arm), we used a generalised linear model (GLM) with a logit link function and a binomial distribution for errors. Due to the dependence between the time spent in each olfactometer arm, the comparison of residence times in the olfactometer arms was analysed by a Wilcoxon matched pairs signed rank test. All analyses were performed with the statistical program R, version 3.1.3 with the package “stat” (R Development Core 2015).
Results
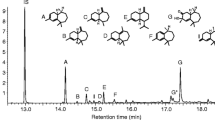
GC-MS analysis of the male volatile extracts showed that 3-methyl-2-butenyl butyrate was present as a major component eluting at 10.1 min (Fig 1), along with leaf volatiles that were also found in the volatile extracts of E. tereticornis leaves that served as control (not shown). GC-MS analysis of the synthetic compound (> 99% pure by GC) showed an identical retention time and mass spectrum than those of the natural compound emitted by males (Fig 1; González et al (2012) and Martins et al (2012) for mass spectra interpretation).
Chromatographic (TIC) traces of Thaumastocoris peregrinus male volatile extracts (with Eucalyptus tereticornis leaves, upper trace) and synthetic 3-methyl-2-butenyl butyrate (lower trace, inverted). The EI mass spectra of natural and synthetic compounds are shown for comparison. Other compounds in the volatile extracts, except for the minor male-emitted compound 3-methyl-3-butenyl butyrate (indicated), correspond to leaf volatiles and air contaminants.
When the behavioural results were evaluated with no separation of males and females, T. peregrinus nymphs showed no preference for male volatile extracts (ext) or synthetic 3-methyl-2-butenyl butyrate (synt). There was no effect of either stimuli on first arm choice (GLMext: z = 0.59, P = 0.56; GLMsynt: z = 1.68, P = 0.09), nor the number of entries to each arm (GLMext: z = 0.0, P = 1.0; GLMsynt: z = 1.28, N = 59, P = 0.20), or the time spent in each arm (Wilcoxonext: Z = 0.31, N = 46, P = 0.76; Wilcoxonsynt: Z = 1.58, N = 59, P = 0.12).
Interestingly, when we incorporate nymph sex into the analysis, the results were different. We found that orientation/attraction toward stimuli and preference between stimulus—control odour cues was dependent on nymph sex (GLM first choice: χ2 = 6.35, df = 1, P = 0.01; GLM number of entries: χ2 = 5.21, df = 1, P = 0.02), but not on the stimuli tested (male volatile extracts vs. synthetic compound; GLM first choice: χ2 = 0.47, df = 1, P = 0.49; GLM number of entries: χ2 = 0.66, df = 1, P = 0.42). Nymphs that moulted into males showed a clear attraction (GLM: z = 2.29, P = 0.03; Fig 2) and preference (GLM: z = 2.22, P = 0.03; Fig 3) toward the olfactometer arm bearing the stimulus, regardless of whether the stimulus was male volatile extracts or the synthetic compound (GLM first choice: χ2 = 0.16, df = 1, P = 0.69; GLM number of entries: χ2 = 0.01, df = 1, P = 0.93). Furthermore, male nymphs spent more time in the olfactometer arm with the stimulus than in the control arm, for both experiments (Wilcoxonext: Z = 1.96, N = 17, P = 0.04; Wilcoxonsynt: Z = 2.11, N = 24, P = 0.03; Fig 4).
First arm choice of Thaumastocoris peregrinus male and female nymphs in Y-tube olfactometer tests with male volatile extracts (black bars) and synthetic 3-methyl-2-butenyl butyrate (grey bars). Bars show the proportion of individuals that chose the stimulus or the control arm (empty bars), and moulted into adults afterwards. Of the 130 nymphs initially tested, N indicates the number of insects that responded, and n indicates the number that moulted into males or females. Asterisks indicate statistically significant differences (GLM, P < 0.05).
Residence time of Thaumastocoris peregrinus male and female nymphs in the stimulus and control arms of the Y-tube olfactometer. Each bar shows the mean residence time in the stimulus arm [male volatile extracts (black bars), synthetic 3-methyl-2-butenyl butyrate (grey bars)] or the control arm (empty bars). N indicates the number of insects that responded, and n indicates the number that moulted into males or females. Error bars indicate standard error. Asterisks indicate statistically significant differences (Wilcoxon matched pairs signed rank test., P < 0.05).
