Abstract
Myzus persicae (Sulzer) is a polyphagous aphid that causes chlorosis, necrosis, stunting, and reduce growth rate of the host plants. In this research, the effects of Zinc sulfate and vermicompost (30%), Bacillus subtilis, Pseudomonas fluorescens, Glomus intraradices, G. intraradices × B. subtilis, and G. intraradices × P. fluorescens compared to control was investigated on the growth characters of Capsicum annuum L. and biological parameters of M. persicae. Different fertilizers caused a significant effect on growth characters of C. annuum and biological parameters of M. persicae. The highest plant growth was observed on Zinc sulfate and B. subtilis treated plants, and the lowest was on control. Increase in the amount of specific leaf area (SLA) (0.502 mm2 mg−1) was significantly higher in the B. subtilis than other fertilizer treatments. The longest (10.3 days) and the shortest (5.3 days) developmental times of M. persicae nymphs were observed on 30% vermicompost and Zinc sulfate treatments, respectively. The lowest adult longevity periods of M. persicae (11.2 and 11.3 days) were observed on G. intraradices × B. subtilis and 30% vermicompost treatments, respectively, and the longest ones (16.4 days) on Zinc sulfate. The highest rate of nymphal mortality and the lowest amount of nymphal growth index (NGI) were recorded on 30% vermicompost. The nymphs reared on Zinc sulfate treatment had the lowest rate of nymphal mortality and the highest amount of NGI. Thus, amending the soil with 30% vermicompost had a significantly negative effect on the biological parameters of M. persicae that can be used as an ecological control tactic for this pest.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bell pepper, Capsicum annuum L., is an important vegetable crop with a good source of nutrients, vitamins A, C, K, and B6, calcium, iron, zinc, fiber natural pigments, and antioxidant compounds important for human health (Greenleaf 1986, Deepa et al 2007, Howard et al 2000). A variety of insects damage the bell pepper during its growth including the green peach aphid, Myzus persicae (Sulzer) (Hem.: Aphididae). It is a polyphagous and a holocyclic aphid that causes chlorosis, necrosis, stunting, flower and fruit abortion, leaf distortion, defoliation, wilting, and reduced growth rate of the plant (Blackman & Eastop 2000, Frantz et al 2004). This aphid is also a vector of potato leaf roll virus (PLRV), potato Y virus (PVY), pepper mottle virus (Pep MoV), tobacco etch virus (TEV), and cucumber mosaic virus (CMV) (Robert et al 2000, Palukaitis & Garcia-Arenal 2003, Fenton et al 2010). Currently, the population of M. persicae is mostly managed with synthetic insecticides. However, after repeated control of the aphid populations with synthetic insecticides, resistant populations are developed through a strong selective pressure (Foster et al 1998, Bolandandam et al 2004). Therefore, other nonchemical control methods need to be integrated for effective control of this aphid. Using organic and bio-fertilizers may prove to be a complementary ecological control method for this pest.
Amending the soil with chemical fertilizer may affect the nutritional quality of plants, which in turn may affect herbivory (van de Rive et al 1972, Bentz et al 1995). Potassium, which is commonly in higher concentration in vermicomposts, might affect the interaction of plant-insect differently. High levels of K have a negative influence on insects such as white backed plant hopper, Sogatella furcifera (Horváth) (Salim 2002), brown plant hopper (BPH), Nilaparvata lugens (Stål) (Samiayyan & Janarathanan 1990), and other leaf hoppers and mites (Parihar & Upadhyay 2001). The negative influence of high levels of K on insect populations is assumed to be related to a reduced carbohydrates and amino acids concentrations (Baskaran et al 1985), and increased silica content and sclerenchyma’s layer in plant (Dale 1988). On the other hand, populations of many insect species have been reported to increase significantly with the increased use of nitrogen and phosphorus fertilizers (Urabe & Sterner 2001, Schade et al 2003, Throop & Lerdau 2004, Hogendorp et al 2006). This might be due to decrease in soluble protein and free sugar content in plants. Patriquin et al (1995) noted that various forms of organic fertilizer applied to the soils may decrease populations of arthropod pests and resultant crop damage.
Vermicompost is a nutrient-rich, microbiologically active organic amendment that equilibrates the release of nutritional components such as nitrogen, soluble potassium, exchangeable calcium, magnesium, and phosphorous that affects quality and yield of crops (Yardim et al 2006). Meanwhile, vermicompost amendment of the soil has shown some negative effects on some phytophagous insects such as Heteropsylla cubana Crawford (Biradar et al 1998); Aproaerema modicella (Deventer) (Ramesh 2000); Manduca quinqemaculata (Haworth) (Yardim et al 2006); M. persicae and Aphis gossypii Glover, Acalymma vittatum (Fabricius) and Diabotrica undecimpunctata Howardi, Tetranychus urticae Koch, and Pseudococcus sp. (Rao 2002, Arancon et al 2006, Edwards et al 2009, Razmjou et al 2011).
