Abstract
House flies, Musca domestica Linnaeus, and stable flies, Stomoxys calcitrans (L.) (Diptera: Muscidae), are common pests on horse farms. The successful use of pupal parasitoids for management of these pests requires knowledge of seasonal fluctuations and biology of the flies as well as natural parasitism levels. However, these dynamics have not been investigated on small equine farms. A 1-year field study began in July 2010, in north central Florida, to determine adult fly population levels and breeding areas on four small equine farms. Weekly surveillance showed that pest flies were present year-round, though there were differences in adult population levels among farms and seasons. Fly development was not confirmed on two of the four small farms, suggesting that subtle differences in husbandry may adversely affect the development of immature flies. In six substrates previously identified as the most common among the farms, stable fly puparia were found overwhelmingly in hay mixed with equine manure and house fly puparia were found in fresh pine shavings mixed with equine manure. Natural parasitism was minimal as expected, but greatest numbers of natural parasitoids collected were of the genus Spalangia. Differences in adult and immature fly numbers recovered emphasizes the need for farm owners to confirm on-site fly development prior to purchase and release of biological control agents. Additionally, due to the low natural parasitism levels and domination of parasitism by Spalangia cameroni, augmentative releases using this species may be the most effective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Filth flies, such as house flies, Musca domestica L., and stable flies, Stomoxys calcitrans (L.) (Diptera: Muscidae), commonly occur on both large and small equine farms in Florida and are considered to be major pests by horse owners. Stable flies and house flies transmit nematodes and pathogens, respectively, that cause habronemiasis (Zumpt 1973), pigeon fever (Spier et al 2004, Barba et al 2015), and other diseases in horses. In addition, stable flies inflict painful bites that can cause an increase in stress hormone (Schwinghammer et al 1986) and general irritation to horses and their owners. Consequently, the use of commercially available pupal parasitoids has increased on equine farms in an effort to reduce fly populations (Rutz & Patterson 1990, USDA 2006, Machtinger & Geden 2013). However, detailed evaluations of the effectiveness of filth fly biological control using pupal parasitoids have not been conducted on any equine farms. There are few records on the population fluctuations and habitat associations of filth flies and natural prevalence and species composition of their parasitoids on small equine farms in Florida.
Implementation of a biological control program using pupal parasitoids requires knowledge of fly seasonal abundance. The seasonal activity of stable flies on large central Florida equine farms generally extends from November through June with a peak in April (Pitzer et al 2011a). Stable fly adults are common in the winter months, decreasing in the late spring, and house flies are more abundant during the summer (LaBrecque et al 1972, Pitzer et al 2011a). It is expected, therefore, that adult fly numbers on small farms would follow similar patterns in response to environmental fluctuations, but this must be determined for biological control to be successful.
Filth fly breeding areas must be identified and monitored so parasitoids can be released before fly populations begin to increase. It is fruitless to release filth fly parasitoids on small equine farms if fly larvae do not develop and pupariate on-site. Large equine farms produce sufficient amounts of organic substances to support continuous filth fly development (Pitzer et al 2011a). Small equine farms generally have few horses; therefore, substrates suitable for oviposition and larval development are often ephemeral.
Dispersal of filth flies can make management difficult. Adult flies may be migrating from off-site locations with no development detected on-site (Ose & Hogsette 2014). Small equine farms producing attractive breeding media may experience increasing fly pressure as adults immigrate to colonize suitable substrates and produce subsequent generations. Conversely, farms with effective cultural control practices that limit larval development may experience increased fly infestations from adults dispersing from off-site locations to forage (Quarterman et al 1954).
The relationship between the parasitoid and host development systems on small equine farms in Florida needs to be better understood if recommendations for use of pteromalid parasitoids for management of house and stable flies are to be improved. To partially fill this knowledge gap, a study was conducted on the filth flies and parasitoid systems of small equine farms in North Central Florida. The objectives were to document fluctuations in adult and immature house and stable fly populations, and document the species composition and seasonal distribution of and the developmental substrates used by filth fly parasitoids.
