Abstract
Among the predators with high potential for use in biological control, the species of the genus Podisus (Hemiptera: Pentatomidae) have received special attention for laboratory rearing, since they feed on different agricultural and forestry pest insects. However, the type of diet offered to insects in the laboratory may affect the viability of populations, expressed essentially by demographic parameters such as survival and fecundity. This study assessed demographic and development aspects in experimental populations of Podisus nigrispinus (Dallas, 1851) fed on larvae of Chrysomya putoria (Wiedemann, 1818) (Diptera: Calliphoridae) as an alternative prey. The demographic parameters fecundity and survival were investigated in life stages of P. nigrispinus with ecological modeling, by applying the Leslie matrix population model, producing histograms of life stages in successive time steps. The functional response of P. nigrispinus was also investigated on seven densities of C. putoria third-instar larvae at 24 and 48 h. The survival of predators that reached adulthood was 65% and the development time from egg to adult was 23.15 days. The predator showed a type III functional response for consumption of C. putoria at 24 and 48 h. The Leslie-matrix simulation of the age structure provided perpetuation of the predator population over time steps and the prey proved to be feasible for use in rearing and maintenance of P. nigrispinus in the laboratory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological control is essential for sustainable management of crop pests, as an alternative to chemical products, which cause environmental damage and lead to the development of genetic resistance (Thacker 2002). However, pest management programs depend on the successful production of natural enemies, which requires systematic rearing to produce satisfactory biological and reproductive performance and high efficiency in the field release (Lemos 2005).
Stinkbugs of the subfamily Asopinae are predators and considered very useful in biological control (Molina-Rugama et al 1998, Oliveira et al 1999, Vivian et al 2002). Although the subfamily includes 63 genera, with 357 species around the world and 23 genera with 100 species present in Neotropics (Grazia et al 2015), little is known about the biology of these species (De Clercq et al 2000). The genus Podisus (Heteroptera: Pentatomidae: Asopinae) is the most important, being widely studied in the Neotropics (De Clercq 2000, Torres et al 2006). The generalist predator Podisus nigrispinus (Dallas, 1851) (Hemiptera: Pentatomidae) is the species most often studied in Brazil (Torres et al 2006) and shows great potential for use in integrated pest management (IPM) for control of larvae in agricultural and forestry areas (De Clercq et al 1998). The species can be found in different environments, including forest, especially on Eucalyptus spp. (Zanuncio et al 1994), and on economically important crops such as soybean (Glycine max L.) (Panizzi et al 1977), cotton (Gossypium hirsutum L.) (Medeiros et al 1998) and tomato (Lycopersicum esculentum Mill.) (Bergam et al 1984), and several prey species can support the growth of this predator that affects the population balance of a large number of insects (O’Neil & Stimac 1988, Wiedenmann & O’Neil 1990), mainly lepidopteran larvae (Woodward et al 1970).
The success of a biological control program depends on the availability of agents for large-scale release. Knowledge of insect nutrition is important to develop an appropriate diet to increase and maintain the population level and to reduce production costs, through the study of the most suitable techniques for breeding and improving the effectiveness of natural enemies in the field (Tauber et al 2000). It is vital to study the most suitable prey species for the production of this predator, enabling mass rearing for multiplication and release in IPM programs.
P. nigrispinus can be reared on several alternative prey species such as Bombyx mori (Linnaeus, 1758) (Lepidoptera: Bombycidae), Musca domestica (Linnaeus, 1758) (Diptera: Muscidae) or Tenebrio molitor (Linnaeus, 1758) (Coleoptera: Tenebrionidae) (Zanuncio et al 1990, 1992a, Zanuncio et al 1991). Although previous studies have compared the performance of P. nigrispinus (Zanuncio et al 2001, 2008) fed on different types of prey, no study has evaluated the use of Chrysomya putoria (Wiedemann, 1818) (Diptera: Calliphoridae) as a food source for this stinkbug, or the effects on demography and population dynamics with ecological modeling by means of computer simulations. The larvae of this blowfly are larger than those of M. domestica, with a mean length of 13.3 mm in the third instar (Oliveira et al 2007). In comparison with other Calliphoridae larvae, C. putoria is similar in size to the larvae of Chrysomya albiceps (Wiedemann, 1819) and is smaller than those Chrysomya megacephala (Fabricius, 1794), but grows faster (Prins 1982).