In contrast, nymphs that moulted into females showed no attraction/orientation (GLM: z = −0.92, P = 0.36; Fig 2) or preference (GLM: z = −1.51, P = 0.13; Fig 3) for any olfactometer arm, either when we tested male volatile extract or synthetic 3-methyl-2-butenyl butyrate (GLM first choice: χ2 = 1.06, df = 1, P = 0.30; GLM number of entries: χ2 = 2.11, df = 1, P = 0.14). Likewise, female nymphs did not spend time differently between the stimulus or control olfactometer arms (Wilcoxonext: Z = − 1.16, N = 29, P = 0.28; Wilcoxonsynt: Z = 0.43, N = 35, P = 0.63; Fig 4).
Number of entries of Thaumastocoris peregrinus male and female nymphs into the arms of the Y-tube olfactometer. Bars show the average number of entries into the stimulus arm bearing male volatile extracts (black bars) or synthetic 3-methyl-2-butenyl butyrate (grey bars), or the control arm (open bars). N indicates the number of insects that responded, and n indicates the number that moulted into males or females. Error bars indicate standard error. Asterisks indicate statistically significant differences (GLM, P < 0.05).
Discussion
Our olfactometer experiments show that male nymphs of the bronze bug, T. peregrinus, are attracted to conspecific adult male volatiles and to synthetic 3-methyl-2-butenyl butyrate, the major volatile emitted by the males (González et al 2012, Martins et al 2012). Male nymphs preferred both volatile stimuli more often than the control, and remained longer in the corresponding arm of the olfactometer, whereas female nymphs showed no preference. Therefore, as previously reported for the adults (González et al 2012), we here show that the attraction of nymphs is gender-specific.
No specific experiments were performed to compare the attraction of male volatile extracts and of the synthetic 3-methyl-2-butenyl butyrate, and no significant differences were found between both stimuli in our model. Nonetheless, the effect of minor pheromonal compounds emitted by the males cannot be discarded, as it is often the case when it comes to pheromone attraction (Greenfield 2002). Indeed, Martins et al (2012) reported that a minor isomer, 3-methyl-3-butenyl butyrate, was also present in adult male extracts in greater amounts than in female extracts. Mixtures of both isomers have not yet been tested for biological activity, and it may be expected that they will show greater attraction than the major volatile alone. In addition, while leaf volatiles were present in all tests and controls, a synergistic effect of plant volatiles and male pheromone cannot be discarded, and deserves further studies.
Male nymphs seem to be specifically orienting towards conspecific male adults by cueing on chemicals that they themselves do not produce. While no volatile collections have been performed on nymphs, it has been shown that extracts of nymph exuviae do not contain 3-methyl-2-butenyl butyrate or related hemiterpene esters (Martins et al 2012), suggesting that nymphs do not produce or emit these chemicals. In addition, this hemiterpene has not been reported from Eucalyptus tree volatiles, and therefore, it may not serve as a host-finding kairomonal cue for the nymphs.
This is the first record of semiochemicals involved in aggregation behaviour between adults and immature stages in Thaumastocoridae. Sensu stricto, an aggregation pheromone attracts both males and females; however, in the case of T. peregrinus, we have observed exclusively same-sex attraction. This unusual phenomenon has been reported for adults of the golden-eyed lacewing, Chrysopa oculata (Chrysopidae), in which only males were attracted to traps baited with male-specific compounds (Zhang et al 2004). It is not uncommon, however, that nymphs are attracted by conspecific adult pheromones in Heteroptera. In several species, male-emitted aggregation pheromones attract nymphs, in addition to luring adults of both sexes. This has been shown for unrelated families such as Pentatomidae (Harris & Todd 1980, Kochansky et al 1989, Weber et al 2014), Alydidae (Leal et al 1995, Higuchi & Hiroaki 1999, Morishima et al 2005, Nakajima et al 2010), Rhopalidae (Schwarz & Gries 2010) and Coreidae (Khrimian et al 2012).