Material and Methods
Small equine farms were defined as those between 2 and 4 ha in size with at least two horses, one stall or shade structure, open pasture, and a single-family residence. Four small equine farms (sites) located in Alachua County, Florida, were used for this study from July 2010 to June 2011. Sites were ≥8 km apart and within 1 km of facilities with cattle or horses. Site A and site B were 8 km apart and site C and site D were 11 km apart. This is characteristic of small equine farms in North and Central Florida. A rain gauge and maximum and minimum thermometer (Taylor Precision, Oak Brook, IL) were installed 90 cm above the ground on wooden stakes at each site.
These sites were managed during the study according to a set of prescribed protocols. Criteria included a density of no more than two horses per 0.4 ha of pasture, routine cleaning of run-in sheds, horses in stalls no more than 12 h per day, daily stall cleaning, no pasture fertilization, and placement and servicing of fly monitoring traps only by the researcher. Each site varied slightly in size and number of horses: site A, 4 ha with two horses; site B, 4 ha with three horses; site C, 2 ha with three horses; and site D, 2 ha with three horses. Pastures were used daily at all sites. Horses were routinely stalled at sites A and B for ≤12 h per day on sand bedding. At sites C and D, horses were stalled for ≥12 h per day on straw bedding. Waste was removed daily from stall or shed areas of sites A, B, and D and deposited in a manure pile. Waste was not managed at site C.
Adult filth fly populations were monitored with six traps placed at each site. For stable flies, three alsynite traps (Olson Products Inc., Medina, OH) (Broce 1988, Hogsette & Ruff 1990) were placed in areas where flies aggregated. These cylindrical corrugated fiberglass traps were mounted 90 cm above the ground on wooden stakes. The outer surface of each trap was covered with an adhesive-coated, clear polypropylene sleeve to capture the attracted flies (Olson Products Inc., Medina, OH). In addition, three Captivator® jar traps, each containing 30 mL Starbar® Fly Terminator Attractant (Farnum Companies Inc., Phoenix, AZ) mixed with 1 L of tap water, were placed within 1 m of the sticky traps to monitor house flies.
Fly populations were sampled weekly on Monday afternoons for 1 year beginning July 2010. On collection dates, sticky sleeves on Olson traps were replaced and used sleeves were processed in the laboratory. Captivator® traps were emptied, and the attractant and water mixtures were then replaced. Contents were processed in the field. Flies collected from all traps at each site were counted and the species, trap location, and date were recorded.
Larval and pupal development areas were identified by examination prior to initiation of the study. Because development areas often are ephemeral on small equine farms, every property was surveyed weekly for active areas of larval and pupal breeding prior to collecting flies from the traps. Development substrates, classified into six types which occurred on all of the sites and are common to small equine farms in Florida, were those described by Machtinger et al (2014). Substrates included (1) hay soiled with urine and manure (Hay), (2) pure manure (Man), (3) 0.3-cm-long pine shavings soiled with urine and manure (<12 h old) (Shav), (4) pine shavings soiled with urine and manure aged >72 h in a manure pile (MP-Shav), (5) soil bedding soiled with urine and manure aged >72 h (MP-Soil), and (6) aged soil from an overgrazed field mixed with urine and manure of variable age (DL).
Attempts were made to collect puparia weekly at each site in the substrates described above. Regardless of availability, the search for puparia was terminated after 1/2 hour or when 100 newly formed (light brown) puparia had been recovered at a site (Romero et al 2010, Pitzer et al 2011a). Puparia were collected to a depth of 10 cm with a trowel (Hogsette et al 2012), returned to the laboratory, washed, and air dried. Puparia that were damaged or had parasitoid emergence holes were discarded (Petersen & Meyer 1985). Dry puparia were placed individually in size 0 gelatin capsules and held at room temperature (22 to 25°C) for emergence of a fly or a parasitoid (Morgan et al 1989). Emerged parasitoids were identified to species using the taxonomic keys of Rueda & Axtell (1985).