Knowing the temporal distribution of life stages in natural enemies or pests is of paramount importance for biological control programs because their life histories may change significantly through the time in response to biological and ecological requirements (Speight et al 2008). Life tables have been used to understand relevant demographic aspects in P. nigripinus emphasizing the influence of different prey on its growth rate (Vivian et al 2002). However, ecological models may also be employed to simulate the temporal distribution of life stages based on the predator performance preying its prey. A usual model to investigate the dynamics of life stage-structured populations is the Leslie matrix (1945, 1948), which has been applied to analyze the transition between different life stages in insects (Nordhein et al 1988, Rosa et al 2011).
Conventionally, functional response is known as the number of prey consumed by an individual predator as a function of prey density (Holing, 1959). The functional response study using C. putoria larvae is useful to better understand stinkbug predation behavior and predator-prey interactions with an alternative prey, reflecting the rate of consumption of individual consumers in response to prey density. The components of the functional response include the length of time during which the predator and prey are exposed to each other, and the successful search rate and handling time (Tostowaryk 1972). Factors that may affect the predation process are prey density, predator density, environmental characteristics, prey defense mechanisms, and predator attack strategies (Holling 1959). The functional response is suppressed when predators are satisfied or when they are prevented from attacking more prey, depending on the behavior of the predator and the prey. Thus, the functional response, the demographic parameters, and population persistence of the species can be assessed by means of key demographic parameters for population dynamics, hoping to learn more about the different population strategies employed by the predator that could affect rearing.
This study evaluated the effect of an alternative prey on demographic parameters of P. nigrispinus in laboratory conditions, using the demography values as components of the Leslie matrix to simulate the temporal distribution of life stages of the predator. The study also aimed to evaluate the functional response of the predator at different prey densities, providing important information to understand the stinkbug’s predatory behavior as well as its development and performance in the laboratory, in order to determine optimum conditions for its rearing.
Material and Methods
Rearing and maintenance of Podisus nigrispinus
The study was conducted in the Laboratory of Insect Ecology of the Escola Superior de Agricultura “Luiz de Queiroz”, Piracicaba, São Paulo state, Brazil, at 25 ± 1°C, RH = 70 ± 10% and 12 h of photophase in BOD incubation chambers throughout the experiments. The eggs were kept on moist cotton pieces in Petri dishes, to maintain humidity until hatching.
A total of 30 individuals of P. nigrispinus were randomly selected after the eggs hatched. First-instar nymphs received only distilled water until the second instar, and were then separated and placed in individual plastic containers of 250 mL, containing glass tubes filled with water and sealed with cotton. The food sources were C. putoria third-instar larvae, offered every 2 days until adult stage.
Rearing and maintenance of the blowflies
Populations of C. putoria were kept in entomological cages 30 × 30 × 30 cm and maintained at 25 ± 1°C, 60 ± 10% RH and photophase of 12 h, and were fed on sugar and water ad libitum. Eggs were obtained by offering about 30 g of raw ground beef to the female blowflies as an oviposition substrate, and raw liver was provided to support the maturation of ovarian follicles (Linhares 1988). Larvae were reared on an artificial diet for blowflies, adapting the methodology used by Leal et al (1982), which uses 2.2 g agar, 30 g yeast extract, 30 g powdered whole milk, 1.5 g casein and 1 g nipagin, added to 60 g raw chicken heart with 270 mL water per 300 g of artificial diet.
