Abstract
Different aspects of human activities can cause environmental change that endanger species persistence, alter species distributions, and lead to changes in antagonistic and mutualistic interactions, whereas deforestation and flooding of riparian forest results in landscapes consisting of patchily distributed riparian forest fragments in a matrix of pastures, plantations, and urban areas. Therefore, we assessed the richness, abundance, and trophic interactions of trap-nesting Hymenoptera and their parasites at four patches of restored riparian forest and at one reference natural fragment, of different sizes and ages, located at the Volta Grande Reservoir, in Minas Gerais and São Paulo states to answer the following questions: (1) Does the richness and abundance of cavity-nesting bees and wasps differ in riparian forest fragments according to the seasonal periods? (2) Does the composition of cavity-nesting bees and wasps vary among restoration and reference sites and between climate seasons (wet and dry)? (3) How do the degrees of specialization of the parasites vary among the patches of forest? We recorded 12 species of wasps, eight of bees, and nine species of parasites. Areas with longer time since restoration (reference site) showed higher species richness. However, the abundance was higher in most recent areas. The composition of bee and wasp assembly has not significantly changed between the climate seasons, although it is different between sampling areas. The richness and abundance were higher in warmer and rainy periods. The rate of bee and wasp mortality was high. The degree of specialization of parasites varies among sampling units, and the network of host-parasite interaction has a modular configuration with generalists and specialists. We concluded that the restored areas with more complex habitat could provide better conditions for the reestablishment of ecological interactions among these insects, the local flora, and other invertebrates, which together contribute to the success of the restored environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Different aspects of human activities can cause global environmental change that endanger species survival, alter species distributions, and lead to changes in antagonistic and mutualistic interactions (Stangler et al 2015, Barlow et al 2007). Deforestation and resulting fragmentation are regarded as a major threat to biodiversity, since they result in landscapes consisting of patchily distributed forest fragments in a matrix of pastures and plantations (Fahrig 2003). But, we still miss how this kind of human impact affects interspecific interactions, such as plant-pollinator (Cane et al 2006), predator-prey, and parasite-host (Klein 2006).
Riparian forests of the Cerrados of Brazil share faunistic and floristic elements with the Atlantic forest (Gibbs and Leitão-Filho 1978, Oliveira-Filho and Ratter 2009). Riparian forests have a highly variable structure, composition, and distribution of species, contributing to different ecological formations based on physiognomy and floristic and structural features (Kageyama et al 2001). From the association between riparian forests and adjacent vegetation formations emerged mixed forests with species characteristic of the two physiognomies (Kageyama et al 2001). Therefore, riparian forests are very flexible to changes in environment. However, some species are too specific to riparian forests and may suffer landscape modification. For example, species adapted to the riparian forest conditions and living in constant shade cannot easily adapt to higher temperatures and lower air moisture in open habitats and do not have many options to escape from rising temperatures and lower humidity (Morato and Campos 2000, Stangler et al 2015). This is the case of species of trap-nesting bees and wasps, which have narrow thermal tolerance and specific requirements for nesting resources (Deutsch et al 2008, Stangler et al 2015).
The assemblage of trap-nesting bees and wasps respond to habitat disturbances in different ways because they have different levels of sensitivity to habitat disturbance (Tscharntke et al 1998, Klein et al 2006, Tylianakis et al 2006). This is very concerning, because bees and wasps fulfill important tasks in ecosystem functions. Specifically, bees are the most important pollinator group of plants (Didham et al 1996, Kremen et al 2007) and wasps are important predators and parasitoids, with considerable economic and agricultural relevance (Tylianakis et al 2005). Their variety of functional niches makes insects and other invertebrates important for the maintenance of vital ecosystem processes (Didham et al 1996). Studies conducted in Neotropical forests have demonstrated that trap-nesting bees are very sensitive to habitat changes that causes reduction on the abundance and diversity. The assemblage of bees is more complex in continuous forest and natural gaps, while wasps seem to prefer small forest remnants and cleared areas (Morato & Campos 2000, Stangler et al 2015). Both groups rely heavily on nesting site availability (Potts et al 2005), specific materials for nest construction (Taki et al 2008), and pollen or arthropod as food resources (Tscharntke et al 1998). The nesting frequencies of trap-nesting Hymenoptera are also known to respond to climate factors such as temperature, precipitation, humidity, and microclimate variables (Morato 2001, Tscharntke et al 1998, Thiele 2005).