Statistical analysis
Field surveillance data were normalized with a natural log transformation and subjected to analysis of variance (ANOVA) using JMP v.9 software (SAS Institute Inc., Cary, NC 2010). Back-transformed values are presented in text and tables. Means of adult fly and puparial collections from the four sites were compared between months using Tukey’s Honestly Different Separation Test (α = 0.05). The average monthly numbers of adult house and stable flies trapped at all four sites are presented graphically as a percent by month of total annual collections. Monthly precipitation and temperature were averaged by month for the sites. Overall percent parasitism was calculated by dividing the number of parasitoids emerged by the total number of undamaged puparia collected. Percent parasitism by species was calculated by dividing the number of parasitoids of each species by the total number of emerged parasitoids.
Results
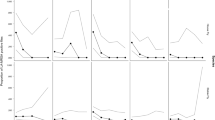
Total numbers of house flies captured at the four sites were highly variable (Table 1) and ranged from 2086 at site C to 21,806 at site D. A grand total of 30,745 house flies was captured (Table 1) and populations were present on all four sites during every month of the study (Table 2). Site D had the greatest variation in mean collection size, ranging from 9 in February 2011 to over 2503 in May 2011 (Table 2). Mean fly captures differed significantly among sites mainly during the warmer months, with the exception of July (Table 2). The greatest percentages of house flies on site C were captured in September 2010 (21.9%) and November 2010 (33.8%). The greatest percentages of house fly captures on sites A and B also occurred in November 2010. However, over 45.9% of total fly captures for the year on site D were recovered in May 2011.
The total number of stable flies captured was 5634 and all sites yielded similar numbers, ranging from 1203 at site A to 1566 at site D (Table 1). Stable fly captures were fewer peak values than house fly captures for most of the collection months, with statistical differences occurring only in April and May (Table 3). The percentages of stable fly captures peaked in March at sites A, B, and C, and in April at site D; populations subsequently decreased at all sites (Table 3).
Searching in the six substrates identified as being the most common on small horse farms, puparia were recovered only from sites C and D (Table 1). The greatest number of house fly puparia (413 of 729) was recovered from fresh pine shavings mixed with horse manure, urine, and peanut hay (Shav) (Table 4). The next largest number (169 of 729) was found in hay mixed with horse manure and urine (Hay) (Table 4). No house fly puparia were recovered from the soil-based substrates, MP-soil and DL, and fresh manure yielded only 2.2% (16 of 729) of recovered house fly puparia.
Stable fly puparia were most abundant (875 of 1078) in the hay mixed with horse manure and urine (Hay) followed by the pine shavings substrates (155 of 1078) (Shav and MP-Shav) (Table 4). Few were collected from the remaining substrates.
Although the hay plus urine plus manure (Hay) and the fresh pine shavings plus urine plus fresh manure (Shav) substrates contained more house and stable fly puparia, collectively, the highest percentage of parasitism (53%) was in puparia from fresh manure (Man) (Table 4). Shav and Hay had only 11.3 and 2.9%, respectively. Of the 729 house fly puparia collected at sites C and D combined, only 37 (5.1%) were parasitized (Table 1). Parasitoids were recovered from 78 (7.2%) of the 1078 stable fly puparia. Spalangia cameroni Perkins was the dominant parasitoid recovered from both fly species. Spalangia endius Walker and Muscidifurax raptor Girault & Sanders were also recovered from house fly puparia and a small number of Aleochora spp. was recovered in stable fly puparia from site C.
As with fly captures, there was considerable variation in environmental conditions by month and site (Fig 1). On all sites, average monthly temperatures ranged from 11.5 to 32.6°C. Total annual precipitation ranged from 94.6 cm (site B) to 101.1 cm (site A). Total precipitation differed by >5 cm among the closely located sites in 7 of the 12 months.