The ecological significance of nymph-adult attraction in true bugs is not clear. One hypothesis aims at explaining the attraction of young nymphs (II instar), and suggests a role in the location of food sources exploited by the adults (Nakajima et al 2010). A second hypothesis relates to the attraction of older nymphs (V instar), and proposes a potential function in finding prospective mates for the newly emerged adults, decreasing mate searching time upon moulting into the adult stage (Harris & Todd 1980, Schwarz & Gries 2010). The former hypothesis would imply that both, male and female nymphs, should be equally attracted to the male pheromone, as it has been shown for the alylid Riptortus linearis (Higuchi & Hiroaki 1999). In the case of a mating advantage for newly emerged adults, it may be possible that only male nymphs are attracted, as it is the case in T. peregrinus.
Additional research in mating behaviour and population structure in T. peregrinus is needed to further understand this intra-gender adult-nymph attraction. Adult and immature females are unaffected by the volatiles, suggesting a social rather that a sexual function for this pheromone. Nonetheless, it is still unknown how T. peregrinus males and females find each other for mating, and this may shed light into the significance of this male-male interaction. Circumstantial observations in our laboratory suggest that males and females simply bump into each other, which is likely to occur given their aggregative behaviour. Upon contacting a female, the males often perform abdominal vibrations, suggesting that close range substrate-driven signals between males and females may be in place. Furthermore, males appear to be ready to mate as soon as they moult into adults (Gonzalo Martínez, pers. obs.), and in this scenario, late-instar male nymphs may benefit from staying close to adult males, where adult females are also likely to occur. Studies in the mating systems of related Cimicomorpha families may provide with hypothesis for explaining male-male interactions in T. peregrinus, for instance, through the establishment of male hierarchies as reported in Coreidae (Eberhard 1998), or by males interfering with female-male chemical communication, as shown for the closely related Miridae (Zhang & Aldrich, 2003).
The potential practical use of attracting T. peregrinus males also deserves further investigation. The gender-specific attraction of adults or late-instar nymphs may provide a suitable tool for monitoring or surveillance/detection programs (Nadel et al 2014). Moreover, the selective attraction of males may open the possibility for controlling T. peregrinus populations by autodissemination of entomopathogenic fungi (Baverstock et al 2010), given that naturally occurring pathogens have been reported (Mascarin et al 2012). However, as it is often the case for heteropteran pheromones (Millar 2005), the practical application of true bug semiochemicals requires extensive basic research in the actual role of the signal in the biology of the insect, as well as applied research for the development of suitable traps and disseminating devices, often requiring a case-by-case approach. T. peregrinus is a highly mobile bug, but it tends to walk rather than to fly, representing a challenge for the application of volatile pheromones in IPM. Given the fairly recent spread of T. peregrinus throughout the Southern hemisphere, most of this research is still in its earlier steps.
References
Baverstock J, Roy H, Pell J (2010) Entomopathogenic fungi and insect behaviour: from unsuspecting hosts to targeted vectors. In: Roy H, Vega F, Chandler D, Goettel M, Pell J, Wajnberg E (eds) The ecology of fungal entomopathogens. Springer, Netherlands, pp 89–102
Branco S, Videira N, Branco M, Paiva MR (2015) A review of invasive alien species impacts on eucalypt stands and citrus orchards ecosystem services: towards an integrated management approach. J Environ Manag 149:17–26. https://doi.org/10.1016/j.jenvman.2014.09.026