Data on communities of trap-nesting bees and wasps and their natural enemies have been used in research on habitat quality (Tscharntke et al 1998), the effects of habitat fragmentation and of landscape complexity on community composition and predator-prey interactions (Steffan-Dewenter et al 2002, Klein et al 2006, Steckel et al 2014, Stangler et al 2015) There is a lack of studies done inside riparian forests that combine the effects of climate and habitat size on communities of trap-nesting bees and wasps since most studies have investigated influences of tropical forests in adjacent agro-ecosystems (Klein et al 2002, 2006) or along land-use gradients (Tylianakis et al 2005, 2006, Batista Matos et al 2013). Moreover, there is a lack of studies investigating the possible interactive effects of small fragments and microclimate on solitary Hymenoptera and their parasitic interactions in tropical restored riparian fragments. Therefore, we assessed the richness and abundance and the network of interactions with its parasites of trap-nesting Hymenoptera at five locations in different sized and aged secondary forests. This was done in order to answer the following questions: (1) Does the richness and abundance of cavity-nesting bees and wasps differ in patches of restored riparian forest according to the seasonal periods? (2) Does the composition of cavity-nesting bees and wasps vary among restoration and reference sites and between seasons (wet and dry)? (3) How do the degrees of specialization of the parasites vary among the patches of riparian forest?
Material and Methods
Study Area
This study was carried out between November 2013 and September 2014 in the Volta Grande Reservoir region in Minas Gerais and São Paulo states (20°01′54″S/48°13′17″W), Brazil. The average annual temperature was 23°C and the average annual precipitation was 1506 mm. According to Alvares et al (2014), Volta Grande belongs to the Tropical AW Köppen climate (with a dry winter) (Fig 1). The landscape consists mostly of pastures and monocultures (sugarcane and rubber tree plantation) (see Martins & Antonini 2016 for more details). We selected five riparian forest fragments (average 17.7 ha ± 4.86; range 3–40 ha), at least 15 km between each, and with similar forest cover in the matrix (approx. 30%). The fragments were created in a single planting event that included between 30 and 40 species of native and exotics trees. Hereafter, following Martins & Antonini (2016), each fragment will be referred to as sampling units (SUs) (Table 1).
Sampling Design
In each of the five sampling units (hereafter SU), 12 plots of 100 m2 were installed in the central area of the fragment. The woodblocks (two by each plot) were placed 1.5 m high in the most central tree, forming a sampling point (hereafter Pt), totaling 120 blocks and 5400 nesting sites in total. Trap nests were black cardboard tubes inserted in holes drilled into wood blocks with a total of 45 holes arranged linearly (Camillo et al 1995). Trap nests were uniform in length (120 mm) but varied in their inner diameters (6–12 mm). The number of trap nesting, in each diameter, was the same.
Occupied cardboard, those closed with soil or plant materials, indicating completed nest construction (Krombein 1967), were collected and taken to the laboratory. New empty cardboards were used to replace those collected. In the laboratory, cardboards brought from the field were kept in a glass assay tube plugged with a cotton wad and kept in the laboratory at room conditions (ca. 15–25°C). Cells that remained closed for a long time were opened to investigate whether a juvenile had died (egg or pre- or post-defecating larvae) or whether it was diapausing. The number and identity of parasites and cleptoparasites also were recorded. Insects were identified and deposited with their nest material in the Entomological Collection of the Laboratório de Biodiversidade, of the Universidade Federal de Ouro Preto.
Analyses
Following Klein et al (2006), we calculated the accumulation curve of species richness of trap-nesting bees and wasps for each SU. Expected values of accumulated richness were obtained from the number of occupied trap nests found in each of the 12 collecting points, inside each SU, using 100 randomizations, with the Jacknife I estimator.
A generalized linear model (GLM) was used to test the relationship between the number of cells built by bees and wasps as well as the richness, abundance, and composition of bees and wasp assemblage with the temperature and precipitation among four seasonal periods following Martins & Antonini (2016). For this, the data were grouped into four categories—start and end of the dry season and the start and end of the wet season. We also tested if the rate of mortality changed between the SUs.
Permutational analysis of variance (PERMANOVA) was used to test the hypothesis that the composition of the bee and wasp assemblage varies between seasons (dry and wet) and between SUs. To test the composition of the bee and wasp assemblage between SUs, the data were grouped into five categories according to the sampling units presented in Table 1. The measure of dissimilarity used was that of Bray-Curtis with 1000 permutations, and to measure the dispersion of the data, multivariate analyses of permutation distance (PERMDISP) were performed. The graphical representation of the variation in the composition of solitary bees and wasps between the five sampling units was shown by analysis of non-metric multidimensional scaling (NMDS). All the statistical analyses were performed using the R software (R Development Core Team 2013).