Discussion
The results of the present study confirm the year-round presence of adult house and stable flies on small equine farms in North Central Florida. However, development of immature flies was variable and natural parasitism was limited. The owners of each site adhered to a uniform set of site management protocols and the distance among sites was no more than 8 km, extending only 40 km from north to south. However, these results highlight the need for managers of small equine farms to personalize management and monitoring of house and stable flies prior to releasing pupal parasitoids as biological control agents, as adult populations and immature fly development were likely influenced by unique environmental conditions, management, and neighboring facilities.
Adult flies were collected throughout the study, but months of peak collection for each species differed. In this study, stable flies were more abundant in the spring months, corroborating the results of Pitzer et al (2011a) on large equine farms (>80 ha) in Central Florida and LaBrecque et al (1972). Though husbandry practices were assumed to be comparable, even small differences in sanitation practices appeared to have a profound effect on numbers of adult flies for both species. Hay round bales placed in the paddock on site C in October 2011 were presumed to be associated with an increase of stable fly collections in November 2011. Additionally, site D began to use pine shavings bedding for a pregnant mare in March 2011 and a subsequent spike in house fly collections was observed following. Adult fly captures on site A increased in September 2010, which corresponded to neighboring cattle being moved to an adjacent property for 4 weeks. Management decisions seemed to have quick and influential impacts on populations of flies and supported the individuality of each small equine farm. Further examination of how changes in management affect fly attraction to small equine farms and subsequent breeding could provide useful information on preemptive control to reduce these risks.
Variation was found among farms in filth fly pupal production. Immature fly development was found at sites C and D, but not at sites A and B, even though suitable fly development substrates (Machtinger et al 2014) were present. Pitzer et al (2011a) found puparia at every study site on large equine farms in Central Florida. However, consistent fly development occurring on large equine farms is likely a result of greater production of waste material, whereas suitable development areas on small farms can be ephemeral, depending on management. In the current study, manure from stalls and other accumulation areas was frequently removed and placed in large manure piles at sites A and B, a practice which was not employed at the other two sites where horses were not consistently stalled. There was a low ratio of horses per acre on both sites A and B as well (approximately 1:5), unlike sites C and D which had a ratio of 3:5 acres, and this may have reduced manure accumulations in pasture areas for sites A and B. These differences suggest that individual small equine farms can differ substantially in fly production based on waste management and thus control of pest flies will differ by facility.
Regardless of immature fly development, we found adult flies on all study sites in our study. This strongly suggests that the adult flies captured on sites without confirmed fly development were dispersing from nearby cattle facilities. Both house and stable flies have been shown to move several miles from development areas (Pickens et al 1967). Movement from pastured cattle to the small equine farms in the current study would not be unexpected. This further emphasizes the need for verification of on-site fly development prior to the use of pupal parasitoids as biological control agents.
Pupal collections of both fly species differed by substrate. The majority of house fly pupal collections in the current study were from the pine shavings mixed with fresh manure. Though fresh manure has been reported to be a preferred substrate of house flies (Broce & Haas 1999), pine shavings mixed with equine waste was found to be preferred for oviposition by house flies by Machtinger et al (2014) in the laboratory. The small percentage of house fly puparia collected from pure fresh manure was likely due to the limited amount available. Most manure was mixed with a bedding material or in a pasture situation where direct sunlight and isolation likely were not conducive to fly development. An overwhelming majority of stable fly collections were from the hay-based substrate with smaller numbers from the fresh pine shavings with urine and manure. The fresh pine shaving substrate with manure and the hay mixed with manure were found to be attractive to stable flies for oviposition by Machtinger et al (2014). Hay round bales have been shown to provide excellent habitat for development of stable fly immatures in pastures (Broce et al 2005), and it is likely that this is also an important breeding substrate on equine farms.
