Abstract
Bemisia tabaci (Genn.) (Hemiptera: Aleyrodidae) has been recognized as an important pest of many agricultural systems including soybean [Glycine max (L.) Merrill] crops. As an alternative to chemical control, the use of resistant genotypes represents an important tool for integrated pest management (IPM). This study aimed to evaluate the biological development of Bemisia tabaci biotype B confined on 13 soybean genotypes under greenhouse conditions. Initially, the nymphal period, complete development period (egg–adult), and the viability of the silverleaf whitefly nymphs were evaluated in all genotypes. Then, four genotypes promising for resistance (‘Jackson,’ UX-2569-159, ‘P98Y11,’ and ‘TMG132 RR’) and a susceptible genotype (PI-227687) were selected for further assays, where two insect populations were compared: a first population from the initial rearing (cabbage plants) and another corresponding to insects previously reared out on the selected genotypes. In addition to the parameters evaluated in preliminary tests, we also determined the viability and incubation period of eggs. Moderate levels of resistance (antibiosis/antixenosis) to B. tabaci biotype B were found in three genotypes. ‘P98Y11’ and ‘TMG132 RR’ were less suitable for insect development, extending the development cycle, and UX-2569-159 caused high nymphal mortality. We did not observe a significant increase in the level of plant resistance by the use of previously stressed insects. This suggests that the evaluation of a single whitefly generation may be sufficient to make correct decisions on promising soybean genotypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bemisia tabaci (Genn.) (Hemiptera: Aleyrodidae) has been extensively studied worldwide since the 1980s, mainly after the reports of outbreaks of biotype B in several countries (De Barro 2011, Oliveira et al 2013). In Brazil, this biotype was found for the first time in the 1990s (Lourenção & Nagai 1994) and has since stood out as an important pest for many crops, including soybean [Glycine max (L.) (Merrill)] (Vieira et al 2011). The attack of this whitefly on different crops in Brazil causes economic losses estimated at approximately 714 million dollars/year (Oliveira et al 2013).
This insect causes direct damage by feeding on phloem sap, compromising development of plants. Indirect damage occurs during the feeding process, favoring the development of sooty mold (Capnodium sp.) and, consequently, reducing the photosynthetic capacity of the plants (Musa and Ren, 2005 Naranjo and Legg 2010, Cameron et al 2013). In addition, this insect has emerged as an important vector of geminivirus (Navas-Castillo et al 2011, Polston et al 2014). In soybean, B. tabaci biotype B transmits Cowpea mild mottle virus (CpMMV) (Thouvenel et al 1982).
Chemical control is used almost exclusively to control this pest. However, this method can select resistant individuals and cause environmental imbalances (Prabhaker et al 2005, Silva et al 2009). Thus, the use of less aggressive methods such as resistant genotypes has become necessary. This method has proven efficiency and great potential to be included into any pest management program (Smith & Clement 2012). There are three categories of plant resistance: antibiosis, antixenosis, and tolerance (Painter 1951). The antibiosis occurs when, upon feeding on a resistant plant, the arthropod biology is impacted by either biophysical or biochemical plant defenses. Common effects of plant antibiosis on arthropods include death of early instars, reduction of adult fecundity, and prolongation of the immature period and life cycle (Panda & Khush 1995). Antixenosis causes adverse effects on insect behavior. Biophysical, biochemical, or both factors present in plants exhibiting antixenosis affect arthropod recognition of the plant as a suitable source of food, oviposition site, or shelter (Kogan 1975, Panda & Khush 1995).
Some work has been conducted in the search for resistant soybean genotypes (Valle & Lourenção 2002, Lima & Lara 2004, Silva et al 2012, Valle et al 2012). However, the main objective of most of the research has been to characterize the occurrence of antixenosis (attractiveness and oviposition preference trials), with few results related to antibiosis (Silva et al 2012). According to Smith (2005), antixenosis and antibiosis often overlap and the distinction between these resistance mechanisms requires the completion of specific tests. In the case of tiny insects such as whiteflies and aphids, this separation becomes even more laborious, due to the difficulty of assessing the insect consumption (Painter 1951).