Host-Parasite Network
We built one host-parasite network for the five fragments. Each network was built by an adjacency matrix A, where “aij” is the number of nests of an individual host species (bee or wasp) “i” parasitized by a parasite species “j.” Then, we evaluated if selective parasite species would parasitize a subset of wasp or bee species parasitized by the generalist parasites (i.e., nested pattern of host-parasite interactions). For this, we estimated nestedness using the NODF metric (Almeida Neto et al 2008) in the ANINHADO program, a program developed by Guimarães and Guimarães (2006). The values of this metric range from 0 (non-nested) to 100 (perfectly nested). In addition, we tested if there were groups of parasite species strongly associated with a particular set of host, as expected in a modular network and less connected to other groups (Guimerá et al 2004). For this, we used the modularity index (M) based on simulated annealing (SA) (range 0–1) (Guimerá et al 2004) using the software MODULAR (Marquitti et al 2014). To characterize the degree of specialization or partitioning, between two parties in the network, we used the H2′ index (Bluthgen et al 2006) that range from 0 (highly generalist) to 1 (highly specialized). We tested the significance of H2′ using Monte Carlo. We used GLM to test whether the number of parasite species has a positive relationship with richness and abundance of hosts.
Results
Trap-Nesting Bee and Wasp Assemblage
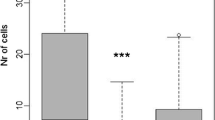
During 11 months, 1271 brood cells were constructed by solitary bees and wasps. The community consisted of eight host bees and 12 host wasps that interacted with three parasitic bees and three parasitic wasps (Table 2). The majority of brood cells (81.5%) were constructed by wasps, while only 18.5% were constructed by bees. In total, 109 cells (8%) were parasitized, being 23 bee cells and 86 wasp cells. The rate of mortality of juvenile was 49.14% for bees and 46.78% for the wasps. Three nests were used both for Centris tarsata and Pachodynerus grandis. The cells of C. tarsata were built first and were located in the end of nest. In these nests, adults of C. tarsata failed to emerge from the nest.
The species accumulation curve indicated that the sampled richness is still somewhat lower than expected (Fig 2). The lower estimated richness was recorded in SU5 (S = 22) with 59.38% of total species. However, SU2 registered 83.10% of the expected species (Table 3).
In SU5 (S = 13), SU2 (S = 12), and SU1 (S = 11), we registered the highest richness of bees and wasps and in SU2 and SU1 the highest abundance (n = 125 and 124 nests built, respectively). In the SU3 and SU4, we found lower richness, but the lower abundance of bees and wasps were observed in the SU4 and SU5 (Table 3).
The composition of trap nesting bees and wasps was different between SUs (PERMANOVA: F = 2.6989, R 2 = 0.45368, P = 0.001; PERMIDISP: F = 2.7324, P = 0.0752) (Fig 3). Only P. grandis was found in all five SUs and Trypoxilum nitidum, C. analis, and Megachile (Melanosaurus) sp. were found in four out the five SUs (Table 2). For P. guadulpensis, Megachile sp., Tetrapedia sp., and Epanthidium sp., they were found only in one sampling unit (SU4, SU1, and SU5, respectively) (Table 2).
Seasonal Periods
There was no difference in species composition among dry and rainy seasons (F = 1.5732; R 2 = 0.08037; P = 0.172). However, abundance of bees and wasps was higher all over the rainy season (F = 10,331, P < 0.0001) (Fig 4(a)). The highest value for total richness was found at the end of the rainy season (F = 9.4704, P < 0.0001) (Fig 4(b)).
Total abundance (a) and richness (b) of trap-nesting bee and wasps for the sampled climate periods in the riparian forests of the Volta Grande Reservoir, Brazil. Ds start of dry season, De end of dry season, Rs start of rainy season, Re end of rainy season. Bars are mean, different letters represents statistical differences.
Some wasp species (P. grandis and Penepodium sp.1) were recorded throughout the sampling period, but the number of constructed cells varied throughout the seasons (Fig 5). Other species such as P. anodontus Willink & Roig-Alsina 1998, Minixi brasilianum, and Penepodium sp2 nested only at the end of rainy season, Hypancistrocerus sp. only at the end of dry season, and P. guadulpensis only at the start of rainy season. Despite the low abundance of Pepsis sp., this species has been recorded in almost all seasonal periods.
