Abstract
Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) represents a pest of economic importance in all Western Hemisphere. This polyphagous species has diverged into two populations that have been mainly recognized with various mitochondrial and nuclear molecular markers and named “the rice” and “the corn” strains. In Colombia, both strains have evolved prezygotic and postzygotic isolation. They differ in tolerance to Bacillus thuringiensis (Cry1Ac and Cry1Ab endotoxins) and the insecticides lambda-cyhalothrin and methomyl. In 2014, a wing morphometric analysis made in 159 individuals from a colony showed that both strains significantly differ in wing shape. The species also exhibits sexual dimorphism in the rice strain as in females wing size is larger than in males. Here, we continued this work with another wing morphometric approach in laboratory-reared strains to calculate wing size and shape heritabilities using a full-sib design and in wild populations to determine if this method distinguishes these strains. Our results show that male heritabilities of both traits were higher than female ones. Wild populations were significantly different in wing shape and size. These results suggest that wing morphometrics can be used as an alternative method to molecular markers to differentiate adults from laboratory-reared populations and wild populations of this pest, particularly in males of this species. Finally, Q ST values obtained for wing size and shape further demonstrated that both strains are genetically differentiated in nature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Morphological characteristics provide an important source of information for several biological areas since changes in morphological traits are usually associated with adaptation and life history diversity in organisms (Dujardin et al 2009, Soto-Vivas et al 2011). In insects, morphological traits are particularly important for systematics and taxonomy (Jaramillo et al 2015), including traits such has horns in scarabaeids, aposematic coloration in wings of Heliconius butterflies, but also inconspicuous traits which are identified by geometric morphometrics (size and shape of the body/wing) (Adams & Funk 1997, Linares 1996, Dujardin et al 2009, Márquez et al 2011, Jaramillo et al 2015).
Most studies based on morphological traits are made on meristic characteristics, and in many cases, they do not offer useful measurements to distinguish populations at intraspecific level (Dujardin 2008). Geometric morphometrics is a widely used method to identify insect cryptic species, races, strains, or populations, representing a rapid and inexpensive approach to find differences between and within species (Soto-Vivas et al 2011, Cañas-Hoyos et al 2014, Jaramillo et al 2015). According to Dujardin (2008, 2010), in modern morphometrics size estimations are now contained in a single variable, the centroid, and shape variation is represented by partial warps that are defined as the relative positions of several landmarks after correcting for their size, position, and orientation. The use of such an approach has proven adequate for the identification of cryptic species or sex differentiation in a number of species (Soto-Vivas et al 2011, Jaramillo et al 2015).
Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) is a polyphagous insect of major economic impact in the Western Hemisphere (Sparks 1979, López-Edwards et al 1999, Prowell et al 2004). This species has genetically differentiated into two strains that are morphologically identical larvae and adults, but with differences in their wing shape (Cañas-Hoyos et al 2014) and genetics (Lu et al 1994, Prowell et al 2004, Nagoshi & Meagher 2003, 2004, Cano-Calle et al 2015). These two populations have mainly been identified with several molecular markers including esterases, polymerase chain reaction restriction fragment-length polymorphism (PCR-RFLP), FR (for rice) tandem repeat fragment, amplified fragment length polymorphism (AFLP), and sequencing of cytochrome oxidase I (COI), ND1, and ND4 mitochondrial genes (Pashley 1986, 1988, Levy et al 2002, Nagoshi & Meagher 2003, 2004, Prowell et al 2004, Groot et al 2008, 2010, Salinas-Hernández & Saldamando-Benjumea 2011). They have evolved prezygotic and postzygotic isolation, as they present temporal, behavioral, and chemical isolation (Pashley & Martin 1987, Pashley et al 1992, 1995, Schölf et al 2011, Groot et al 2008, 2010, Saldamando-Benjumea et al 2014), and at the postzygotic level, both strains produce F1 and F2 generations that had a significant reduction in the fitness of several of their life-history traits (Velásquez-Vélez et al 2011). These strains are broadly distributed, being recorded in Argentina, Brazil, USA, Puerto Rico, French Guyana, Jamaica, several islands from the Caribbean, Honduras, Ecuador (Murúa et al 2008, Busato et al 2004, Prowell et al 2004), and Colombia (Vélez-Arango et al 2008, Saldamando & Vélez-Arango 2010, Cano-Calle et al 2015).