Experiments and analysis
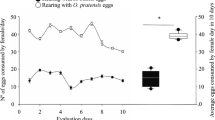
To evaluate the influence of prey on the demographic parameters and reproductive performance, an experiment was conducted using couples of P. nigrispinus. After emergence, males and females were separated to obtain the egg masses, in order to determine the number of eggs per female. Pairs were formed 3 days after the females emerged, because of their post-emergence fragility, to ensure that they would survive and reach sexual maturity (Zanuncio et al 1992b). Third-instar larvae were offered to the predator and the larvae available were double the amount initially offered every 2 days. The fecundity and egg viability were estimated, to evaluate the reproductive performance. New eggs were recorded daily, in order to obtain the standard egg production during the lifetime of P. nigrispinus, for use in the matrix of life stages. Survival between life stages of the stinkbug was estimated and used as an estimate for the probability of reaching successive stages in the Leslie matrix (Leslie 1945, 1948).
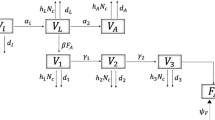
The algebraic manipulation software MATLAB 8.0 (MathWorks, 2004) was used to define a classification model of life stages for 90 generations, entering the life stages of P. nigrispinus. The Leslie matrix (Leslie 1945, 1948) was used to describe the population dynamics and life history from the data for survival between the life stages and the fertility estimated in the laboratory. Six stages were defined (1, 2, 3, 4, 5, 6), and the sixth stage of the adult predator F and fecundity. The shape of the matrix is shown below:
In the matrix, F represents the values for fecundity of the population. Each column represents one life stage of the pest. The values for fecundity of instar stages 1 to 4 were considered to be equal to zero for these immatures. Two values of fecundity were used in the matrix to represent two levels of fecundity during the adult stage of females, initial and final, according to the estimates previously mentioned. The values of S determine the probability that the insect will survive during an earlier life stage and will reach the next stage. The values used in the simulation were based on laboratory experiments. The simulation was performed for populations at successive times by inserting the matrix recurrence in discrete time as: N t+1 = M*N t , where N t and N t+1 describe the populations at successive times. Thus, the population N t+1 depends on the population N t as affected by fecundity and survival in the matrix. The initial values used are the same used in the experiments.
The functional response experiment used a completely randomized design composed of seven densities: 1, 2, 3, 4, 6, 7 or 9 larvae of C. putoria per female individual of the predator fasted for 24 h. Individual females were placed in plastic containers of 250 mL. The mean daily consumption of prey at each density was estimated, and then subjected to analysis of variance. Consumption after 24 and 48 h was evaluated taken into account prey densities, and when significant, a nonlinear regression analysis was performed. It was performed three replicates per treatment. The parameters of the nonlinear function were estimated through polynomial logistic regression (Allison 2005), for the maximum-likelihood method (Juliano 2001). The data were fitted to this function that describes the number of prey consumed as a function of density. The polynomial function is written as:
where B 0 , B 1, B 2 and B 3 are the parameters estimated through the nonlinear function. L c determines the consumption of C. putoria larvae and L i the initial density of prey.
Results and Discussion
The survival (nymphal viability) of individuals that reached adulthood was 65 ± 0.02%, in which mortality was higher in early instars (second and third instars), probably because the prey was larger than the predator, resulting in higher mortality initially and then declining from the fourth instar. The development time from egg to adult was 23.15 ± 0.57 days (Table 1).
Mean sex ratio (female/female + male) was estimated, resulting in 0.4, from which were obtained 20.5 ± 6.36 egg masses. The number of eggs per egg mass was 27.25 ± 1.23 and the mean total number of eggs was 475 ± 73.53. The longevity of females was 13.25 ± 0.64 days and the egg viability was 69%.
Figure 1 shows the Leslie-matrix model simulation, which produced a histogram series over 90 successive time steps, using the results for survival of the nymphal stages and the fecundity data for P. nigrispinus obtained with C. putoria third-instar larvae as an alternative prey. The data showed the population distribution of all stinkbug stages at each time step. The simulation suggested high viability in the laboratory production and indicated that the predator population could be supported indefinitely.