Centris (Heterocentris) analis (Fabricius 1804) was the bee species that constructed the higher number of cells (n = 76) and also the only one registered during the entire sampling period (Fig 6). The other bee species showed a pattern of more “seasonal” occupation. Centris tarsata for example was recorded only at the end of dry season, Megachile sp. only at the end of rainy season, Euglossa melanotricha during the end of rainy and start of dry period, Tetrapedia sp. only at the start of rainy season, and Epanthidium sp. only at the start of dry season and C. terminata only being absent at the start of rainy season.
Host-Parasite Interaction
Nine parasitic species were recorded, and there was more than one species in seven trap nests. Wasp species of P. grandis (n = 35), T. nitidum (n = 22), and Penepodium sp1 (n = 10) were the most parasitized species. However, for Penepodium sp1, we found only one parasite species. We found 25 host-parasite interactions, 23% of the possible interactions. The average number of interactions was 1.2. Parasite species overlap interactions with hosts in 23%, while host species overlap in 22%.
The network was more modular (M = 0.56; P = 0.05) and less nested (NODF = 29.6; P = 0.30) than expected by chance. Four groups were identified in the host-parasite network (Fig 7); two groups were formed by one parasite species (Melittobia sp. and Coelioxys sp.) each with three interacting host species, a group formed by one host species (C. tarsata) interacting with two species of parasites, and a group formed by several species of parasite sharing various hosts. Although the most abundant parasites (Chrysis sp., Anthrax sp1, and Mellitobia sp.) have shared most abundant hosts (P. grandis and T. nittidum), there was a higher preference of Mellitobia sp. for Penepodium sp1 and Macrosiagon sp. for P. grandis. The parasite Coelioxys sp. formed, together with their host bees, a module. Although modular, the network showed low specialization (H′obs = 2.71; H′ran = 3.2; p < 0.001). On the sampling units SU2 and SU4, we registered more specialized parasites to hosts (H2 = 1.000 and 0.75, respectively). For sampling unit SU3, the value of H2 was 0.56 and for SU1 was 0.45. For sampling unit SU5, we do not find any specialized parasites to hosts and the value of H2 was 0.
Network of interactions between parasites and their hosts. The squares represent the hosts and the circles represent parasites, form sizes is related to the number of interactions that each species and thickness of the lines represents the number of times that these interactions have been carried out. Names of parasites and hosts according to Table 2.
Discussion
Bee and Wasp Assemblage
The number of trap nests occupied by solitary bees and wasps quantified in this study was relatively high compared with data from other studies in Brazilian Forests (e.g., Aguiar & Martins 2002, Alves-dos-Santos 2003, Buschini et al 2006, Loyola & Martins 2006, Aguiar et al 2005, Pires et al 2012), which is not expected for recovered riparian forests. The abundance of trap-nesting wasps in recovered riparian forests was much higher than that of bees. Wasp dominance was also observed in previous studies in Costa Rica (Stangler et al 2015), Central Amazon (Morato & Campos 2000), and Northern (Batista Matos et al 2013, Melo & Zanella 2012, Aguiar et al 2005) and Southern Brazil (Loyola & Martins 2006).
Our results are in accordance with some studies that show that bees are commonly found in continuous forest and natural gaps, while wasps are common to small forest remnants and cleared areas (Oliveira & Campos 1996, Morato & Campos 2000). The characteristics of the matrix that surround patches of habitat have significant effects over the biodiversity in different types of landscapes, spatial scales, and taxonomic groups (Martins & Antonini 2016). There is evidence that the type of matrix influences individual survival and reproduction as well as the structure and dynamics of communities, especially interspecific relationships (Prevedello & Vieira 2009). The matrix around the sampling units also could explain the dominance of wasps. The sampling units were located in a very anthropogenic matrix formed mainly by grasslands and sugarcane plantations. After 30 years of recovery, the landscape naturally became a mosaic of environments that strongly influenced the restoration of the riparian forest fragments. According to Fried et al (2005), field edges provided connectivity and facilitated wasp movements between trap nests and source habitats where dispersal started. It is important to notice that species of the subfamily Eumeninae (most representative group in this study) also showed more preference for open places (Jennings and Housewart 1984). According to these authors, the presence of ruderal plants improve the availability of resources (nectar and prey).
The curves of species accumulation showed that the expected bee and wasp richness in all of the five sampling units is higher than that observed. However, for equal sampling effort, we trapped greater species richness in the more “complex” area (SU5), possibly reflecting resource availability. The habitat heterogeneity of SU5 may support more potential niches and is likely to support food webs with greater range (Lassau et al 2005, Stangler et al 2015). However, in SU5, we observed lower abundance of nests of bees and wasps in traps. Besides that cavity-nesting bees and wasps depend on nesting sites in natural habitats, they can forage in multiple habitats including crop fields (Fye 1972). So, the lack of nesting sites could explain the higher abundance of occupied trap nests, in the SU1 and SU2, where the forest was recovered recently (10 years).