In Colombia, the most recent study based on the molecular characterization of both strains demonstrated the corn strain was associated to corn, sorghum, cotton, and sugarcane, while the rice strain was found in rice and grasses (Cano-Calle et al 2015). Studies in Colombia have indicated a reduced gene flow in between these strains (Saldamando & Vélez-Arango 2010) and found differences in insecticide susceptibility among them—the rice strain being more tolerant to lambda-cyhalothrin and methomyl, while the corn strain being more tolerant to Bacillus thuringiensis (Cry1Ac, Cry1Ab) endotoxins (Ríos-Díez & Saldamando-Benjumea 2011, Ríos-Díez et al 2012). Such molecular, ecological, and biological differences in between both strains from Colombia were also detected at the morphological level. Cañas-Hoyos et al (2014)) showed that corn and rice strains significantly differed in their wing shape, but not in size (measured by their centroid size). However, individuals of the corn strain tended to be larger than individuals of the rice strain. They also reported sex dimorphism in the rice strain according to wing size—females were larger than males. Given that geometric morphometrics appear to be an alternative to differentiate such strains, we aimed (a) to estimate heritability values of wing shape and size in S. frugiperda strains, as these estimators are used to determine a morphological trait as a strong genetic component and (b) to carry out a wing morphometric analysis in field specimens collected at the Tolima department (Central Colombia) to certify that this approach is useful to distinguish in between these strains from field-collected specimens.
Material and Methods
Insect collection and rearing
Samplings of S. frugiperda took place in April 2009 to obtain individuals used for the heritability estimations and in May 2013 to obtain individuals used as field populations for the wing morphometric differentiation. Samples were made in corn and rice farms in north and central Tolima, where >100 larvae were sampled randomly at each site as in Cañas-Hoyos et al (2014). Briefly, corn populations were sampled in the municipalities of Mariquita (location San Felipe) (05°06′42.08″N, 74°53′35.03″W) and San Luís (04°12′48.2″N, 75°06′29″W). Rice populations were sampled in the municipalities of Ambalema (04°42′26.8″N, 74°50′47.9″W) and Buenos Aires (04°20′46.6″N, 75°05′43.3″W). Larvae were reared under controlled conditions (28 ± 5°C and 70% RH) until adult emergence. A subsample of 30% of the larvae collected was subjected to diagnostic PCR-RFLP of the mitochondrial gene COI for species identification (Vélez-Arango et al 2008).
Adults were kept in cages of 20 × 20 × 30 cm3, and males and females were separated before mating to establish crosses between virgin individuals to obtain the first parental generation. For aeration of each cage, the top was covered by a mesh cloth. In these cages, a piece of paper was placed for oviposition. Food was provided via a cotton plug saturated with a 1:1 mixture of honey and water, changed every day. Cages were checked every other day for oviposition and adult mortality. Egg masses were collected and deposited in plastic cups (3 cm in height and 1.5 cm in diameter). Once the larvae hatched, five larvae were placed in individual plastic cups with an artificial diet of pinto beans (Arévalo-Maldonado & Zenner de Polanía 2009), replaced every 3 days. As larvae pupated, pupae were placed in plastic cups 13 cm in height and 6 cm in diameter until adult emergence. Each pupa was sexed and adults were used to initiate a new generation. After establishing a colony with larvae collected from each site, one to one crosses between adults were used to obtain parental generations of the corn and the rice strains.