Plant growth promoting rhizobacteria (PGPR) that are used as biofertilizater, phytostimulant, and biocontrol (Bloemberg & Lugtenberg 2001) also might induce systemic resistance (ISR) or systemic acquired resistance (SAR) in crop plants against different pathogens and some insect pests (Hammerschmidt & Kuc 1995, Vidhyasekaran & Muthamilan 1999). The use of PGPR has increased corn resistance to the corn earworm, Helicoverpa zea (Hübner) (Bong & Sikorowski 1991), cucumber beetles (Zehnder et al 1997), and cotton bollworm Helicoverpa armigera (Hübner) (Qingwen et al 1998).
Arbuscular mycorrhizal (AM) fungi are widespread microorganisms associated symbiotically with the roots of more than 80% of terrestrial plants (Smith & Read 2008). Glomus intraradices is a member of the AM fungus that is used as a soil inoculant in agriculture and horticulture (Schuessler & Walker 2010). Mycorrhizal fungi increase the host plant’s ability to absorb the mineral elements from the soil, especially phosphorus and non-sources available to them (Kristek et al 2005).
Our objective was to evaluate the effects of some chemical, organic, and bio-fertilizers on the life table parameters of M. persicae under the laboratory conditions. The results could be used as a complementary ecological pest management program for M. persicae on the bell pepper.
Material and Methods
This research was conducted during 2014 in the greenhouse and laboratory of the Department of Plant Protection, College of Agriculture and Natural Resources, University of Mohaghegh Ardabili, Ardabil, Iran. The sandy loam soil which used to grow plants was collected from a fallow potato field in Ardabil plain. The chemical and bio-fertilizers were obtained from the Iranian Soil and Water Research Institute in Karaj. The cattle manure vermicompost was obtained from the Pars Koud Company, Gorgan, Iran.
Plant and insect sources
Seeds of bell pepper, C. annuum (cv. California Wonder), were grown in 3-L-volume pots in an aphid-free greenhouse set at 25 ± 5°C, 60 ± 5% RH and 14:10 h (L:D), and when the plants reached 4–6 leaf stage, they were used in the experiment. A colony of M. persicae, was collected from a tomato field in Meshkin-Shahr (Ardabil province) and transferred to the potted plants raised in the greenhouse under the above mentioned conditions. To maintain a suitable aphid colony, every week some aphids were transferred from infected plants to new young plants. After rearing the aphid for three generations on the pepper plant, they were used in the experiments.
Greenhouse experiments
The experiment was conducted in the greenhouse under the above stated conditions with seven treatments in a completely randomized design experiment. Treatments were (1) bell pepper plants grown in the field collected soil and then sprayed with Zinc sulfate fertilizer at the concentration of 0.001 at 4–6 expanded leaves stages; (2) bell pepper plants grown in the same soil amended with 30% vermicompost (which contained 1.8% N, 3.9 mg/kg P, pH = 7.3, and EC = 2.2 ds/m) before planting; (3) bell pepper seeds treated with Pseudomonas fluorescens strain 187 as PGPR treatments (1 mL/seed) with the rate of 1 × 107 CFU/mL; (4) bell pepper seeds treated with Bacillus subtilis, as PGPR treatments (1 mL/seed) with the rate of 1 × 107 CFU/mL and planted in pots containing the field collected soil; (5) bell pepper seeds treated with G. intraradices (5 g/seed) with 250 propagul/g and planted in pots containing the field collected soil; (6) bell pepper seeds treated with the combination of G. intraradices and P. fluorescens fertilizers; and (7) bell pepper seeds treated with the combination of G. intraradices and B. subtilis. Control plants were planted in pots containing only the field collected soil without adding any fertilizer.
Measurement of growth characters of C. annuum
For evaluating the effect of different fertilizer treatments on plant growth, indices such as plant height, leaf area, and leaf dry weight of bell pepper were measured at the flower stages on two randomly selected plants per treatment. Individual leaf area was measured with a leaf area meter AM300 (AM300 portable leaf area meter, ADC Bioscientific Ltd., Hoddesdon, UK). Leaf dry weights (DWs) were determined after they dried at 60°C for 3 days until constant weight was obtained. Specific leaf area (SLA) was calculated from leaf area and leaf dry weights determinations as follows (Evans 1972): SLA = leaf area per plant/leaf DWs per plant
In this study, also leaf chlorophyll was determined twice a week over the season on the eighth leaves per plot using a chlorophyll meter (SPAD-502, Minolta, Japan).