In this paper, we investigated the biological aspects of silverleaf whitefly on Brazilian soybean genotypes promising for resistance to soybean pests (Valle & Lourenção 2002, Silva et al 2012, 2014) and American genotypes that are resistant to other sucking insects (Hill et al 2004, 2006, Prochaska et al 2013). In addition to traditional tests comparing insect survival and development in a specific germplasm, we also evaluated insects previously confined to soybean genotypes showing whitefly resistance in order to test its stability in more than one insect generation.
Material and Methods
The study was conducted in a greenhouse at the College of Agronomic Sciences (UNESP), Botucatu, SP, Brazil (22°85′Sʺ latitude, 48°26′Wʺ longitude). The 13 soybean genotypes evaluated in the experiments, their respective genealogies, and the justification for choosing them are described in Table 1.
Bemisia tabaci biotype B rearing
The population of B. tabaci biotype B, originally obtained from the culture of the Instituto Agronômico de Campinas (IAC), was maintained in a screen cage (2.0 × 2.5 × 2 m) covered with plastic sheeting and shade cloth, with the lateral and frontal parts protected with white anti-aphid screens (200 mesh). The insects were provided cabbage (Brassica oleracea var. acephala L.) grown in plastic pots with a capacity of 2.5 L. Molecular characterization of the insect was performed periodically during the experiment to confirm the biotype according to Walsh et al (1991), Simon et al (1994), and De Barro et al (2003).
Preliminary test
The soybean genotypes were grown in pots (3 L), filled with a sterilized substrate composed of soil, coarse sand, and organic matter (cattle manure) at a ratio of 1:1:1. The plants were maintained in greenhouses, free from insect infestation.
Initially, a test with all 13 soybean genotypes was conducted (Table 1) in a greenhouse (temperature of 25.3°C, with a maximum of 31.4°C and a minimum of 17.3°C; average relative humidity of 58%, with a maximum of 95% and a minimum of 38%; natural photophase), during the month of September 2014. Three plants of each genotype in the vegetative stage V1–V2 (Fehr & Caviness 1997) were isolated in metal cages (35 cm diameter × 55 cm height), covered with voile, and infested with approximately 50 pairs of whitefly (individuals from initial culture) for a period of 24 h. The insects were collected using a mouth aspirator (11 cm high and 4 cm in diameter), giving preference to whitefly pairs, since, according to Byrne & Bellows Junior (1991), insect couples usually stay paired.
After 24 h of infestation, the adult insects were removed and two leaflets per plant were examined with the aid of a stereoscopic microscope (magnification ×40), and 30 eggs (with normal color and shape) were left on the abaxial surface of each leaflet. The leaflets of the first fully developed leaf were chosen. The surplus eggs were removed using flexible cotton swabs (Cotonete®, Brazil) (Cruz et al 2014). A completely randomized design was adopted, and each leaflet containing 30 eggs represented a repetition (a total of six per genotype). The following biological parameters of the insect were evaluated: duration of nymphal period, the egg to adult development, and the viability of the immature phase (from adult emergence).
Test with selected genotypes
From the results of the initial test, four genotypes that indicated some type of resistance (‘Jackson,’ UX-2569-159, ‘P98Y11,’ and ‘TMG132 RR’) and one susceptible genotype (PI-227687) were selected for further biological performance tests, which compared two populations of whitefly: one with individuals from the initial culture (cabbage plants) and one with insects reared on the respective selected genotypes.
To obtain the insects reared on the selected genotypes, two plants of each genotype (vegetative stage V2–V3) were subjected to infestation by whiteflies (insects from initial culture) for 24 h as described above. After this period, the adult insects were removed and the plants were monitored until the emergence of adults, which were used in the next stage of the experiment.