Wing data analysis
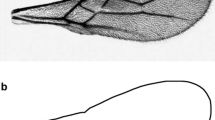
Left wings were analyzed in 161 laboratory reared specimens [n = 14 rice strain females (RSF), n = 16 corn strain females (CSF), n = 7 rice strain males (RSM), and n = 8 corn strain males (CSM)], n = 116 F1(1) individuals and in 75 field individuals collected in corn and rice farms in Tolima (n = 38 from corn fields and n = 37 from rice fields). To visualize wing venations, all wings were soaked in sodium hypochlorite 5.5% (30 s) and water (1 min), and scales were removed with an entomological brush. Wings were mounted by standard techniques and photographed using a Nikon 990 digital camera fitted to a Nikon SMS 800 stereomicroscope following Cañas-Hoyos et al (2014).
Fifteen anatomical markers were recognized as landmarks of type I (Bookstein 1991). They were identified on each wing on digital photographs (Fig 1). These landmarks were chosen because they were considered homolog points since they were easily detected, were reproducible, and were compared to the S. frugiperda morphometric colony data studied by Cañas-Hoyos et al (2014). Similar to Cañas-Hoyos et al (2014), the coordinates of these landmarks were digitized by using the software COO V. 39 (Dujardin 2010). The isometric estimator known as centroid size (CS) was used for size comparisons. CS is defined as the square root of the sum of the squared distances between the center of the configuration of landmarks and each individual landmark (Bookstein 1991). Raw coordinates were submitted to generalized Procrustes analyses (GPA) to generate “partial warp” (PW) scores and relative warp (RW) scores (Rohlf 1990, Rohlf & Slice 1990) as shape variables. GPA was used to remove the non-biological variability in the individual including metrical scales, orientation, and position of the individual wing in the photograph (Bookstein 1991). Both CS and shape variables were estimated with the software MOG V. 82 (Dujardin 2010).
The precision in the digitalization of the reference points for both colony and field specimens was computed as 1 − R, where R corresponds to two consecutive measurements performed in the same points on three images that were randomly chosen. R provides information of the proportion of variance generated between measurements, between individuals and the total variance (Cañas-Hoyos et al 2014). To calculate repeatability of the data, the estimators of centroid size and shape variables were used in this work.
Full-sib nested half-sib and partial factorial mating designs were conducted to generate families with half-sibling relationships (sharing a male, where one male was crossed to three females) for estimation of wing size and shape heritabilities (Falconer 1989). Wing size heritability was calculated from estimations obtained of the centroid size (CS) and wing shape heritability estimations from the first relative warp (R1) (Simmons & Kotiaho 2007). Heritabilities of CS and R1 were calculated based on a nested ANOVA test where the measurement variable (either CS or R1) was considered together with two nominal variables (females and offspring). These nominal variables were nested; therefore, each value of one nominal variable (the subgroups composed by the females and the offspring) is found in combination with only one value of the higher-level nominal variable (in this case the males) (Falconer 1989). Nested ANOVA tests produce three sources of genetic variation for the estimation of heritabilities: between sires (MSS = σ 2 w + K σ 2 d + dK σ 2 s), between dams (MSD = σ 2 w + K σ 2 d), and within progenies (MSW = σ 2 w) and three estimations of heritability considering (a) the sire component (h 2 = 4 σ 2 s/σ 2 T), (b) the dam component (h 2 = 4 σ 2 d/σ 2 T), and (c) sire + dam components (h 2 = 2 (σ 2 s + σ 2 d)/σ 2 T), where MSS = mean square of the sires, MSD = mean square of the dams, MSW = mean square of the progeny, σ 2 w = within progeny component of the variance, σ 2 s = between sire component of the variance, σ 2 d = between dam component of the variance, s = number of sires, d = number of dams per sire, and k = number of offspring per dam, where in this study k = 3 (siblings), d = 3 (dams), and s = 15 (sires) (7 sires for the rice strain and 8 for the corn strain) (Falconer 1989). To estimate the heritability of wing shape, the estimator RW1 was chosen because it produced the highest value of repeatability in Cañas-Hoyos et al (2014) study (97% in average). Nested ANOVA tests based on wing shape and size of S. frugiperda strains were obtained with the software Minitab 15 (Minitab 2007).