Determination of the life table parameters of M. persicae
Developmental time, survivorship of immature stages, fecundity, and longevity of the resultant adults were studied on 50 wigless adult aphids per treatment that were randomly chosen from the original greenhouse colony. Each adult aphid was confined in a clip cage (2 cm diameter × 1 cm height) on a leaf surface) the first fully expanded leaf from the top of the plant) with a suitable ventilation in a growth chamber that was set at 25 ± 2°C, 65 ± 5% RH, and 16:8 (L:D) h. They were permitted to produce nymphs for 24 h, and then the adult aphids were eliminated from the leaf clip cages. Each plant received one aphid nymph that was confined to the first true leaf. These nymphs were monitored daily to assess the aphid’s performance on different treatments. After maturity and when the reproduction started, adult fecundity and mortality were recorded daily, and the offsprings were removed from each leaf cage until the death of all adult aphids. The mortality parameters including entropy (H) and life expectancy (e x ) were calculated according to Carey (2001):
Also the reproduction parameters of M. persicae including gross fecundity rate (GFR), net fertility rate (NFrR), and the mean number of nymphs produced per female per day were calculated for the treatments (Carey 1993):
Mean number of nymphs produced per female per day = \( \frac{\sum_{\upalpha}^{\upbeta}{\mathrm{M}}_{\mathrm{x}}}{\left(\upvarepsilon -\upomega \right)} \), where l x is the days lived in interval x and x + 1; M x is the average number of offsprings produced by female at age x; α is the age of female at the first nymph; β is the age of female at the production of the last nymph; and ε − ω is the female longevity.
The nymphal growth index (NGI) was calculated by dividing the survival rate of the immature stage (l x ) by the period of each immature stage (T) (Setamou et al 1999):
Data analysis
All data were tested for normality by Kolmogorov-Smirnov method before analysis. The data were subjected to the one-way analysis of variance (ANOVA) using the statistical software of Minitab 16.0 (Minitab Inc. 1994). Statistical differences among means were compared using the Tukey post hoc Honestly Significant Difference (HSD) test at P < 0.05. Difference in each parameter value on fertilizer treatments were tested for significance by estimating variances through the jackknife procedure (Sokal & Rohlf 1981, Meyer et al 1986).
Results
Treatment effects on growth characters and chlorophyll content of C. annuum
The results showed that the plant growth significantly increased in all fertilizer treatments, but there were significant differences across treatments (Fig 1). The highest plant height was observed on Zinc sulfate (18.9 cm) and on B. subtilis (18.55 cm) treatments, respectively, and the lowest plant height (10.3 cm) was observed on control plant (F = 24.2; df = 7, 31; P < 0.01) (Fig 1A). Also, different fertilizer treatments affected the leaf area of the bell pepper differently (F = 7.9; df = 7, 31; P < 0.01). The highest leaf area was observed on treatments G. intraradices × P. fluorescens (349.8 mm2/plant) and G. intraradices × B. subtilis (329.1 mm2/plant), respectively, while the lowest leaf area (170.4 mm2/plant) belonged to the control plant (Fig 1B). Different fertilizer treatments significantly affected the leaf dry weight of C. annuum (F = 4.2; df = 7, 31; P < 0.01). The highest leaf dry weight (114.2 mg) occured on B. subtilis treatment, while the lowest amount (72.0 mg) was observed on control treatment. Also, different fertilizer treatments significantly affected the SLA (F = 61.46; df = 7, 31; P < 0.01) (Fig 1C). Bacillus subtilis treatment significantly increased the amount of SLA (0.502 mm2 mg−1) than the other treatments, and the lowest amount of SLA was observed on treatments G. intraradices × P. fluorescens and G. intraradices × B. subtilis (0.258 and 0.253 mm2 mg−1, respectively) (Fig 1D). Chlorophyll content of the leaves of bell pepper was affected differently with different fertilizer treatments (F = 16.9; df = 7, 31; P < 0.01). The highest (53.4) and the lowest (36.8) chlorophyll reading were obtained on B. subtilis and control treatments, respectively (Fig 1E).
Treatment effects on biology of M. persicae
Developmental time of immature stages of M. persicae was significantly affected by different fertilizer treatments (Table 1). There were significant differences among developmental time of nymph on different treatments (F = 25.0; df = 7, 193; P < 0.01). The longest (10.3 days) and the shortest (5.3 days) developmental times of M. persicae nymph were observed on vermicompost and Zinc sulfate treated plants, respectively. Also, there were significant differences among adult longevity of M. persicae on different fertilizer treatments (F = 2.6; df = 7, 195; P < 0.01). The shortest (11.2 and 11.3 days) and the longest (16.4 days) adult longevity period of M. persicae were observed on the G. intraradices × B. subtilis and vermicompost, respectively, and Zinc sulfate treated plants, respectively (Table 1).