In the next part of the experiment, six plants were used for each genotype, three being subjected to infestation with insects collected from the initial culture and three that were infested with insects from the respective plant genotypes that were evaluated. The methodology used was the same as described for the first test. This step was also developed in the greenhouse, but under different conditions (28.5°C, with a maximum of 35.2°C and a minimum of 21.5°C; average relative humidity of 64%, with a maximum of 95% and minimum of 37%; natural photophase), during the month of October 2014. We used a completely randomized design in a factorial 5 × 2 (five soybean genotypes and two populations), with six replications for each combination, and each leaflet containing 30 eggs represented a repetition (total of six per genotype). The parameters evaluated were incubation period and egg viability, duration of nymphal period, the egg to adult development, and the viability of the immature phase.
Statistical analysis
The data were subjected to ANOVA using the F test. The normality and homogeneity of the data were assessed with the Shapiro–Wilk and Levene tests, respectively. Whenever the F test was significant, means were compared by the Tukey test (α = 5), using the statistical software Statistical Analysis System (SAS, version 9.2). The original data for viability (x) were arcsine-transformed (x + 0.5)1/2.
Results and Discussion
In the preliminary test, ‘TMG132 RR,’ ‘P98Y11,’ ‘TMG1176 RR,’ UX-2569-159, ‘Jackson,’ and ‘IAC-100’ prolonged (14.9–15.2 days) the nymphal period of B. tabaci biotype B, differing from PI-227687 and ‘IAC-19’ (Table 2). The genotypes ‘TMG132 RR’ and ‘P98Y11’ prolonged the developmental period from egg to adult (Table 2). This effect may have occurred due to a lower suitability of these genotypes as a food source for the insect, suggesting antibiosis. This mechanism of resistance is usually associated with plant biochemical factors, such as the presence of free amino acids, fatty acids, and fibers in the leaflets, which may have adverse effects on an insect that attempts to colonize it, affecting the biological performance of the insect (Smith & Chuang 2014). However, the associated expression of antixenosis should not be disregarded, since we did not evaluate the food consumption of insects.
As discussed by some authors (Panda & Khush 1995, Diaz-Montano et al 2006, Stout 2013), plants expressing high levels of antixenosis can also cause deleterious effects (e.g., underdevelopment, high mortality, and life cycle delay) on insect biology, suggesting the occurrence of antibiosis. The distinction of the two mechanisms requires precise quantification of food intake, which in the case of whitefly makes the evaluation more difficult.
Although soybean PI-227687 has been reported as having multiple resistance mechanisms against crop pests (Smith 1985, Silva et al 2014), in this work, this genotype was associated with the most accelerated development of B. tabaci biotype B from egg to adult. This result was similar to those observed by Lima & Lara (2004) and Silva et al (2012), which also verified the susceptibility of PI-227687 to B. tabaci biotype B. The shorter time required by the whitefly to develop on this genotype, combined with reports of its higher attractiveness and oviposition preference by the insect (Valle & Lourenção 2002), indicate that PI-227687 is highly susceptible to colonization by the whitefly.
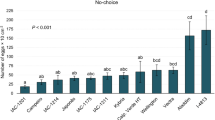
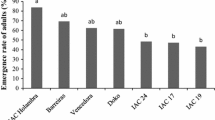
Regarding the adult emergence (Table 2), UX-2569-159 showed the lowest adult emergence, differing from ‘IAC-17,’ PI-227687, PI-274453, ‘Conquista,’ ‘TMG1176 RR,’ ‘TMG132 RR,’ and IAC-100. This suggests the occurrence of resistance (antibiosis/antixenosis) in UX-2569-159 because of the low viability in the younger stage of the insects. The expression of antibiosis in UX-2569-159 genotype was also recently reported to the soybean aphid (Baldin et al 2016). The inadequate ingestion of compounds produced by the plant may have caused physiological changes in the whitefly, resulting in different levels of mortality over the course of its development (Baldin & Beneduzzi 2010). The ‘Jackson’ genotype, which showed nymphal viability similar to UX-2569-159, has previously been mentioned as having antibiosis against the soybean aphid A. glycines, reducing fertility, longevity, and viability of insects relative to other genotypes (Li et al 2004). These authors also report the occurrence of antibiosis in ‘Dowling’; however, in this study, this genotype was found to be favorable to the development of insects.