Differences between S. frugiperda strains according to wing shape and size was tested in field populations collected in corn and rice fields in 2013; for that, the contribution of the size to the differences in wing shape was evaluated by using a multivariate regression. This contribution is commonly named “Allometric effect” (Dujardin 2010). When the allometric effect is significant, the common allometric slope hypothesis is contrasted by using a covariance analysis (MANCOVA). When the model is not rejected (i.e., the groups showed common allometric slopes), an adjustment has to be performed for the size to determine if the data continued showing differences in shape without the effect produced by the wing size (Dujardin 2010). Also, to determine differences in wing shape between both strains, a discriminant analysis was obtained with the program PAD V. 98 (Dujardin 2010) based on Mahalanobis distances of each wing to the mean shape of each group. Finally, a validated classification for each strain was separately performed for females and males using PAD V. 98 (Márquez et al 2011, Cañas-Hoyos et al 2014).
Finally, Q ST was calculated here based on CS and RW1 data obtained from field S. frugiperda populations of the corn and the rice strains collected in Tolima department. For that, the following formula was used: Q ST = Var bet/Var bet + 2 Var with, where Var bet = variance between populations in this case between the corn vs the rice strains and Var with = variance within each population, meaning within populations of the corn strain or the rice strain (Freeland 2005). This Q ST is comparable to F ST since it represents the degree to which populations are genetically differentiated. When Q ST > F ST, this means that quantitative traits have differentiated to a greater extent than would be expected by genetic drift alone. When Q ST = F ST, the quantitative trait is selectively neutral, and when Q ST < F ST, this means that morphological differentiation in a population is less than that would be attributable to drift alone, and so the same phenotype is being selected for multiple populations (Freeland 2005).
Results
Laboratory colony
Given that repeatability measurements (R) determine the precision levels on the identification of the 15 wing-landmarks used here (Fig 1), these estimators were calculated for CS and RW1 and where R = 0.99 for CS. Shape variables repeatabilities were also very high for the first eight relative warps found in S frugiperda in all males and females of both strains from the laboratory colony (Table 1).
Values of the centroid size (CS) and relative warps (R1 and R2) were used to calculate the heritability of wing CS (size) and RW1 (shape) with a nested ANOVA for each trait (Tables 2 and 3) and produced the following values for CS and RW1: σ 2 d = 0.866, σ 2 s = 644.8, h 2 d = 0.4668, h 2 s = 0.00062, and h 2 d + h 2 s = 0.2340 for CS and σ 2 d = 0.000017, σ 2 s = 5.93 * 10−6, h 2 d = 0.3334, h 2 d = 0.1163, and h 2 d + s = 0.1958 for RW1. These results suggest that both wing size and shape heritabilities are larger for males than for females, and thus inheritance of both traits is affected by mainly males and not females in this species.
Field populations
Wing morphometrics analyses were also performed in 75 individuals of both S. frugiperda strains collected in nature where n = 25 CSF, n = 22 CSM and n = 12 RSF, n = 16 RSM. Significant differences in wing size based on the results given by the MANCOVA test (Wilks = 0.12, F = 1, 56, p = 0.3) and the Euclidian distances (DE = 0–010, p = 0.05) were found from specimens from both strains collected from the field. Likewise, differences between wing shapes were also found in these strains, as the linear regression produced significant results (r 2 = 0.028, p = 0.05) and the discriminant analysis (where the first function explains 100% of the total variance) (Wilks = 0.119) and the Mahalanobis distance was 5.55 (Figs 2 and 3).
Finally, Q ST values obtained for CS and RW1 from wild populations were Q ST = 0.385 and Q ST = 0.3261, respectively. These values demonstrate that populations of S. frugiperda from the corn and the rice strains are genetically differentiated, and this differentiation can also be attributable to differences in morphological traits between these two strains from the Tolima department.