Entropy
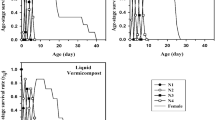
The entropy (H) of M. persicae on control, B. subtilis, vermicompost, P. fluorescens, Zinc sulfate, G. intraradices, G. intraradices × P. fluorescens, and G. intraradices × B. subtilis treatments treated plants were 0.48, 0.45, 0.43, 0.42, 0.41, 0.41, 0.40, and 0.32, respectively.
Life expectancy
Life expectancies (e x ) of M. persicae reared on various fertilizer treated plants are given on Fig 2. The life expectancy of M. persicae at the first days was 17.3, 17.0, 16.9, 16.7, 15.9, 15.6, 15.2, and 14.5 on G. intraradices × P. fluorescens, G. intraradices × B. subtilis, control, Zinc sulfate, P. fluorescens, B. subtilis, G. intraradices, and vermicompost (30%) treated plants, respectively. The lowest and the highest e x belonged to the vermicompost and Zinc sulfate treated plants, respectively.
Treatment effects on population growth parameters of M. persicae
There were significant differences on the number of offspring produced per female per day on different fertilizer treated plants (F = 40.6; df = 7, 188; P < 0.01). The lowest number of offspring/reproduction/day (0.30) was observed on vermicompost and no significant difference between G. intraradices × B. subtilis and G. intraradices × P. fluorescens, respectively, while the highest (1.1) was observed on Zinc sulfate and control treated plants. There were also significant differences among GFR of M. persicae on different fertilizer treatments (F = 9.5; df = 7, 188; P < 0.01). The lowest GFR (11.5) was observed on vermicompost treatments, and there were no significant difference among G. intraradices, B. subtilis, G. intraradices × B. subtilis, and G. intraradices × P. fluorescens treated plants. Also, the highest GFR (30.3) of M. persicae was observed on Zinc sulfate treated plants, and there were no significant difference between control and P. fluorescens. There were significant differences among NFrR of M. persicae on different fertilizer treated plants (F = 30.7; df = 7, 188; P < 0.01). The lowest NFrR (5.0) was observed on 30% vermicompost treatments, but there were no significant difference between G. intraradices × B. subtilis and G. intraradices × P. fluorescens treated plants. The highest value of NFrR (21.3) was observed on Zinc sulfate treated plants. The highest rate of nymphal mortality (60.0%) and the lowest amount of NGI (3.8) was recorded on vermicompost treated plants. Also, the nymphs reared on Zinc sulfate treated plants had the lowest rate of nymphal mortality (40.0%) and the highest amount of NGI (11.3) (Table 2).
Discussion
Currently, healthy and safe food free from toxic residues is demanded by consumers, especially with respect to freshly consumed vegetables like C. annuum. Therefore, to avoid hazardous chemicals against insect pests of such crops, certain protective or curative procedures could be implied using different non-chemical methods to reduce the pest population and resultant damage. According to the results obtained in this research, the different fertilizer treatments varied in their effects on the growth characters and leaf chlorophyll of C. annuum as well as the biological parameters of M. persicae.
We found that when the foliage of C. annuum treated with Zinc sulfate or when the soil is amended with B. subtilis treatments, plant growth was improved compared to the control. Zinc is an essential element for the functioning of many enzymes, as well as for the synthesis of tryptophan, a precursor of indole acetic acid (IAA) in plants, and Zinc deficiency causes a reduction in RNA synthesis and ribosome stability (Spiegel-Roy & Goldschmidt 2008). Also, Zinc deficiency is one of the most widespread mineral nutritional problems that affect normal development of plants (Pinton et al 1993, Mullins et al 1992). Bacillus sp. grows very rapidly and occupies the court of infection preventing pathogen spores to reach susceptible tissues in competition for spaces (Wolk & Sarkar 1994). This might be due to the induction of systemic resistance as the main mechanism of activity on the plant (Ramamoorthy et al 2001, Xing et al 2003, Abdel-Kader et al 2012). Thus, increasing plant growth of C. annuum on Zinc sulfate and B. subtilis treatments can be positively correlated with the amount of nutrient matter in the soil.
In this research, the highest leaf chlorophyll reading was observed on plants treated with B. subtilis, and the lowest reading was observed on control plant. The results of the correlation analysis revealed significant relationships between B. subtilis treatment in the soil and total chlorophyll content and plant height. The increased level of the total chlorophyll concentration in the leaves of the B. subtilis treated plants might be due to the increase of growth retardant on delaying leaf senescence and hence keeping the green pigment from degradation (Wafsy & El-Din 1995). Thus, bio-fertilizers are more effective than chemical fertilizer in improving plant growth and leaf chlorophyll content of C. annuum. It has been shown that conventional fertilizers (NPK) significantly increase the plant growth characters of pepper plant compared to all sources of organic manure and control (Gopinath et al 2009, Olaniyi & Ojetayo 2010, Abu-Zahra 2012).