Based on the preliminary results, five genotypes were selected for further performance assays: ‘TMG132 RR’ and ‘P98Y11’ (highest average egg–adult development); UX-2569-159, and ‘Jackson’ (low adult emergence and life cycle prolongation). PI-227687 was kept as the susceptible standard. Egg viability was not influenced by the origin of insects used for plant infestation, with an average hatch rate of over 90% (Table 3). The interaction between genotype and the origin of the population was significant for the incubation period. The genotypes ‘TMG132 RR,’ PI-227687, UX-2569-159, and ‘Jackson’ were shown to increase the incubation period of the insect when the infestation was made up of insects from those genotypes.
The interaction between the population and genotype was not significant for the nymphal period (Table 3). Thus, regardless of the population used for infestation, the only observed effect was related to genotypes, with insects exposed to ‘P98Y11’ and ‘TMG132 RR’ having a more prolonged nymphal period than those exposed to PI-227687, which was the most favorable to the development of nymphs. Regarding the development period from egg–adult (Table 3), no significant interaction was observed and only the effect of the genotypes was observed, with the individuals fed on ‘P98Y11’ and ‘TMG132 RR’ presenting the largest extension of that phase. These results support those found in the preliminary test, in which the insects reared on these genotypes also required more time to complete their cycle, suggesting the expression of antibiosis/antixenosis.
The variation in the egg–adult development period differed between trials (Tables 2 and 4). This difference probably occurred due to the different environmental conditions under which the experiments were conducted, with the highest average temperature in the second test. Previous studies have shown that in soybean the length of egg–adult development of B. tabaci is shorter in temperatures around 26–28°C (Albergaria & Cividanes 2002, Silva et al 2012). Whitefly development time also varies with the host plant, being shorter in soybean, cabbage, cowpea, and tomato (17–22 days) and longer in poinsettia and cassava (25–27 days) (Villas-Bôas et al 2002, Lima & Lara 2004, Cruz et al 2014).
The interaction was not significant for the adult emergence (Table 4). However, effects were observed between the origins of the insects used for infestation, with lower levels of adult emergence observed in the descendants of insects reared on soybean genotypes than in the descendants of insects of the original culture reared on cabbage. In absolute values, ‘TMG132 RR’ showed the greatest difference, with 82.4 and 64.7% emergence for the descendants of individuals from the initial culture and individuals reared on the genotype itself, respectively.
Considering all of the results from the second test (Tables 3 and 4), a significant increase in the level of plant resistance by the use of previously stressed insects was not observed. Overall, ‘P98Y11’ and ‘TMG132 RR’ were less suitable for insect development, prolonging their development cycle, which indicates the occurrence of antibiosis/antixenosis in these genotypes. UX-2569-159 also demonstrated to be resistant, causing high nymphal mortality. These genotypes may present resistance factors in their constitution, which can be explored in soybean breeding programs for resistance to whiteflies. The absence of significant difference in the performance of individuals confined by one or two generations indicates that the expression of resistance in these soybean genotypes is stable for up to two generations of B. tabaci biotype B. Thus, the evaluation of a single whitefly generation may be sufficient to make correct decisions on promising soybean genotypes.