Discussion
In the present study, heritability values for the wing size and shape of the corn and rice strains of S. frugiperda populations from Central Colombia (Tolima department) were estimated. The results show that heritability of CS (size) was greater than heritability of RW1 (shape) in both males and females. In addition, male heritabilities (h 2 d = 0.4668 for CS, h 2 d = 0.3334 for RW1) were higher than female heritabilities, suggesting that male genetic components play an important role in the inheritance of wing size and shape in this species. According to Falconer (1989), heritability values close to 0.5 are high, but values close to 0 are very low, and thus in our study, female heritabilities were very low even though they were under laboratory conditions. Since heritabilities are estimators used in quantitative genetics to determine how much of the phenotypic variation (VP = VG + VE where VE = environmental variation) is explained by the genetic variation (VG) of a quantitative trait (h 2 = VG/VP) (Falconer 1989), their values are important to decide whether a quantitative trait is affected by the environmental factors such as temperature, type of food, cage sizes, and inter- and intraspecies competition, among others. In our case, inheritance of wing size and shape in males is less affected by environmental factors, but female wing size and shape are more likely to be affected. Further studies with S. frugiperda should consider larger populations and different environmental conditions to test whether heritabilities are easily influenced by such conditions.
This study corroborates previous analyses made by Cañas-Hoyos et al (2014), who suggested wing morphometrics were useful to differentiate the rice from the corn strain. In this work, field populations of the corn and rice strains were significantly different based on both wing shape and size according the MANCOVA and discriminant analysis. These results further demonstrate the possibility of using a morphometric approach to distinguish these two strains as an alternative method to molecular markers when adults are collected from the field or the laboratory, as these methods can be expensive and time consuming. However, no morphological tools have so far been discovered for strain differentiation at the larval stage, and thus molecular markers are still needed for them (Cañas-Hoyos et al 2014).
Wing morphometrics and heritability estimations have also been used in Drosophila gouveai to demonstrate that morphometric parameters had a substantial genetic additive component in their phenotypic variance and that wing morphometric parameters were appropriate quantitative markers for assessing morphological differences among populations (Marsola-Moraes & Melo Sene 2004). We used a different method to calculate heritabilities of wing size and shape in S. frugiperda as nested ANOVAs were estimated based on a half-sib design (Falconer 1989). For this reason, heritability values from natural populations were not obtained in this work since progeny from parental crosses sampled in 2013 were not reared in the laboratory.
In another study made in Drosophila suboscura, the authors found that wild and laboratory-reared flies differ in heritability values of wing shape as this estimator was 90% from laboratory populations and 15% from natural populations collected in 1989, but these values differ from populations collected in 1988 as they found that heritability of wing size was 14% from laboratory populations and 7% from wild populations (Orengo & Prevosti 1999). The results obtained by Orengo & Prevosti (1999) are relevant for further estimations of wing heritabilities in S. frugiperda strains as this study was based in one collection of corn and rice strain larvae made in 2013, future studies based on wing morphometrics should consider several collections made in different years to remeasure heritabilities of wing size and shape in this moth.
Studies based on wing morphometrics have also been done in other Lepidoptera species that are considered pests, including Tecia solanivora in which sexual dimorphism was evidenced in this potato pest based on their wing size and shape (Hernández et al 2010). In Synneuria sp. a geometridae species from Chile, Benítez et al (2011) found that natural populations of this insect did not differ in wing shape or size but their sexes were clearly separated by their sizes compared to other cryptic and sibling species. However, none of these studies have estimated heritability values of wing shape and size as performed here. This work demonstrated that that inheritance of wing size and shape in S. frugiperda is modulated more by males than females and thus female wing size and shape can be affected by environmental factors. Also, wild populations of these two stains were significantly different in wing size and shape, and thus, this tool has been useful in both laboratory (Cañas-Hoyos et al 2014) and field specimens of this pest in order to differentiate these two strains.