Carpintero DL, Dellapé PM (2006) A new species of Thaumastocoris Kirkaldy from Argentina (Heteroptera: Thaumastocoridae: Thaumastocorinae). Zootaxa 1228:61–68
Ciesla WM (2011) Forest entomology: a global perspective. Blackwell, Oxford, p 400. https://doi.org/10.1002/9781444397895
Eberhard WG (1998) Sexual behavior of Acanthocephala declivis guatemalana (Hemiptera: Coreidae) and the allometric scaling of their modified hind legs. Ann Entomol Soc Am 91(6):863–871. https://doi.org/10.1093/aesa/91.6.863
Garcia A, Figueiredo E, Valente C, Monserrat VJ, Branco M (2013) First record of Thaumastocoris peregrinus in Portugal and of the neotropical predator Hemerobius bolivari in Europe. Bull Insectol 66:251–256
González A, Calvo MV, Cal V, Hernández V, Doño F, Alves L, Gamenara D, Rossini C, Martínez G (2012) A male aggregation pheromone in the bronze bug, Thaumastocoris peregrinus (Thaumastocoridae). Psyche 2012:7
Greenfield MD (2002) Signalers and receivers: mechanisms and evolution of arthropod communication. Oxford University Press, Oxford, p 414
Harris VE, Todd JW (1980) Male-mediated aggregation of male, female and 5th instar southern green stink bugs and concomitant attraction of a tachinid parasite, Trichopoda pennipes. Entomol Exp Appl 27(2):117–126. https://doi.org/10.1111/j.1570-7458.1980.tb02955.x
Haynes KF, Millar JG (2012) Methods in chemical ecology. Volume 2: bioassay methods. Springer, United States
Higuchi H, Hiroaki N (1999) Attraction of conspecific adults and nymphs by adults of Riptortus linearis (Fabricius) (Heteroptera: Alydidae). Appl Entomol Zool 34(4):455–458. https://doi.org/10.1303/aez.34.455
Jacobs DH, Neser S (2005) Thaumastocoris australicus Kirkaldy (Heteroptera: Thaumastocoridae): a new insect arrival in South Africa, damaging to Eucalyptus trees: research in action. S Afr J Sci 101:233–236
Jolivet P (1992) Insects and plants: parallel evolution and adaptations. Sandhill Crane Press, Gainesville, FL, p 190
Khrimian A, Fay HAC, Guzman F, Chauhan K, Moore C, Aldrich JR (2012) Pheromone of the banana-spotting bug, Amblypelta lutescens lutescens Distant (Heteroptera: Coreidae): identification, synthesis, and field bioassay. Psyche 2012:8
Kochansky J, Aldrich JR, Lusby WR (1989) Synthesis and pheromonal activity of 6,10,13-trimethyl-1-tetradecanol for predatory stink bug, Stiretrus anchorago (Heteroptera: Pentatomidae). J Chem Ecol 15(6):1717–1728. https://doi.org/10.1007/BF01012260
Leal W, Higuchi H, Mizutani N, Nakamori H, Kadosawa T, Ono M (1995) Multifunctional communication in Riptortus clavatus (Heteroptera: Alydidae): conspecific nymphs and egg parasitoid Ooencyrtus nezarae use the same adult attractant pheromone as chemical cue. J Chem Ecol 21(7):973–985. https://doi.org/10.1007/BF02033802
Martínez G, López L, Cantero G, González A, Dicke M (2014) Life-history analysis of Thaumastocoris peregrinus in a newly designed mass rearing strategy. Bull Insectol 67:199–205
Martins CB, Soldi RA, Barbosa LR, Aldrich JR, Zarbin PH (2012) Volatile chemicals of adults and nymphs of the Eucalyptus pest, Thaumastocoris peregrinus (Heteroptera: Thaumastocoridae). Psyche 2012:6
Mascarin GM, Duarte VS, Brandao MM, Delalibera Jr. I (2012) Natural occurrence of Zoophthora radicans (Entomophthorales: Entomophthoraceae) on Thaumastocoris peregrinus (Heteroptera: Thaumastocoridae), an invasive pest recently found in Brazil. J Invertebr Pathol 110:401–404, 3, DOI: https://doi.org/10.1016/j.jip.2012.03.025
Millar JG (2005) Pheromones of true bugs. In: Schulz S (ed) The chemistry of pheromones and other semiochemicals II. Springer-Verlag, Berlin Heidelberg, pp 37–84
Morishima M, Tabuchi K, Ito K, Mizutani N, Moriya S (2005) Effect of feeding on the attractiveness of Riptortus clavatus (Thunberg) (Heteroptera: Alydidae) males to conspecific individuals. Jpn J Appl Entomol Zool 49(4):262–265. https://doi.org/10.1303/jjaez.2005.262