The most abundant species of wasps occurred in almost all sampling areas, especially species of Trypoxylon nitidum and P. grandis. It is important to notice that this is the first record of P. grandis in Cerrados of Brazil. The high abundance of potter wasps reflects greater availability of preys (Klein et al 2002), which is higher for areas of intense land use. So far, the occurrence of this species in trap nests had been recorded only in Atlantic Rainforest (Teixeira 2011).
We did not expect lower abundance of C. tarsata, which was considered numerical dominant species both in Atlantic Forest (Aguiar & Garófalo 2004) and in Cerrado (Mesquita et al 2009, Pires et al 2012). C. tarsata is able to successfully nest if the proper nest sites are available, even in areas of open vegetation. However, the number of nests founded by this species was much lower compared to C. analis. At the sampling units studied, C. analis seems to be more efficient in resource use “cavity,” as well as having founded more nests in four of the five areas and nests during the entire sampling period.
Seasonality
Nest occupation seems to be directly associated with climatic factors since higher temperatures led to an increased breeding activity of bees and wasps. According to Stangler et al (2015) and Batista Matos et al (2013), rainfall and temperature are key conditions regulating bees and wasps nesting through the availability of resources, mainly prey and pollen for the supply of the cells. In fact, the more abundant species occupied fewer trap nests when the temperature and rainfall were lower. Wasps are known to markedly respond to relative humidity and temperature (Batista Matos et al 2013). For example, Ancistroceroides sp., Hypancistrocerus sp., and P. guadulpensis were also affected by seasonality and nested in a single period. These Eumeninae species prey upon Lepidoptera larvae that are common in agroforests (Buschini & Buss 2010).
No significant correlation was found between temperature, rainfall, and nesting abundance of bee species in our study, probably because they obtain access to complementary resources such as nesting materials or nutrients (Ries & Sisk 2004). Another possible explanation is that the species composition, in our riparian forest fragments, shifted in favor of species adapted to environmental disturbances (Stangler et al 2015).
Host-Parasite Interaction
Parasitoid species richness and abundance were well explained by both bee and wasp species richness and abundance, and a higher number of parasitized nests were found in SU5, SU4, and SU3 where the most abundant species were also recorded. Bees and wasps formed, together with its associated parasites, an interactive network, with some species-specific interactions previously reported besides some new associations. More specialist species as Mesocheira sp. only parasitized nests of C. tarsata, and this association has been previously recorded (Aguiar & Garófalo 2004, Aguiar et al 2005, Gazola and Garófalo 2009). Anthrax and Coelioxys are generalists and parasitized nests of C. analis, Megachile (Melanosauros) sp., and several species of wasps (Gazola & Garófalo 2009). Macrosiagon sp. parasitized only nests of wasps. According to Krombein (1967) and Callan (1981), this genus usually parasitizes the nests of Eumeninae, being rarer in Sphecidae, which confirms our results. This preference may be related to the type of resource used by the host to feed the immature.
The most abundant parasitic genus (Melittobia, Anthrax sp1, and Chrysis sp1) also presented the higher number of hosts, as T. nitidum and P. grandis. Although Anthrax sp1 had presented preference for parasitize P. grandis and Mellitobia sp. for Penepodium sp1.
In this study, the interaction network formed by the parasites and their hosts seems to follow the hypothesis of asymmetric abundance proposed by Vazquez et al (2007). According to this hypothesis, the abundance of species in the community determinates the frequency and the power of interaction networks, resulting in asymmetric structures.
The formation of sub-groups (modules) on the network of host-parasite interaction observed in this study is expected in communities where morphological, functional, and phylogenetical constraints determined by evolutionary history prevail (Lewinsohn et al 2006). Somehow, the modular presentation in a network of interactions may be related to the evolutionary history of the community and the pressures suffered by these species (Poulin 2010, Lewinsohn et al 2006).