Additionally, Q ST values obtained here demonstrate that S. frugiperda strains are genetically different based on two morphological wing traits: the centroid size (CS) and the relative warp 1 (RW1). Previous studies based on a genetic characterization of both strains from Tolima obtained by Saldamando & Vélez-Arango (2010) estimated PhiPt = 0.42 for the mitochondrial gene cytochrome oxidase I (COI) and PhiPt = 0.17 for the tandem repeated sequence FR (for rice) in 246 individuals of both strains collected in 2007 and 2008. Since Q ST values estimated here were 0.385 for CS and 0.326 for RW1 and represent similar values to 0.42 and larger values than 0.17, our results suggest that these two quantitative traits have differentiated to a greater extent than the genetic traits analyzed by Saldamando & Vélez-Arango (2010). Q ST values obtained here suggest that directional selection is favoring different populations in nature (Freeland 2005) meaning that corn strain phenotypes are morphologically differentiating from rice strain phenotypes. Given that n = 75 individuals were employed in this work, it is necessary to estimate this parameter in further investigations to corroborate the results obtained with S. frugiperda from Tolima as geometric morphometrics has been useful to also determine genetic differentiation between strains.
In conclusion, we demonstrated that S. frugiperda strains exhibit higher heritability values for males than for females in wing size and shape according to a morphometric analysis carried out in a colony. Researchers should use males rather than females to differentiate these two strains with this method. Spodoptera frugiperda females produced lower heritability values for both wing shape and size; thus, further studies should include a larger sample size to recalculate these heritability values. Wild individuals of both strains significantly differed in wing size and shape, and therefore, geometric morphometrics represents a promising tool to recognize specimens of each strain from field-collected specimens. However, molecular markers are still needed to distinguish both strains at larvae level. Finally, both wing size and shape have shown that corn and rice strains of S. frugiperda are genetically differentiated signifying that not only molecular markers but also geometric morphometrics have demonstrated that the species is diverging in two subpopulations: the corn and the rice strains.
References
Adams DC, Funk DJ (1997) Morphometric inferences on sibling species and sexual dimorphism in Eochlamisus bebbianae beetles: multivariate applications of the thin-plate spline. Syst Biol 46:180–194
Arévalo-Maldonado H, Zenner de Polanía I (2009) Evaluation of meridic diets for rearing Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) under laboratory conditions. Rev Udcaactual Divulg Cient 12:91–100
Benítez HA, Parra LE, Sepulveda E, Sanzana JM (2011) Geometric perspectives of sexual dimorphism in the wing shape of Lepidoptera: the case of Synneuria sp. (Lepidoptera: Geometridae). J Entomol Res Soc 13:53–60
Bookstein FL (1991) Morphometric tools for landmark data. Geometry and biology. Cambridge University Press, Cambridge
Busato GR, Grützmacher AD, de Oliveira AC, Vieira EA, Zimmer PD, Kopp MM, Bandeira JDM, Magalhanes TR (2004) Analysis of the molecular structure and diversity of Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) populations associated to the corn and rice crops in Rio Grande do Sul State, Brazil. Neotrop Entomol 33:709–716
Cañas-Hoyos N, Márquez EJ, Saldamando-Benjumea CI (2014) Differentiation of Spodoptera frugiperda (Lepidoptera: Noctuidae) corn and rice strains from Central Colombia: a wing morphometric approach. Ann Entomol Soc Am 107:575–581