Different studies have shown that fertilizer can have contradictive effects on growth, reproduction, and survival of insects feeding on the treated plants. Meyer (2000) proposed that soil nutrient availability not only affects the amount of damage that plants receive from herbivores but also enhances the ability of plants to recover from herbivores. Chau & Heong (2005) reported that organic fertilizers have positive effects on rice plant growth and the population of stem borer (SB) and leaf folder (LF) were reduced more on rice plants treated with chicken and hog manure compost and organic fertilizer compared to rice plants treated with chemical fertilizer. Also, Ramesh et al (2005) concluded that organic crops are more tolerant to insect attacks compared to non-organic plants. Organic rice is reported to have a thicker cell wall and lower levels of free amino acid than conventional rice. Our results indicated that the developmental time of nymph of M. persicae on vermicompost treated plants was higher than Zinc sulfate treated plants. van Lenteren & Noldus (1990) noted that the longer developmental time of an insect on a host is an indicative of its unsuitability for the insect. This could be due to increased secondary compounds (such as flavonoids, anthocyanin, and total phenol) on the leaves of C. annuum. In our experiments, the level of secondary compounds in the leaves of C. annuum increased significantly on vermicompost versus Zinc sulfate treatments (Mardani-Talaee et al 2016b). Edwards et al (2009) reported that phenolic substance contents of vermicompost cause change in feeding response of pests. The phenolic compounds from the wild ground nut, Arachis hypogaea L., retard the developmental rate of Spodoptera litura Hübner (Stevenson et al 1993). Also, the phenolics in plant tissues decrease the consumption rates of tissues by a geometrid caterpillar, Epirrita autumnata Borkhausen (Haukioja et al 2002).
The growth index (GI) depicts the effect of food quality on both survival rate and developmental time of an insect (Setamou et al 1999). NGI of M. persicae was lower on vermicompost treatment compared to Zinc sulfate treatment. Also, the highest and the lowest rates of nymphal mortality were recorded on vermicompost and Zinc sulfate treatments, respectively. Results of other studies have demonstrated that fertilizer treatment affect fecundity, life table parameters, and physiological performance of herbivorous insects (Edwards et al 2009), especially sap-sucking insects (Oliveira et al 2014, Mardani-Talaee et al 2016a). Amending the soil with fertilizers increases the level of organic matters of the soil and increased biological interactions lead to relative host plant resistance to pest damages (Luong & Heong 2005). Reduced NGI and increased mortality rate on vermicompost treated plants may be the result of lower suitability of vermicompost treated plants to M. persicae that can have negative effects on growth and survival, as well as the rate of ovipositon days of M. persicae.
Field studies have shown that the addition of vermicompost to soil significantly reduces the populations of the psyllid, Heteropsylla. cubana Crawford (Biradar et al 1998), the sucking insect Aproaerema modicella Deventer (Ramesh 2000), beetles (Acalymma vittatum (F.) and Diabrotica undecimpunctata Howardii), spider mites (T. urticae), mealybugs (Pseudococcus sp.), and aphids (M. persicae (Sulzer) and Myzus quinquemaculata (Haworth)) on several crops (Rao 2002, Yardim et al 2006, Arancon et al 2006).
In our studies, the lowest net fecundity of aphid was observed on vermicompost treated plants. Kale et al (1992) found that amending the soil with vermicompost may result in a significant increase in the abundance of N-fixers actinomycetes and other spore forming fungi compared to the soil supplemented with inorganic fertilizers. Soil enzyme activity is also significantly increased by vermicompost addition as compared to equivalent rates of mineral fertilizers (Marinari et al 2000, Arancon et al 2006, Saha et al 2008).
The entropy parameter provides a practical summary measure for characterizing differences in shapes of life to small changes in mortality rate among the different age group among cohorts (Carey 1993). The results showed that the survival schedules of M. persicae were convex on different fertilizer treatments (H < 0.5), suggesting that the probability of dying was higher in late ages as compared with early ones.
In summary, significant differences were observed on the life history parameters of M. persicae fed C. annuum cultivated in amended soil with bio-fertilizers. Amending the soil with vermicompost was more effective than chemical fertilizer in inducing antibiosis to green peach aphid. The observed effects were decreasing gross and net fecundity, increased developmental time and mortality rate, decreased adult longevity and NGI index, as well as life expectancies (e x ). These findings revealed that amending the soil with vermicompost at the rate of 30% under the greenhouse conditions can reduce aphid damage and can be helpful in ecological management of the pest in combination with other control tactics.