Nadel R, Wingfield M, Scholes M, Garnas J, Lawson S, Slippers B (2014) Population dynamics of Thaumastocoris peregrinus in Eucalyptus plantations of South Africa. J Pest Sci:1–10
Nadel RL, Noack AE (2012) Current understanding of the biology of Thaumastocoris peregrinus in the quest for a management strategy. Int J Pest Manage 58(3):257–266. https://doi.org/10.1080/09670874.2012.659228
Nakajima Y, Sakuma M, Sasaki R, Fujisaki K (2010) Adaptive traits of Riptortus pedestris nymphs (Heteroptera: Alydidae) for locating host plants. Ann Entomol Soc Am 103(3):439–448. https://doi.org/10.1603/AN09144
Noack AE, Cassis G, Rose HA (2011) Systematic revision of Thaumastocoris Kirkaldy (Hemiptera: Heteroptera: Thaumastocoridae). Zootaxa 3121:1–60
Paine TD, Steinbauer MJ, Lawson SA (2011) Native and exotic pests of Eucalyptus: a worldwide perspective. Annu Rev Entomol 56(1):181–201. https://doi.org/10.1146/annurev-ento-120709-144817
R Development Core (2015). R: A language and environment for statistical computing. Foundation for Statistical Computing, Vienna, https://www.R-project.org
Schoonhoven LM, Van Loon JJ, Dicke M (2005) Insect-plant biology. Oxford University Press, Oxford, p 421
Schwarz JJ, Gries G (2010) 2-Phenylethanol: context-specific aggregation or sex-attractant pheromone of Boisea rubrolineata (Heteroptera: Rhopalidae). Can Entomol 142(05):489–500. https://doi.org/10.4039/n10-027
Slater JA (1973) A contribution to the biology and taxonomy of Australian Thaumastocoridae with the description of a new species (Hemiptera: Heteroptera). Aust J Entomol 12(2):151–156. https://doi.org/10.1111/j.1440-6055.1973.tb01653.x
Speight M, Hunter M, Watt A (2008) Ecology of insects: concepts and applications. Oxford University Press, Oxford, p 628
Strong DR, Lawton JH, Southwood TRE (1984) Insects on plants—community patterns and mechanisms. Blackwell Scientific Publications, Oxford, p 313
Suma P, Nucifora S, Bella S (2014) New distribution record of the invasive bronze bug Thaumastocoris peregrinus Carpintero and Dellapé (Heteroptera, Thaumastocoridae) in Italy. EPPO Bull 44(2):179–182. https://doi.org/10.1111/epp.12122
Weber DC, Leskey TC, Cabrera Walsh G, Khrimian A (2014) Synergy of aggregation pheromone with methyl-(E,E,Z)-2,4,6-decatrienoate in attraction of Halyomorpha halys (Hemiptera: Pentatomidae). J Econ Entomol 107(3):1061–1068. https://doi.org/10.1603/EC13502
Wheeler AG Jr (1980) The mirid rectal organ: purging the literature. Fla Entomol 63(4):481–485. https://doi.org/10.2307/3494532
Zar J (2010) Biostatistical analysis. Pearson Prentice Hall International, New Jersey, p 663
Zhang QH, Aldrich JR (2003) Male-produced anti-sex pheromone in a plant bug. Naturwissenschaften 90(11):505–508. https://doi.org/10.1007/s00114-003-0466-8
Zhang QH, Chauhan K, Erbe E, Vellore A, Aldrich J (2004) Semiochemistry of the goldeneyed lacewing Chrysopa oculata: attraction of males to a male-produced pheromone. J Chem Ecol 30(9):1849–1870. https://doi.org/10.1023/B:JOEC.0000042406.76705.ab
Acknowledgments
We thank Gissel Cantero for technical assistance with insect maintenance.
Funding
This work was supported by the National Agency for Science and Innovation of Uruguay (ANII), projects FSA-2009-3133 and FSA-2013-13033. We also thank ANII for a post-doctoral fellowship to HFG.
Author information
Authors and Affiliations
Additional information
Edited by Raul Laumann – Cenargen/Embrapa
Rights and permissions
About this article
Cite this article
Calvo, M.V., Groba, H.F., Martínez, G. et al. Attraction of Male Nymphs to Adult Male Volatiles in the Bronze Bug Thaumastocoris peregrinus Carpintero & Dellape (Heteroptera: Thaumastocoridae). Neotrop Entomol 47, 835–841 (2018). https://doi.org/10.1007/s13744-017-0576-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-017-0576-1