Cano-Calle D, Arango-Isaza RE, Saldamando-Benjumea CI (2015) Molecular Identification of Spodoptera frugiperda (Lepidoptera: Noctuidae) corn and rice strains in Colombia by using aPCR-RFLP of the mitochondrial gene cytochrome oxidase I (COI) and a PCR of the gene FR (for rice). Ann Entomol Soc Am 108:172–180
Dujardin JP (2008) Morphometrics applied to medical entomology. Infect Genet Evol 8:875–890
Dujardin JP (2010) COO, MOG and COV for Windows. Institut de Recherches pour le Développement. IRD, France
Dujardin JP, Costa D, Bustamante N, Jaramillo N, Catalá S (2009) Deciphering morphology in Triatominae: the evolutionary signals. Acta Trop 110:101–111
Falconer DS (1989) Introduction to quantitative genetics, 3rd edn. Longman. Sci and Teach. London, 438 p
Freeland J (ed) (2005) Molecular ecology. Jonh Wiley & Sons, West Sussex, 388p
Groot AT, Marr M, Scholf G, Lorenz S, Svatos A, Heckel DG (2008) Host strain specific sex pheromone variation in Spodoptera frugiperda. Front Zool 5:20
Groot AT, Marr M, Heckel DG, Schöfl G (2010) The roles and interactions of reproductive isolation mechanisms in fall armyworm (Lepidoptera: Noctuidae host strains. Ecol Entomol 35(S1):105–118
Hernández N, Barragán AR, Dupas S, Silvain JF, Dangles O (2010) Wing shape variations in an invasive moth are related to sexual dimorphism and altitude. Bull Entomol Res 100:529–541
Jaramillo N, Dujardin JP, Calle-Londoño D, Fonseca-Gonzalez I (2015) Geometric morphometrics for the taxonomy of 11 species of Anopheles (Nyssorhynchus) mosquitoes. Med Vet Entomol 29:26–36
Levy HC, Garcia-Maruniak A, Maruniak JE (2002) Strain identification of Spodoptera frugiperda (Lepidoptera: Noctuidae) insects and cell line: PCR-RFLP of cytochrome oxidase subunit I gene. Fla Entomol 85:186–190
Linares M (1996) The genetics of the mimetic coloration in the butterfly Heliconius cydno weymeri. J Hered 87:142–149
López-Edwards M, Hernández-Mendoza J, Pescador-Rubio L, Molina-Ochoa A, Lezma-Gutierrez J, Hamm JJ, Wiseman BR (1999) Biological differences between five populations of fall armyworm (Lepidoptera: Noctuidae) collected from corn in Mexico. Fla Entomol 82:254–262
Lu YJ, Ochert GD, Isenhour DJ, Adang MJ (1994) Molecular characterization of a strain-specific repeated DNA sequence in the fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae). Insect Mol Biol 3:123–130
Márquez E, Jaramillo N, Gómez-Palacio A, Dujardin JP (2011) Morphometric and molecular differentiation of a Rhodnius robustus-like form from R. robustus Larousse, 1927 and R. prolixus Stal, 1859 (Hemiptera, Reduviidae). Acta Trop 120:103–109
Marsola-Moraes E, Melo Sene F (2004) Heritability of wing morphology in a natural population of Drosophila gouveai. Genetica 121:119–123
Minitab (2007) Minitab Inc., version 15.0. State College, PA. (www.minitab.com)
Murúa MG, Vera MT, Abraham S, Juarez ML, Prieto S, Head GP, Willinki E (2008) Fitness and mating compatibility of Spodoptera frugiperda (Lepidoptera: Noctuidae) populations from different host plant species and regions in Argentina. Ann Entomol Soc Am 101(3):639–649
Nagoshi RN, Meagher RL (2003) Fall armyworm FR sequences map to sex chromosomes and their distribution in the world indicate limitations in interstrain mating. Insect Mol Biol 12:453–456
Nagoshi RN, Meagher RL (2004) Seasonal distribution of fall armyworm (Lepidoptera: Noctuidae) host strains in agricultural and turf grass habitats. Environ Entomol 33:881–889
Orengo DJ, Prevosti A (1999) Wing size heritability in Drosophila suboscura. Heredity 82:100–106
Pashley DP (1986) Host-associated genetic differentiation in fall armyworm (Lepidoptera: Noctuidae): a sibling species complex? Ann Entomol Soc Am 79:898–904
Pashley DP (1988) The current status of fall armyworm host strains. Fla Entomol 71:227–234
Pashley DP, Martin JA (1987) Reproductive incompatibility between host strains of the fall armyworm (Lepidoptera: Noctuidae). Ann Entomol Soc Am 80:731–733