References
Abdel-Kader MM, El-Mougy NS, Lashin SM (2012) Efficacy of different plant resistance inducers against downy and powdery mildew diseases of pepper under plastic houses conditions. J Appl Sci Res 8:3415–3423
Abu-Zahra T (2012) Vegetative, flowering and yield of sweet pepper as influenced by agricultural practices. Middle East J Sci Res 11:1220–1225
Arancon NQ, Edwards CA, Yardim EN, Oliver T, Byrnem RJ, Keeney G (2006) Suppression of two-spotted spidermite (Tetranychus urticae), mealy bugs (Pseudococcus sp.) and aphid (Myzus persicae) populations and damage by vermicomposts. Crop Prot 26:29–39
Baskaran P, Narayanasamy P, Pari A (1985) The role of potassium in incidence of insect pests among crop plants, with particular reference to rice. In: Role of potassium in crop resistance to insect pests. Research series no. 3. Potash Research Institute of India, Guragaon, pp 63–68
Bentz JA, Reeves IJ, Barbosa P, Francis B (1995) Nitrogen fertilizer effect on selection, acceptance and suitability of Euphorbia pulcherrima (Euphorbiaceae) as a host plant to Bemisia tabaci (Homoptera: Aleyrodidae). Environ Entomol 24:40–45
Biradar AP, Sunita ND, Teggelli RG, Devaranavadgi SB (1998) Effect of vermicomposts on the incidence of subabul psyllid. Insect Environ 4:55–56
Blackman RL, Eastop VF (2000) Aphids on the World’s crops, Second edn. John Wiley & Sons with the Natural History Museum, London
Bloemberg GV, Lugtenberg BJJ (2001) Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr Opin Pl Biol 4:343–350
Bolandandam J, Barker H, Fenton B (2004) Differences in potato leafroll transmitting ability of individual genotypes of Scottish Myzus persicae with different susceptibilities to Lambda- cyhalothrin insecticide. Proc. 15th Intl. Plant Protect. Cong., Beijing, China
Bong CFJ, Sikorowski PP (1991) Effects of cytoplasmic polyhedrosis virus and bacterial contamination on growth and development of the corn earworm, Helicoverpa zea. J Invertebr Pathol 57:406–412
Carey JR (1993) Applied demography for biologists with special emphasis on insects. Oxford University Press Inc., 205 p
Carey JR (2001) Insect biodemography. Annu Rev Entomol 46:79–110
Chau LM, Heong KL (2005) Effects of organic fertilizers on insect pest and diseases of rice. Omonrice 13:26–33
Dale D (1988) Plant mediated effects of soil mineral stress on insects. In: Heinrich EA (ed) Plant stress insect interactions. John Wiley & Sons New York, USA, pp 35–110
Deepa N, Kaur C, George B, Singh B, Kapoor HC (2007) Antioxidant constituents in some sweet pepper (Capsicum annuum L.) genotypes during maturity. LWT-Food Sci Technol 40:121–129
Edwards CA, Arancon NQ, Vasko-Bennett M, Askar A, Keeney G, Little B (2009) Suppression of green peach aphid (Myzus persicae) (Sulz.), citrus mealy bug (Planococcus citri) (Risso), and two spotted spider mite (Tetranychus urticae) (Koch.) attacks on tomatoes and cucumbers by aqueous extracts from vermicomposts. Crop Prot 28:1–14
Evans GC (1972) The quantitative analysis of plant growth. Blackwell Scientific, Oxford
Fenton B, Kasprowicz L, Malloch G, Pickup J (2010) Reproductive performance of asexual clones of the peach-potato aphid, Myzus persicae, (Homoptera: Aphididae), colonising Scotland in relation to host plant and field ecology. Bull Entomol Res 100:451–460
Foster SP, Denholm I, Harling ZK, Moores GD, Devonshire AL (1998) Intensification of insecticide resistance in UK field populations of the peach-potato aphid, Myzus persicae (Hemiptera: Aphididae) in 1996. Bull Entomol Res 88:127–130
Frantz DJ, Gardner J, Hoffmann PM, Jahn MM (2004) Greenhouse screening of Capsicum accessions for resistance to green peach aphid (Myzus persicae). Hort Science 39:1332–1335
Gopinath KA, Saha S, Mina BL, Pande H, Srivastva AK, Gupta HS (2009) Bell pepper yield and soil properties during conversion from conventional to organic production in India Himalayas. Sci Hortic 122:339–345
Greenleaf WH (1986) Breeding vegetable crops, chapter 3. In: Basset MJ (ed) Pepper breeding. The AVI Publishing Company Inc., Westport, Connecticut, pp 67–134