References
Albergaria NMMS, Cividanes FJ (2002) Ecological life table of Bemisia tabaci (Genn.) B-biotype (Hemiptera: Aleyrodidae). Neotrop Entomol 31:359–363
Baldin ELL, Beneduzzi RA (2010) Characterization of antibiosis and antixenosis to the whitefly silverleaf Bemisia tabaci B biotype (Hemiptera: Aleyrodidae) in several squash varieties. J Pest Sci 83:223–229
Baldin ELL, Marchi-Werle L, Pannuti LER, Lourenção AL, Heng-Moss TM, Hunt TE (2016) Evaluating categories of resistance in soybean genotypes from the United States and Brazil to Aphis glycines (Hemiptera: Aphididae). Fla Entomol 99: (in press)
Byrne DN, Bellows Junior TS (1991) Whitefly biology. Annu Rev Entomol 36:431–457
Cameron R, Lang EB, Annan IB, Portillo HE, Alvarez JM (2013) Use of fluorescence, a novel technique to determine reduction in Bemisia tabaci (Hemiptera: Aleyrodidae) nymph feeding when exposed to benevia and other insecticides. J Econ Entomol 106:597–603
Cruz PL, Baldin ELL, Castro MJP (2014) Characterization of antibiosis to the silverleaf whitefly Bemisia tabaci biotype B (Hemiptera: Aleyrodidae) in cowpea entries. J Pest Sci 87:639–645
De Barro PJ (2011) Bemisia tabaci, the capacity to invade. In: Thompson WMO (ed) The whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae) interaction with geminivirus-infected host plants. Springer Dordrecht, London, pp. 181–204
De Barro PJ, Scott KD, Graham GC, Lange CL, Schutze MK (2003) Isolation and characterization of microsatellite loci in Bemisia tabaci. Mol Ecol Notes 3:40–43
Diaz-Montano JD, Reese JC, Schapaugh WT, Campbell LR (2006) Characterization of antibiosis and antixenosis to the soybean aphid (Hemiptera: Aphididae) in several soybean genotypes. J Econ Entomol 99:1884–1889
Fehr WR, Caviness CE (1977) Stages of soybean development. Iowa State University Cooperative Extension Service Special Rep. 80. Iowa State University, Ames, IA., USA
Hill CB, Li Y, Hartman GL (2004) Resistance to the soybean aphid in soybean germplasm. Crop Sci 44:98–106
Hill CB, Li Y, Hartman GL (2006) Soybean aphid resistance in soybean Jackson is controlled by a single dominant gene. Crop Sci 46:1606–1608
Kogan M (1975) Plant resistance in pest management. In: Metcalf RL, Luckmann WH (eds) Introdution to insect pest management. Wiley, New York, pp. 103–146
Li Y, Hill CB, Hartman GL (2004) Effect of three resistant soybean genotypes on the fecundity, mortality, and maturation of soybean aphid (Homoptera: Aphididae). J Econ Entomol 97:1106–1111
Lima ACS, Lara FM (2004) Resistance of soybean genotypes to the silverleaf whitefly Bemisia tabaci (Genn.) biotype B (Hemiptera: Aleyrodidae). Neotrop Entomol 33:71–75
Lourenção AL, Nagai H (1994) Surtos populacionais de Bemisia tabaci no Estado de São Paulo. Bragantia 53:53–59
Musa PD, Ren S (2005) Development and reproduction of Bemisia tabaci (Homoptera: Aleyrodidae) on three bean species. Insect Sci 12:25–30
Naranjo SE, Legg JP (2010) Biology and ecology of Bemisia tabaci. In: Stansly PA, Naranjo SE (eds) Bemisia: bionomics and management of a global pest. Springer, Dordrecht, The Netherlands, pp. 105–107
Navas-Castillo J, Fiallo-Olivé E, Sánchez-Campos S (2011) Emerging virus diseases transmitted by whiteflies. Annu Rev Phytopathol 49:219–248
Oliveira CM, Auad AM, Mendes SM, Frizzas MR (2013) Economic impact of exotic insect pests in Brazilian agriculture. J Appl Entomol 137:1–15
Painter RH (1951) Insect resistance in crop plants. McMillan, New York, p. 520
Panda N, Khush GS (1995) Host plant resistance to insects. CABI, Wallingford, p. 431
Polston JE, De Barro P, Boykin LM (2014) Transmission specificities of plant viruses with the newly identified species of the Bemisia tabaci species complex. Pest Manag Sci 70:1547–1552