An increased specialization of the parasites to their hosts was observed in the SU2 area (H2 = 1.000), but this may not be representing greater specialization, as both the richness and abundance in this area were low, compared with the other sample units. However, in SU4, we found a level of specialization slightly lower than in SU2 but the richness and abundance were higher. According to Vazquez et al (2007), the abundance of species within the community could be a major factor mediating the frequency and power of interactions in the network. Also, according to Pereira-Peixoto et al (2016), isolated habitats have less specialized natural enemies because generalist species are likely to survive in structurally poor isolated habitats, because they are able to reproduce on a variety of available host species. The specialized parasitoid species are dependent on their specific host species and thus are more likely to benefit from habitats that are structurally more diverse. This is similar to our results for SU5 compared, e.g., with SU2 and SU4.
We can conclude that the restored riparian forests constitute important areas of habitat for bees and wasps that nest in pre-existing cavities. In addition, that the seasonality strongly influences the richness and abundance of these insects and their associated parasites is probably due to differences in the amount and types of habitat features offered. Finally, we can conclude that the community establishment of these Aculeata was possibly also linked to the vegetation structure (type of surrounding matrix, width, and growth) which plays an important role in the group’s occupation of riparian forests.
References
Aguiar CML, Garófalo CA (2004) Nesting biology of Centris (Hemisiella) tarsata (Hymenoptera, Apidae, Centridini). Rev Brasi Zool 21:477–486
Aguiar AJC, Martins CF (2002) Abelhas e vespas solitárias em ninhos-armadilha na Reserva Biológica Guaribas (Mamanguape, Paraíba, Brasil). Revta bras Zool 19(Supl.1):101–116. doi:10.1590/S0101-81752002000500005
Aguiar CML, Garófalo CA, Almeida GF (2005) Trap-nesting bees (Hymenoptera, Apoidea) in áreas of dry semideciduous Forest and caatinga, Bahia, Brasil. Rev Brasil Zool 22:1030–1038
Almeida Neto M, Guimarães P, Guimarães PR, Loyola RD, Ulrich W (2008) A consistent metric for nestedness analysis in ecological systems: reconciling concept and measurement. Oikos 117:1227–1239
Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G (2014) Koppen's climate classification map for Brazil. Meteorol Z 22:711–728
Alves-dos-Santos I (2003) Trap-nesting bees and wasps on the university campus in Sao Paulo, southeastern Brazil (Hymenoptera, Aculeata). J Kansas Entomol Soc 76(2):328–334
Barlow J, Gardner TA, Araujo IS, Avila-Pires TC, Bonaldo AB, Costa JE, Esposito MC, Ferreira LV, Hawes J, Hernandez MIM, Hoogmoed MS, Leite RN, Lo-Man-Hung NF, Malcolm JR, Martins MB, Mestre LAM, Miranda-Santos R, Nunes-Gutjahr AL, Overal WL, Parry L, Peters SL, Ribeiro-Junior MA, da Silva MNF, da Silva MC, Peres CA (2007) Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proc Natl Acad Sci U S A 104:18555–18560
Batista Matos MC, Sousa-Souto L, Almeida RS, Teodoro AV (2013) Contrasting patterns of species richness and composition of solitary wasps and bees (Insecta: Hymenoptera) according to land-use. Biotropica 45:73–79
Bluthgen N, Menzel F, Bluthgen N (2006) Measuring specialization in species interactions networks. Ecology 14:6–9
Buschini MLT, Buss CE (2010) Biological aspects of different species of Pachodynerus (Hymenoptera; Vespidae; Eumeninae). Braz J Biol 70:623–629
Buschini MLT, Niesing F, Wolff LL (2006) Nesting biology of Trypoxylon (Trypargilum) lactitarse Saussure (Hymenoptera, Crabronidae) in trap-nests in southern Brazil. Braz J Biol 66:919–929
Callan EMC (1981) Further records of Macrosiagon (Coleoptera: Rhipiphoridae) reared from eumenid and sphecid wasps in Australia. Australian Entomol Mag 7:81–83
Camillo E, Garofalo CA, Serrano JC, Muccillo G (1995) Diversidade e abundância sazonal de abelhas e vespas solitárias em ninhos armadilhas (Hymenoptera: Apocrita: Aculeata). Rev Bras Entomol 39:459–470