Pashley DP, Hammond AM, Hardy TN (1992) Reproductive isolating mechanisms in fall armyworm host strains (Lepidoptera: Noctuidae). Ann Entomol Soc Am 85:400–405
Pashley DP, Hardy TN, Hammond AM (1995) Host effects on developmental and reproductive traits in fall armyworm strains (Lepidoptera: Noctuidae). Ann Entomol Soc Am 88:748–755
Prowell DP, McMichael M, Silvain JF (2004) Multilocus genetic analysis of host use, introgression, and speciation in host strains of fall armyworm (Lepidoptera: Noctuidae). Ann Entomol Soc Am 97:1034–1044
Ríos-Díez JD, Saldamando-Benjumea CI (2011) Susceptibility of Spodoptera frugiperda (Lepidoptera: Noctuidae) strains from Central Colombia to two insecticides, methomyl and lambda-cyhalothrin: a study of the genetic basis of resistance. J Econ Entomol 104:1698–1705
Ríos-Díez JD, Siegfried B, Saldamando-Benjumea CI (2012) Susceptibility of Spodoptera frugiperda (Lepidoptera: Noctuidae) strains from Central Colombia to Cry1Ac and Cry1Ab. Southwest Entomol 7:281–293
Rohlf FJ (1990) Rotational fit (Procrustes) methods. In: Rohlf FJ, Bookstein FL (Eds) Proceedings of the Michigan Morphometrics Workshop. University of Michigan Museum of Zoology, Ann Arbor, Mich, p 227–236
Rohlf FJ, Slice DE (1990) Extensions of de Procrustes method for the optimal superimposition of landmarks. Syst Zool 39:40–59
Saldamando CI, Vélez-Arango AM (2010) Host plant association and genetic differentiation of corn and rice strains of Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) in Colombia. Neotrop Entomol 39:921–929
Saldamando-Benjumea CI, Estrada-Piedrahíta K, Velásquez-Vélez MI, Bailey RI (2014) Assortative mating and lack of temporality between corn and rice strains of Spodoptera frugiperda (Lepidoptera, Noctuidae) from Central Colombia. J Insect Behav 27:555–566
Salinas-Hernández H, Saldamando-Benjumea CI (2011) Haplotype identification within Spodoptera frugiperda J. E. Smith (Lepidoptera: Noctuidae) corn and rice strains from Colombia. Neotrop Entomol 40:421–430
Schölf G, Dill A, Heckel DG, Groot AT (2011) Allochronic separation versus mate choice nonrandom patterns of mating between fall armyworm host strains. Am Nat 177:470–485
Simmons LW, Kotiaho JS (2007) Quantitative genetic correlation between trait and preference supports a sexually selected sperm process. PNAS 42:16604–16608
Soto-Vivas A, Liria J, De Luna E (2011) Morfometría geométrica y filogenia en Rhodniini (Hemiptera, Reduvidae) de Venezuela. Acta Zool Mex 27:87–102
Sparks AN (1979) A review of the biology of the fall armyworm. Fla Entomol 62:82–86
Velásquez-Vélez MI, Saldamando-Benjumea CI, Ríos-Díez JD (2011) Reproductive isolation between two populations of Spodoptera frugiperda (Lepidoptera, Noctuidae) collected in corn and rice fields from Central Colombia. Ann Entomol Soc Am 104:826–833
Vélez-Arango AM, Arango RE, Villanueva D, Aguilera E, Saldamando CI (2008) Identificación de biotipos de Spodoptera frugiperda (Lepidoptera: Noctuidae) mediante marcadores mitocondriales y nucleares. Rev Colomb Entomol 34:145–150
Acknowledgments
The financial support to Clara Saldamando-Benjumea was granted by Universidad Nacional de Colombia, sede Medellín under the grant numbers 17836 and 18608. The collection of the larvae and genetic access were provided by the Ministry of the Environment of Colombia, permit number 4120E1-44703 (24 April 2008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Roberto A Zucchi – ESALQ/USP
Rights and permissions
About this article
Cite this article
Cañas-Hoyos, N., Márquez, E.J. & Saldamando-Benjumea, C.I. Heritability of Wing Size and Shape of the Rice and Corn Strains of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Neotrop Entomol 45, 411–419 (2016). https://doi.org/10.1007/s13744-016-0393-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-016-0393-y