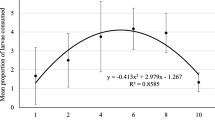
The mean consumption of prey at 24 h, in the functional response experiment of the predator females feeding on larvae of C. putoria, was 0.86 (86%) for density (d) = 1; 0.63 for d = 2; 0.67 for d = 3; 0.48 for d = 4; 0.42 for d = 6, 0.31 for d = 7; and 0.36 for d = 9 larvae. At 48 h, the mean consumption was 0.9 for d = 1; 0.85 for d = 2; 0.84 for d = 3; 0.67 for d = 4; 0.7 for d = 6; 0.51 for d = 7; and 0.56 for d = 9 larvae.
The polynomial logistic regression analysis model indicated that the functional response pattern for the consumption of C. putoria by the predator was type III, since the resulting linear coefficient was positive. The type III functional response was fitted to the data with the statistical nonlinear regression protocol for obtaining adjustment to type III, with the explained variance of 78% for 24 h of exposure of the prey to the predator. The polynomial logistic regression analysis for predator consumption at 48 h also showed a type III functional response, due to the linear coefficient found with the explained variance of 87%. In type III functional response, the attack rate speeds up initially and then slows down when the predator is satiating.
The predator developed to adulthood and successfully reproduced when fed on C. putoria as an alternative prey, which provided adequate nutritional quality to meet the physiological requirements of the predator. The results obtained from simulations with the Leslie matrix demonstrate the importance of fecundity to ensure the perpetuation of P. nigrispinus in the laboratory.
Food resource is an important component of the environment, and directly influences the distribution and abundance of insects, affecting biological processes such as fertility, longevity, rate of development and behavior (Zanuncio et al 1991). In this sense, particularly the knowledge of predation dynamics under experimental conditions is essential for mass rearing of predators for field release as part of IPM (Parra et al 2002).
The size of the prey affected nymphal viability in the early stages of development, resulting in higher mortality. Prey size also influenced the durations of the second and third instars (4.5 ± 0.34 and 3.92 ± 0.28 days, respectively) because of the difficulty and longer time needed to capture and manipulate prey for later ingestion. An alternative procedure in the rearing of the predator in its early stages of development to improve the survival rates could be to offer smaller larvae of C. putoria (first-instar or second-instar larvae).
In the fourth and fifth instars, closer to adulthood, mortality decreased (mean viability of 65%) and development was faster (3.91 ± 0.25 and 4.81 ± 0.26 days, respectively). The viability of P. nigrispinus nymphs fed on larvae of Alabama argillacea (Hübner, 1818) (Lepidoptera: Noctuidae) was 98.96% (Lemos et al 2003), 64% with Spodoptera frugiperda (J.E. Smith, 1797) (Lepidoptera: Noctuidae) (Oliveira et al 2004), and 51.84% when fed Musca domestica (Lemos et al 2003). dos Santos et al (1995) reported that P. nigrispinus prefers to predate on small larvae of A. argillacea rather than on large ones, which can better defend themselves.
The reproductive functions of the predator were prioritized and probably resulted in the low longevity of the females (13.25 ± 0.64 days) because the energy used in egg production reduced the longevity. The mean numbers of egg masses and the number of eggs per mass were 20.50 ± 6.36 and 27.25 ± 1.23, respectively. With larvae of S. frugiperda, females produced on average 14.89 egg masses with 30.06 eggs, and with larvae of T. molitor they produced 17 egg masses with 19.06 eggs (Oliveira et al 2004). The mean fecundity of females was 475 eggs, higher than that of females fed with larvae of M. domestica, which produced 162.90 eggs (Molina-Rugama et al 1997); or females fed with larvae of T. molitor, which produced 325 eggs; or with larvae of S. frugiperda, which produced 447.62 eggs (Oliveira et al 2004). The viability of the eggs was high, but lower, for example, when compared to 75% for alternately T. molitor and M. domestica (Zanuncio et al 2001).