Hammerschmidt R, Kuc J (1995) Induced resistance to disease in plants. Kluwer Academic Publishers, Dordrecht, p 182
Haukioja E, Ossipov V, Lempa K (2002) The interactive effects of leaf maturation and phenolics on consumption and growth of a geanetrid moth. Futrand Exp Appl 104:125–136
Hogendorp BK, Cloyd RA, Swiader JM (2006) Effect of nitrogen fertility on reproduction and development of citrus mealybug, Planococcus citri Risso (Homoptera: Pseudococcidae), feeding on two colors of coleus, Solenostemon scutellarioides L. Environ Entomol 35:201–211
Howard LR, Talcott ST, Brenes CS, Villalon B (2000) Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum spp.) as influenced by maturity. J Agric Food Chem 48:1713–1720
Kale RD, Mallesh BC, Kubra B, Bagyaraj DJ (1992) Influence of vermicompost application on the available macronutrients and selected microbial populations in a paddy field. Soil Biol Biochem 24:1317–1320
Kristek S, Kristek A, Pavlovic H (2005) The influence of mycorrhizal fungi (Glomus sp.) on field pea plant survival and growth in drought caused stress conditions. Pl Soil Environ 51:385–389
Luong MC, Heong KL (2005) Effects of organic fertilizers on insect pest and diseases of rice. Omonrice 13:26–33
Mardani-Talaee M, Zibaee A, Nouri-Ganblani G, Razmjou J (2016a) Chemical and organic fertilizers affect physiological performance and antioxidant activities in Myzus persicae (Hemiptera: Aphididae). Invert Surviv J 13:122–133
Mardani-Talaee M, Nouri-Ganblani G, Razmjou J, Hassanpour M, Naseri B, Asgharzadeh A (2016b) Effects of chemical, organic and bio-fertilizers on some secondary metabolites in the leaves of bell pepper (Capsicum annuum) and their impact on life table parameters of Myzus persicae (Hemiptera: Aphididae). J Econ Entomol 109:1–10
Marinari S, Masciandaro G, Ceccanti B, Grego S (2000) Influence of organic and mineral fertilizers on soil biological and physical properties. Bioresour Technol 72:9–17
Meyer GA (2000) Interactive effects of soil fertility and herbivory on Brassica nigra. Oikos 22:433–441
Meyer JS, Ingersoll CG, McDonald LL, Boyce MS (1986) Estimating uncertainty in population growth rates: jackknife vs. bootstrap techniques. Ecology 67:1156–1166
Minitab lnc. 1994. Philadelphia, PA, USA. www.minitab.com
Mullins MG, Bouquet A, Williams LE (1992) Biology of the grapevine. Cambridge University Press, Cambridge
Olaniyi JO, Ojetayo AE (2010) The effect of organomineral and inorganic fertilizers on the growth, fruit yield and quality of pepper (Capsicum frutescence). J Anim Pl Sci 8:1070–1076
Oliveira MD, Barbosa PRR, Silva-Torres CSA, Silva RR, Barros EM, Torres JB (2014) Reproductive performance of striped mealy bug Ferrisia virgata Cockerell (Hemiptera: Pseudococcidae) on water-stressed cotton plants subjected to nitrogen fertilization. Arth Plant Int 8:461–468
Palukaitis P, Garcia-Arenal F (2003) Cucumber mosaic virus. AAB. Descriptions of Plant Viruses, No. 400
Parihar SBS, Upadhyay NC (2001) Effect of fertilizers (NPK) on incidence of leaf hoppers and mite in potato crop. Insect Environ 7:10–11
Patriquin DG, Baines D, Abboud A (1995) Diseases, pests and soil fertility. In: Cook HF, Lee HC (eds) Soil Management in Sustainable Agriculture. Wye College Press, Wye, pp 161–174
Pinton R, Cakmak I, Marschner H (1993) Effect of zinc deficiency on proton fluxes in plasma membrane-enriched vesicles isolated from bean roots. J Exp Bot 44:623–630
Qingwen Z, Ping L, Gang W, Qingnian C (1998) The biochemical mechanism of induced resistance of cotton to cotton bollworm by cutting of young seedling at plumular axis. Acta Phytophylacica Sin 25:209–212
Ramamoorthy V, Viswanathan R, Raguchander T, Pkakasam V, Samivappan R (2001) Induction of systemic resistance by plant growth promoting rhizobacteria in crop plants against pests and diseases. Crop Prot 20:1–11
Ramesh P (2000) Effects of vermicomposts and vermicomposting on damage by sucking pests to ground nut (Arachis hypogea). Indian J Agr Sci J 70:334
Rao KR (2002) Induced host plant resistance in the management of sucking insect pests of groundnut. Ann Pl Protect Sci 10:45–50