Cane JH, Minckley R, Roulston T, Kervin L, Williams NM (2006) Complex responses within a desert bee guild (Hymenoptera: Apiformes) to urban habitat fragmentation. Ecol Appl 16:632–644
Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR (2008) Impacts of climate warming on terrestrial ectotherms across latitude. PNAS 105:6668–6672
Didham RK, Ghazoul J, Stork NE, Davis AJ (1996) Insects in fragmented forests: a functional approach. Trends Ecol Evol 11:255–260
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Fried JH, Levey DJ, Hogsette JA (2005) Habitat corridors function as both drift fences and movement conduits for dispersing flies. Oecologia 143:645–651
Fye RE (1972) The effect of forest disturbances on populations wasps and bees in northwestern Ontario (Hymenoptera: Aculeata). Canad Entomol 104:1623–1633
Gazola AL, Garófalo CA (2009) Trap-nesting bees (Hymenoptera: Apoidea) in Forest fragments of the state of São Paulo. Genet Mol Rev 8:607–622
Gibbs P, Leitão-Filho HF (1978) Floristic composition of an area of gallery forest near Moji-Guaçu, state of São Paulo, SE Brazil. Rev Brasil Bot 1:151–156
Guimarães PR, Guimarães P (2006) Improving the analyses of nestedness for large sets of matrices. Environ Modell and Sofl 21:1512–1513
Guimerá R, Sales-Pardo M, Amaral LAN (2004) Modularity from fluctuations in random graphs and complex networks. Phys Rev 70:25–101
Jennings DR, Housewart MW (1984) Predation by eumenid wasps (Hymenoptera: Eumenidae) on spruce budworm (Lepidoptera: Tortricidae) and other lepidopterous larvae in spruce-fir forests of Maine. Ann Entornol Soc Amer 77:39–45
Kageyama PY, Gandara FB, Oliveira RE, Moraes LFD (2001) Restauração da mata ciliar- Manual para recuperação de áreas ciliares e microbacias. Semads, Rio de Janeiro 104 p
Klein AM, Steffan-Dewenter I, Buchori D, Tscharntke T (2002) Effects of land-use intensity in tropical agroforestry systems on coffee flower-visiting and trap-nesting bees and wasps. Conserv Biol 16:1003–1014
Klein AM, Steffan-Dewenter I, Tscharntke T (2006) Rain forest promotes trophic interactions and diversity of trap-nesting hymenoptera in adjacent agroforestry. J Anim Ecol 75:315–323
Kremen C, Williams NM, Aizen MA, Gemmill-Herren B, LeBuhn G, Minckley R, Packer L, Potts SG, Roulston T, Steffan-Dewenter I, Vazquez DP, Winfree R, Adams L, Crone EE, Greenleaf SS, Keitt TH, Klein A-M, Regetz J, Ricketts TH (2007) Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land-use change. Ecol Lett 10:299–314
Krombein KV (1967) Trap-nesting wasps and bees: life histories and associates. Smithsonian, Inst Press 570 p
Lassau SA, Cassis G, Flemons PKJ, Wilkie L, Hochuli DF (2005) Using high-resolution multi-spectral imagery to estimate habitat complexity in open-canopy forests: can we predict ant community patterns? Ecography 28:495–504
Lewinsohn TM, Prado PI, Jordano P, Bascompte J, Olesen JM (2006) Structure in plant-animal interaction assemblages. Oikos 113:174–184
Loyola RD, Martins RP (2006) Trap-nest occupation by solitary wasps and bees (Hymenoptera: Aculeata). Neotrop Entomol 35:41–48
Marquitti FMD, Guimaraes PR, Pires MM, Bittencourt LF (2014) Modular: software for the autonomous computation of modularity in large network sets. Ecography 37:221–224
Martins R, Antonini Y (2016) Can pollination syndromes indicate ecological restoration success in tropical forests? Restor Ecol 24:373–380
Melo RR, Zanella FCV (2012) Dinâmica de Fundação de Ninhos por Abelhas e Vespas Solitárias (Hymenoptera, Aculeta) em Área de Caatinga na Estação Ecológica do Seridó. Rev Bras Ciênc Agrár 7:657–662
Mesquita TM, Vilhena AMGF, Augusto CS (2009). Ocupação de ninhos-armadilha por Centris (Hemisiella) tarsata Smith, 1874 e Centris (Hemisiella) vittata Lepeletier, 1841 (Hymenoptera: Apidae: Centridini) em áreas de Cerrado. Bios J 25:124–132
Morato EF (2001) Efeitos de fragmentação florestal sobre vespas e abelhas solitárias na Amazônia Central. II. Estratificação Vertical. Rev Bras Zool 18:737–747
Morato EF, de O Campos LA (2000) Efeitos da fragmentação florestal sobre vespas e abelhas solitárias em uma área da Amazonia Central. Rev Bras Zool 17:429–444
Oliveira ML, Campos LAO (1996) Preferência por estratos florestais e por substâncias odoríferas em abelhas Euglossinae (Hymenoptera, Apidae). Rev Brasil de Zool 4:1075–1085