The results of the functional response were compared at 24 and 48 h of prey consumption, in order to determine if time affects the functional relationships. When fed on third-instar larvae of C. putoria, the predator showed a type III functional response at 24 and 48 h. The increase in the number of prey reflected in the predator consumption, stabilizing the attack rate with increasing prey density. At lower densities, the predator needs to move over larger areas to find new prey, slowing its rate of consumption. Some factors affecting the functional response include the size of the prey and its defense capability, which need to be taken into account to better understand the interaction (Azevedo & Ramalho 1999). Third-instar larvae of C. putoria are larger in relation to the predator, and their mobility provides some protection from capture and manipulation.
Therefore, the predator efficiency alone does not completely define the interaction. The defensive behavior of the prey needs to be taken into consideration, because the predatory capacity may decrease with increasing size of the prey (dos Santos et al. 1995). According to Gaylord & Weston (2008), the handling time of the prey varies according to the sizes of both the prey and predator: the greater the weight/size of the prey, the longer the time that the predator spends in handling it.
Oliveira et al (2001) obtained a type II functional response at 24 h of experiment with P. nigrispinus females fed on A. argillacea. Zanuncio et al (2008) used this predator females fed on larvae of S. frugiperda with and without defensive ability, and obtained type II and type I functional responses, due to changing defensive conditions, showing that the functional response interactions are dynamic and may change under specific conditions. These authors also observed that the lower the density, the longer the time spent searching for new prey and the lower the consumption rate. They noted, however, high consumption in small densities after 24 h, and in most cases, the predator fed on all prey offered during this period.
Type I is the linear functional response in which the attack rate increases linearly with the density of prey while type II functional response is typical of predators that specialize in few prey, which the attack rate increases at a decelerated rate to the density of prey until becoming constant (Holling 1959, Juliano 2001).
The results indicate that consumption fell at 48 h, which can probably be explained by the decreased encounter rate, the increased handling time, and predator satiation. At higher densities, the daily consumption was more homogeneous, with increased consumption during the first 24 h after fasting, but without consuming all of each individual prey.
In general, the predators are affected by the quality of prey offered (Strohmeyer et al 1998). They use different prey to maximize energy savings depending on the encounter rates relative to the size of the prey, with larger prey at first providing more nutritional benefit (Charnov 1976). However, there may be higher energy costs to capture large prey and greater resistance and possible aggression. The differences in the development of the predator are probably responses to the nutritional quality of the prey. A suitable prey will support more rapid development, a higher survival rate, and greater reproductive success of the predator, resulting in rapid population growth in a short time (Beddington et al 1976), which is essential for mass rearing for biological control programs.
The larvae of C. putoria are easy to rear, develop rapidly, and the results indicate that are a suitable prey for rearing and maintaining populations of P. nigrispinus in the laboratory over time. Leslie-matrix simulation of the age structure of the predator population, fed on C. putoria third-instar larvae as alternative prey, provided a satisfactory outcome, as the generations of the predator population were perpetuated for over 90 time steps, indicating a high number of individuals based on the fecundity data.