Razmjou J, Mohammadi M, Hassanpour M (2011) Effect of vermicompost and cucumber cultivar on population growth attributes of the melon aphid (Hemiptera: Aphididae). J Econ Entomol 104:1379–1383
Robert Y, Trefor Woodford JA, Ducray-Bourdin DG (2000) Some epidemiological approaches to the control of aphid-borne virus diseases in seed potato crops in northern Europe. Virus Res 71:33–47
Saha S, Mina BL, Gopinath KA, Kundu S, Gupta HS (2008) Relative changes in phosphatase activities as influenced by source and application rate of organic composts in field crops. Bioresour Technol 99:1750–1757
Salim M (2002) Effects of potassium nutrition on growth, biomass and chemical composition of rice plants and on host-insect interaction. Pak J Agric Res 17:14–21
Samiayyan K, Janarathanan R (1990) Influence of K in combination with N on the incidence of BPH in rice. Potassium Res 6:36–41
Schade JD, Kyle M, Hobbie SE, Fagan WF, Elser JJ (2003) Stoichiometric tracking of soil nutrients by a desert insect herbivore. Ecol Lett 6:96–101
Setamou M, Schulthess F, Bosque-Perez NA, Poehling HM, Borgemeister C (1999) Bionomics of Mussidia nigrivenella (Lepidoptera: Pyralidae) on three host plants. J Chem Ecol 89:465–471
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Elsevier, New York
Sokal RR, Rohlf FJ (1981) The principles and practice of statistics in biological research. Biometry, New York, 843 pp
Spiegel-Roy P, Goldschmidt E (2008) Biology of citrus. Cambridge University Press, p 140–184
Stevenson PC, Anderson JC, Blaney WM, Simmonds MSJ (1993) Developmental inhibition of Spodoptera litura (Fab.) larvae by a novel caffeoylquinic acid from the wild ground, Arachis paraguariensis (Chodat and Hassl.). J Chem Ecol 19:2917–2933
Throop HL, Lerdau MT (2004) Effects of nitrogen deposition on insect herbivory: implications for community and ecosystem processes. Ecosystems 7:109–133
Urabe J, Sterner RW (2001) Contrasting effects of different types of resource depletion on life- history traits in Daphnia. Funct Ecol 15:165–174
van de Rive JAC, Murtry JA, Huffaker CB (1972) Ecology of mites and their natural enemies: a review: III. Biology, ecology, and pest status, and host plant relations of tetranychids. Hilgardia 41:354–432
van Lenteren JC, Noldus LPJJ (1990) Whitefly plant relationship: behavioral and ecological whitefly their bionomics. In: Gerling D (ed) Pest status and management. Intercept, Andover
Vidhyasekaran P, Muthamilan M (1999) Evaluation of powder formulation of Pseudomonas yuorescens Pf1 for control of rice sheath blight. Biocontrol Sci Techn 9:67–74
Wafsy E, El-Din (1995) Growth regulators and flowering. Academic Bookshop, Modern Egyptian Press, p 503–510
Wolk M, Sarkar S (1994) Antagonism in vitro of Bacillus sp., against Rhizoctonia solani and Pythium spp. Anz Schädlingskd Pfl Umwelt 67:1–5
Xing L, Ding Z, Wenxiang Y, Li D, Daqun L (2003) A study on the effect of Bacillus on downy mildew of cucumber. Pl Prot 29:25–27
Yardim EN, Arancon NQ, Edwrads CA, Oliver TO, Byrne R (2006) Suppression of hornworm (Manduca quinqemaculata) and cucumber beetles (Acalymma vittatum and Diabotrica undecimpunctata) populations and damage by vermicomposts. Pedobiologia 50:23–29
Zehnder G, Kloepper J, Yao C, Wei G (1997) Induction of systemic resistance in cucumber against cucumber beetles (Coleoptera: Chrysomelidae) by plant growth promoting rhizobacteria. J Econ Entomol 90:391–396
Acknowledgments
This research is financially supported by the University of Mohaghegh Ardabili, (Ardabil, Iran), which is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by J B Torres – UFRPE
Rights and permissions
About this article
Cite this article
Mardani-Talaee, M., Razmjou, J., Nouri-Ganbalani, G. et al. Impact of Chemical, Organic and Bio-Fertilizers Application on Bell Pepper, Capsicum annuum L. and Biological Parameters of Myzus persicae (Sulzer) (Hem.: Aphididae). Neotrop Entomol 46, 578–586 (2017). https://doi.org/10.1007/s13744-017-0494-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-017-0494-2