Oliveira-Filho AT, Ratter JA (2009) Padrões florísticos das matas ciliares daregião dos cerrados e a evolução das paisagens do Brasil central durante oquaternário tardio. In: Rodrigues RR, Leitão-Filho HR (eds) Matas Ciliares: conservação e recuperação. Universidade de São Paulo, São Paulo 320 p
Pereira-Peixoto MH, Pufal G, Staab M, Martins CF, Klein AM (2016) Diversity and specificity of host-natural enemy interactions in an urban-rural interface. Ecological Entomology 41(3):241–252. doi:10.1111/een.12291
Pires EP, Pompeu DC, Silva MS (2012) Nidificação de vespas e abelhas solitárias (Hymenoptera: aculeata) na reserva biológica Boqueirão, Ingaí, Minas Gerais. Bioscience Journal 28:302–310
Potts SG, Vulliamy B, Roberts S, O’Toole C, Dafni A, Ne’eman G, Willmer P (2005) Role of nesting resources in organizing diverse bee communities in a Mediterranean landscape. Ecol Entomol 30:78–85
Poulin R (2010) Network analysis shining light on parasite ecology and diversity. Trends Parasitol 26:492–498
Prevedello AJ, Vieira MV (2009) Does the type of matrix matter? A quantitative review of the evidence. Biodiv.Conserv. 19:1205–1223
R Development Core Team (2013) R: a language and environment for statistical computing. Version 2.13. User’s guide and aplication published: http://www.R-project.org
Ries L, Sisk T (2004) A predictive model of edge effects. Ecology 85:2917–2926
Stangler ES, Hanson PE, Steffan-Dewenter I (2015) Interactive effects of habitat fragmentation and microclimate on trap-nesting Hymenoptera and their trophic interactions in small secondary rainforest remnants. Biodivers Conserv 24:563–577
Steckel J, Westphal C, Peters MK, Bellach M, Rothenwoehrer C, Erasmi S, Scherber C, Tscharntke T, Steffan-Dewenter I (2014) Landscape composition and configuration differently affect trap-nesting bees, wasps and their antagonists. Biodivers Conserv 172:56–64
Steffan-Dewenter I, Münzenberg U, Bürger C, Thies C, Tscharntke T (2002) Scale-dependent effects of landscape structure on three pollinator guilds. Ecology 83:1421–1432
Taki H, Viana BF, Kevan PG, Silva FO, Buck M (2008) Does forest loss affect the communities of trap nesting wasps (Hymenoptera: Aculeata) in forests? Landscape versus local habitat conditions. J Insect Conserv 12:15–21
Teixeira FM (2011) Aculeata (Insecta, Hymenoptera) em ninhos-armadilhas em diferentes tipos fitofisionômicos de Mata Atlântica no Estado do Rio de Janeiro, 2011. Tese Doutorado em ecologia e recursos naturais), Universidade Estadual do Norte Fluminense Darcy Ribeiro, Campos dos Goytacazes, 107 p
Thiele R (2005) A new species of Ctenioschelus Romand from Costa Rican dry forest (Hymenoptera : Apidae : Ericrocidini). J Kans Entomol Soc 78:272–276
Tscharntke T, Gathmann A, Steffan-Dewenter I (1998) Bioindication using trap-nesting bees and wasps and their natural enemies: community structure and intrations. J Apl Ecol 35:708–719
Tylianakis JM, Klein A-M, Tscharntke T (2005) Spatiotemporal variation in the diversity of Hymenoptera across a tropical habitat gradient. Ecology 86:3296–3302
Tylianakis JM, Tscharntke T, Klein AM (2006) Diversity, ecosystem function, and stability of parasitoid host interactions across a tropical habitat gradient. Ecology 87:3047–3057
Vazquez DP, Melian CJ, Willians NM, Bluthgen N, Krasnov BR, Poulin R (2007) Species abundance and asymmetric interaction strength in ecological networks. Oikos 116:1120–1127
Acknowledgments
We highly acknowledge Graziela França for their support for the statistical data analysis and Marcel Gustavo Hermes for the wasp identification, FAPEMIG-CEMIG—Grant 03055/11 for the financial support and scholarship provided to GJA, and Conselho Nacional de Desenvolvimento Científico e Tecnológico for the scholarships provided to YA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Fernando B Noll – UNESP
Rights and permissions
About this article
Cite this article
Araujo, G.J., Fagundes, R. & Antonini, Y. Trap-Nesting Hymenoptera and Their Network with Parasites in Recovered Riparian Forests Brazil. Neotrop Entomol 47, 26–36 (2018). https://doi.org/10.1007/s13744-017-0504-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-017-0504-4