Spalangia cameroni accounted for the overwhelming majority of the emerged parasitoids collected. The high recovery of S. cameroni is consistent with the results of Pitzer et al (2011a) who recovered nearly 100% Spalangia spp. in the 2-year study on large equine farms in Central Florida. Spalangia cameroni prefers loose substrates (Smith & Rutz 1991) and has demonstrated an ability to parasitize deeper in substrates, such as those on the equine farms where puparia were collected in the present study. In addition, S. cameroni appears to be effective in locating and parasitizing hosts in equine-associated substrates (Machtinger et al. 2015, Machtinger & Geden 2013, Pitzer et al 2011b). The dominance of S. cameroni in this study and that of Pitzer et al (2011b) supports the use of this species for augmentative releases on equine farms in Florida.
Overall, the natural parasitism of house and stable fly puparia was at a minimal level, as expected. This corresponded with Pitzer et al (2011a) who found between 5 and 18% parasitism in house fly puparia and 7 to 18% parasitism in stable fly puparia on large equine farms. However, this is low compared to a study conducted by Romero et al (2010) on the University of Florida Dairy Research Unit where natural parasitism was >26%. This discrepancy is possibly due to the long-term establishment of the substrates at the Dairy Research Unit versus the ephemeral substrates at the small equine farms. Parasitism was highest in puparia from pure manure and the pine shavings + urine and manure (<12 h old), but there were too few samples with recovered parasitoids to determine if this pattern would continue in the field. It is likely that the temporary nature of suitable fly breeding substrates on small equine farms does not support high levels of parasitoid reproduction, so targeted parasitoid releases may be beneficial for maintaining controlling levels.
Temperature and rainfall have been important factors influencing dispersal, development areas, and adult fly prevalence (Larsen & Thomsen 1940, Elvin & Krafsur 1984, Krafsur et al 1985, Hogsette & Farkas 2000). Additionally, development and oviposition of parasitoids have been determined at various optimal temperatures which are species dependent (Mann et al 1990, Geden 1999). This is the first study to our knowledge to use site-specific weather stations at each equine farm in North and Central Florida. Temperature and rainfall did not differ significantly in annual totals. There was, however, variability among sites on collection dates. Although sites were close to one another, monthly precipitation totals among sites differed by as much as 5 cm. Pupal collections were too inconsistent to determine if variations in environmental conditions among sites had significant influences on total fly captures and breeding. However, on sites where flies develop, these site-specific environmental conditions could account for differences in fly emergence.
The discrepancy between large on-site adult fly numbers and low to non-existent on-site immature fly numbers is indicative of adult immigrations among farms. This emphasizes the need for farm owners to confirm on-site fly development prior to purchase and release of biological control agents. If adult flies are immigrating to the equine farm, management practices should be focused on trapping and exclusion of adults; biological control and improved sanitation measures may be more effective when fly breeding is found on the property. Due to the low natural parasitism levels and domination of parasitism by S. cameroni, augmentative releases using this species may be the most effective. The use of pupal parasitoids on equine farms has not been evaluated for effectiveness in the field but certainly warrants investigation.