References
Allison PD (2005) Fixed effects regression methods for longitudinal data using SAS. SAS Institute, Cary
Azevedo FR, Ramalho FS (1999) Efeitos da temperatura e da defesa da presa no consumo pelo predador Supputius cincticeps (Stäl) (Heteroptera: Pentatomidae). Pesq Agropec Bras 34(2):165–171
Beddington JR, Free CA, Lawton JH (1976) Concepts of stability and resilience in predator-prey models. J Anim Ecol 45:791–816
Bergam EC, Imenes SO, Hojo D, Campos TB, Takemtsu AP, Macellaro MLFS (1984) Levantamento da entomofauna na cultura do tomateiro (Lycopersicon esculentum). Biológico 50:209–236
Charnov EL (1976) Optimal foraging, the marginal value theorem. Theo Pop Biol 9:129–136
De Clercq P (2000) Predacious stinkbug (Pentatomidae: Asopinae). In: Schaefer CW, Panizzi AR (eds) Heteroptera of economic importance. Cambridge University, Cambridge, pp 737–789
De Clercq P, Merlevede F, Mestdagh I, Vandendurpel K, Mohaghegh J, Degheele D (1998) Predation on the tomato looper Chrysodeixis chalcites (Esper) (Lep., Noctuidae) by Podisus maculiventris (Say) and Podisus nigrispinus (Dallas) (Het., Pentatomidae). J Appl Entomol 122:93–98
De Clercq P, Mohaghegh J, Tirry L (2000) Effect of host plant on the functional response of the predator Podisus nigrispinus (Heteroptera: Pentatomidae). Biol Control 18:65–70
Gaylord AD, Weston PA (2008) Influence of prey size and environmental factors on predation by Podisus maculiventris (Hemiptera: Pentatomidae) on viburnum leaf beetle (Coleoptera: Chrysomelidae). Can Entomol 140:192–202
Grazia J, Panizzi AR., Greve C, Schwertner CF, Campos LA, Garbelotto TDA, Fernandes JAM (2015) Stink Bugs (Pentatomidae). In: True bugs (Heteroptera) of the Neotropics. Springer Netherlands. p 681–756
Holling CS (1959) Some characteristics of simple types of predation and parasitism. Can Entomol 91(7):385–398
Juliano SA (2001) Nonlinear curve fitting: predation and functional response curves. In: Scheiner SM, Gurvethich J (eds) Design and analysis of ecological experiments, vol 2., pp 178–196
Leal TTS, Prado AP, Antunes AJ (1982) Rearing the larvae of the blowfly Chrysomya chloropyga (Wiedemann) (Diptera, Calliphoridae) on oligidic diets. Rev Bras Zool 1:41–44
Lemos WP (2005) Fitofagia do predador Brontocoris tabidus (Heteroptera: Pentatomidae) no campo: aspectos morfo-fisiológicos e populacionais. PhD. Thesis, Universidade Federal de Viçosa, Viçosa, Brasil
Lemos WP, Ramalho FS, Serrão JE, Zanuncio JC (2003) Effects of diet on development of Podisus nigrispinus (Dallas) (Heteroptera: Pentatomidae), a predator of cotton leafworm. J Appl Entom 127:389–395
Leslie PH (1945) The use of matrices in certain population mathematics. Biometrika 33(3):183–212
Leslie PH (1948) Some further notes on the use of matrices in population mathematics. Biometrika 35(3):213–245
Linhares AX (1988) The gonotrophic cycle of Chrysomya megacephala (Diptera, Calliphoridae) in the laboratory. Rev Bras Entomol 32:383–392
Medeiros RS, Lemos WP, Ramalho FS (1998) Efeitos da temperatura no desenvolvimento de Podisus nigrispinus (Dallas) (Heteroptera, Pentatomidae), predador do curuquerê-do-algodoeiro (Lepidoptera, Noctuidae). Rev Bras Entomol 42:121–130
Molina-Rugama AJ, Zanuncio JC, Torres JB, Zanuncio TV (1997) Longevidad y fecundidad de Podisus nigrispinus (Heteroptera: Pentatomidae) alimentado con Musca domestica (Diptera: Muscidae) y frijol. Rev Biol Trop 45(3):1125–1130