Prabhaker N, Castle S, Henneberry TJ, Toscano NC (2005) Assessment of cross-resistance potential to neonicotinoid insecticides in Bemisia tabaci (Hemiptera: Aleyrodidae). Bull Entomol Res 95:535–543
Prochaska TJ, Pierson LM, Baldin ELL, Hunt TE, Heng-Moss TM, Reese J (2013) Evaluation of reproductive stage soybeans for resistance to soybean aphid (Hemiptera: Aphididae). J Econ Entomol 106:1036–1044
Silva LD, Omoto C, Bleicher E, Dourado PM (2009) Monitoring the susceptibility to insecticides in Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) populations from Brazil. Neotrop Entomol 38:116–125
Silva JPGF, Baldin ELL, Souza ES, Lourenção AL (2012) Assessing Bemisia tabaci (genn.) biotype B resistance in soybean genotypes: antixenosis and antibiosis. Chilean J Agric Res 72:516–522
Silva JPGF, Baldin ELL, Canassa VF, Souza ES, Lourenção AL (2014) Assessing antixenosis of soybean entries against Piezodorus guildinii (Hemiptera: Pentatomidae). Arth-Plant Int 8:349–359
Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P (1994) Evolution, weighting, and phylogenetic utility of mitochondrial gene-sequences and a compilation of conserved polymerase chain-reaction primers. Ann Entomol Soc America 87:651–701
Smith CM (1985) Expressions, mechanisms and chemistry of resistance in soybean, Glycine max L. (Merr.) to the soybean looper, Pseudoplusia includens (Walker). Insect Sci Appl 6:243–248
Smith CM (2005) Plant resistance to arthropods. Springer Science & Business, Dordrecht, The Netherlands, p. 423
Smith CM, Chuang WP (2014) Plant resistance to aphid feeding: behavioral, physiological, genetic and molecular cues regulate aphid host selection and feeding. Pest Manag Sci 70:528–540
Smith CM, Clement SL (2012) Molecular bases of plant resistance to arthropods. Annu Rev Entomol 57:309–328
Stout MJ (2013) Reevaluating the conceptual framework for applied research on host-plant resistance. Insect Sci 20:263–272
Thouvenel JC, Monsarrat A, Fauquet C (1982) Isolation of Cowpea Mild Mottle Virus from disease soybeans in the Ivory Coast. Plant Dis 66:336–337
Valle GE, Lourenção AL (2002) Resistance of soybean genotypes to Bemisia tabaci (Genn.) biotype B (Hemiptera: Aleyrodidae). Neotrop Entomol 31:285–295
Valle GE, Lourenção AL, Pinheiro JB (2012) Adult attractiveness and oviposition preference of Bemisia tabaci biotype B in soybean genotypes with different trichome density. J Pest Sci 85:431–442
Vieira SS, Bueno AF, Bueno RCOF, Hoffman-Campo CB (2011) Resistance of soybean genotypes to Bemisia tabaci (Genn.) biotype B (Hemiptera: Aleyrodidae). Neotrop Entomol 40:117–122
Villas-Bôas GL, França FH, Macedo N (2002) Biotic potential of Bemisia argentifolii to different host plants. Hort Bras 20:71–79
Walsh PS, Metzger DA, Higuchi R (1991) Chelex-100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. BioTechniques 4:506–513
Acknowledgments
The authors acknowledge the São Paulo Research Foundation (FAPESP) (Proc. 2013/13672-7) for financial support, as well as the Coordination for the Improvement of Higher Education Personnel (CAPES) for a doctoral scholarship granted to the first author and the National Council for Scientific and Technological Development (CNPq) for the productivity in research fellowship granted to the second author.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Lessando Moreira Gontijo – UF
Rights and permissions
About this article
Cite this article
Cruz, P.L., Baldin, E.L.L. Performance of Bemisia tabaci Biotype B on Soybean Genotypes. Neotrop Entomol 46, 210–215 (2017). https://doi.org/10.1007/s13744-016-0445-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-016-0445-3