Molina-Rugama AJ, Zanuncio JC, Pratissoli D, Cruz I (1998) Efeito do intervalo de alimentação na reprodução e na longevidade do predador Podisus nigrispinus (Dallas) (Heteroptera: Pentatomidae). An Soc Entomol Bras 27:77–84
Nordhein EV, Hogg DB, Chen SY (1988) In: Berger J, Fienberg S, Gani J, Krickeberg K, Singer B (eds) Lecture notes in statistics. Springer, Berlin, p 491
Oliveira HN, Pratissoli D, Edruzzi EP, Espindula MC (2004) Desenvolvimento do predador Podisus nigrispinus alimentado com Spodoptera frugiperda e Tenebrio molitor. Pesq Agropec Bras 39(10):947–951
Oliveira HN, Zanuncio JC, Sossai MF, Pratissoli D (1999) Body weight increment of Podisus nigrispinus (Stal) (Heteroptera: Pentatomidae), fed on Tenebrio molitor L. (Coleoptera: Tenebrionidae) or Musca domestica L. (Diptera: Muscidae). Brenesia 51:77–83
Oliveira JEM, Torres JB, Carrano-Morreira AF, Zanuncio JC (2001) Efeito da densidade de presas e do acasalamento na taxa de predação de fêmeas de Podisus nigrispinus (Dallas) (Heteroptera: Pentatomidae) em condições de laboratório e campo. Neotrop Entomol 30(4):647–654
Oliveira MS, Mello RP, Queiroz MMC (2007) Morfologia e duração dos ínstares larvais de Chrysomya putoria (Wiedemann) (Diptera, Calliphoridae), em laboratório. Rev Bras Entomol 51(2):239–245
O’Neil IR, Stimac IL (1988) Measurement and analysis of arthropod predation on velvet bean caterpillar, Anticarsia gemmatalis (Lepidoptera: Noctuidae), in soybeans. Environ Entomol 17(5):821–826
Panizzi AR, Corrêa BS, Gazzoni DL, Oliveira EB, Newman GG, Turnipseed SB (1977) Insetos da soja no Brasil. Embrapa Soja, Londrina, p 20
Parra JRP, Botelho PSM, Corrêa-Ferreira BS, Bento JMS (2002) Controle biológico no Brasil: parasitóides e predadores. São Paulo, Manole, p 609
Prins AJ (1982) Morphological and biological notes on six South African blowflies (Diptera, Calliphoridae) and their immature stages. Ann S Afr Mus 90:201–217
Rosa GS, Costa MIS, Corrente JE, Silveira LVA, Godoy WAC (2011) Population dynamics, life stage and ecological modeling in Chrysomya albiceps (Wiedemann) (Diptera: Calliphoridae). Neotrop Entomol 40(2):181–189
dos Santos TM, Silva EM, Ramalho FS (1995) Desenvolvimento ninfal de Podisus connexivus Bergroth (Hemiptera: Pentatomidae) alimentado com curuquerê-do-algodoeiro. Pesq Agropec Bras 30(2):163–167
Speight MR, Hunter MD, Watt AD (2008) Ecology of insects, concepts and applications. Wiley-Blackwell, UK
Strohmeyer HH, Stamp NE, Jatzomski CM, Bowers D (1998) Prey species and prey diet affect growth of invertebrate predators. Ecol Entomol 23:68–79
Tauber MJ, Tauber C, Daane KM, Haggen KS (2000) Commercialization of predators: recent lessons from green lacewing (Neuroptera: Chrysopidae). Am Entomol 26(1):26–38
Thacker JRM (2002) An introduction to arthropod pest control. University Press, Cambridge
The MathWorks INC. Matlab and Simulink (2004) The MathWorks Inc. Natick, Massachusetts, p 429
Torres JB, Zanuncio JC, Moura MA (2006) The predatory stinkbug Podisus nigrispinus: biology, ecology and augmentative releases for lepidopteran larval control in Eucalyptus forests in Brazil. CAB Rev: Perspect Agric, Vet Sci, Nutr Nat Resour 15:01–18
Tostowaryk W (1972) The effect of prey defense on the functional response of Podisus modestus (Hemiptera: Pentatomidae) to densities of the sawflies Neodiprion swainei and N. pratti banksianae (Hymenoptera: Neodiprionidae). Can Entomol 104:61–69
Vivian LM, Torres JB, Barros R, Veiga AFSL (2002) Tasa de crecimiento poblacional del chinche depredador Podisus nigrispinus (Heteroptera: Pentatomidae) y de la presa Tuta absoluta (Lepidoptera: Gelechiidae) en invernadero. Rev Biol Trop 50:145–153
Wiedenmann RN, O’Neil RJ (1990) Body weight of Podisus maculiventris (Say) under various feeding regimens. Can Entomol 122:285–294
Woodward TE, Evans JW, Eastop VF (1970) Hemiptera. In: Britton EB (ed) The insects of Australia, 1st edn. Melbourne University Press, Melbourne, p 1028
Zanuncio JC, Alves JB, Leite JEM, Silva NR, Sartório RC (1990) Desenvolvimento ninfal de Podisus connexivus (Bergroth, 1891) (Hemiptera: Pentatomidae) alimentado com dois hospedeiros alternativos. Rev Árvore 14(2):164–174
Zanuncio JC, Freitas MF, Alvesand JB, Leite JEM (1992a) Fecundidade de fêmeas de Podisus connexivus (Bergroth, 1891) (Hemiptera: Pentatomidae) em diferentes hospedeiros. An Soc Entomol Bras 20(2):369–378
Zanuncio JC, Didonet J, Santos GP, Zanuncio TV (1992b) Determinação da idade ideal para o acasalamento de fêmeas de Podisus connexivus, Bergroth, 1891 (Hemiptera: Pentatomidae) visando uma criação massal. Rev Árvore 16:362–367
Zanuncio JC, Alves JB, Zanuncio TV, Garcia JF (1994) Hemipterous predators of eucalypt defoliator caterpillars. Forest Ecol Manag 65:65–73
Zanuncio JC, Molina-Rugama AJ, Serrão JE, Pratissoli D (2001) Nymphal development and reproduction of Podisus nigrispinus (Heteroptera: Pentatomidae) fed with combinations of Tenebrio molitor (Coleoptera: Tenebrionidae) pupae and Musca domestica (Diptera: Muscidae) larvae. Biocontrol Sci Tech 11(3):331–337
Zanuncio JC, Domingues Da Silva CA, Rodrigues De Lima E, Pereira FF, Ramalho FS, Serrão JE (2008) Predation rate of Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae with and without defense by Podisus nigrispinus (Heteroptera: Pentatomidae). Braz Arch Biol Technol 51:121–125
Zanuncio TV, Batalha VC, Zanunci JC, Santos GP (1991) Parâmetros biológicos de Podisus connexivus (Bergroth, 1891) (Hemiptera: Pentatomidae) em alimentação alternada com lagartas de Bombyx mori e larvas de Musca domestica. Rev Árvore 15(3):308–316
Acknowledgments
Support for this research was provided by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP - 2011/10829-7). We are grateful to the Laboratório de Biologia dos Insetos and the Laboratório de Resistência de Artrópodes a Táticas de Controle of the Departamento de Entomologia e Acarologia (ESALQ-USP) and to the Centro de Tecnologia Canavieira (CTC) for providing larvae to feed the stinkbugs. We also thank the anonymous reviewers for important comments and suggestions that helped us to improve the clarity of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Marcelo N Rossi – UNIFESP
Rights and permissions
About this article
Cite this article
Botteon, V.W., Neves, J.A. & Godoy, W.A.C. Functional Response and Matrix Population Model of Podisus nigrispinus (Dallas, 1851) (Hemiptera: Pentatomidae) fed on Chrysomya putoria (Wiedemann, 1818) (Diptera: Calliphoridae) as Alternative Prey. Neotrop Entomol 46, 137–143 (2017). https://doi.org/10.1007/s13744-016-0440-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-016-0440-8