References
Barba M, Stewart AJ, Passler T, Wooldridge AA, van Santen E, Chamorro M, Cattley R, Hathcock T, Hogsette JA, Hu XP (2015) Experimental transmission of Corynebacterium pseudotuberculosis serovar equi in horses by house flies. J Vet Intern Med 29:636–643
Broce AB (1988) An improved Alsynite trap for stable flies, Stomoxys calcitrans (Diptera: Muscidae). J Med Entomol 25:406–409
Broce AB, Haas MS (1999) Relation of cattle manure age to colonization by stable fly and house fly (Diptera: Muscidae). J Kansas Entomol Soc 72:60–72
Broce AB, Hogsette JA, Paisley S (2005) Winter feeding sites of hay in round bales as major developmental sites of Stomoxys calcitrans (Diptera: Muscidae) in pastures in spring and summer. J Econ Entomol 98:2307–2312
Elvin MK, Krafsur ES (1984) Relationship between temperature and rate of ovarian development in the house fly, Musca domestica L. (Diptera: Muscidae). Ann Entomol Soc Am 77:50–55
Geden CJ (1999) Host location by house fly (Diptera: Muscidae) parasitoids in poultry manure at different moisture levels and host densities. Environ Entomol 28:755–760
Hogsette JA, Farkas R (2000) Secretophagous and hematophagous higher Diptera. In: Papp L, Darvas B (eds) Contributions to a Manual of Palearctic Diptera, Volume 1 General and Applied Dipterology. Science Herald, Budapest, pp 769–792
Hogsette JA, Ruff JP (1990) Comparative attraction of four different fiberglass traps to various age and sex classes of stable fly (Diptera: Muscidae) adults. J Econ Entomol 83:883–886
Hogsette JA, Urech R, Green PE, Skerman A, Elson-Harris MM, Bright RL, Brown GW (2012) Nuisance flies on Australian cattle feedlots: immature populations. Med Vet Entomol 26:46–55
Krafsur ES, Black WC, Church CJ, Barnes DA (1985) Age composition and seasonal phenology of house fly (Diptera: Muscidae) populations. J Med Entomol 22:515–523
LaBrecque GC, Meifert DW, Weidhaas DE (1972) Dynamics of house fly and stable fly populations. Fla Entomol 55:101–106
Larsen EB, Thomsen M (1940) The influence of temperature on the development of some species of Diptera. Vidensk Medd Dansk Naturh Foren Bd 104:1–75
Machtinger ET, Geden CJ (2013) Host location by Spalangia cameroni (Hymenoptera: Pteromalidae) in equine associated substrates. Biol Control 65:130–134
Machtinger ET, Geden CJ, Hogsette JA, Leppla NC (2014) Development and oviposition preference of filth flies (Diptera: Muscidae) in six substrates from Florida equine facilities. J Med Entomol 51:1144–1150
Machtinger ET, Geden CJ, Teal PE, Leppla NC (2015) Comparison of host-seeking behavior of the filth fly pupal parasitoids, Spalangia cameroni and Muscidifurax raptor (Hymenoptera: Pteromalidae). Environ Entomol 44:330–337
Mann JA, Axtell RC, Skinner RE (1990) Temperature dependent development and parasitism rates of four species of Pteromalidae (Hymenoptera) parasitoids of house fly (Musca domestica) pupae. Med Vet Entomol 4:245–253
Morgan PB, Hoyer H, Patterson RS (1989) Life history of Spalangia cameroni (Hymenoptera: Pteromalidae) a microhymenopteran pupal parasitoid of muscoid flies (Diptera: Muscidae). J Kansas Entomol Soc 62:381–386
Ose GA, Hogsette JA (2014) Spatial distribution, seasonality and trap preference of stable fly, Stomoxys calcitrans L. (Diptera: Muscidae), adults on a 12-hectare zoological park. Zoo Biol 33:228–233
Petersen JJ, Meyer JA (1985) Evaluation of methods presently used for measuring parasitism of stable flies and house flies (Diptera: Muscidae) by Pteromalid wasps (Hymenoptera: Pteromalidae). J Kansas Entomol Soc 58:84–90
Pickens LG, Morgan NO, Hartsock JC, Smith JW (1967) Dispersal pattern of the house fly affected sanitations and weather in rural Maryland. J Econ Entomol 60:1250–1255
Pitzer JB, Kaufman PE, Hogsette JA, Geden CJ, Tenbroeck SH (2011a) Seasonal abundance of stable flies and filth fly pupal parasitoids (Hymenoptera: Pteromalidae) at Florida equine facilities. J Econ Entomol 104:1108–1115
Pitzer JB, Kaufman PE, Geden CJ, Hogsette JA (2011b) The ability of selected pupal parasitoids (Hymenoptera: Pteromalidae) to locate stable fly hosts in soiled equine bedding substrate. Environ Entomol 40:88–93
Quarterman KD, Mathis W, Kilpatrick JW (1954) Urban fly dispersal in the area of Savannah, Georgia. J Econ Entomol 47:405–412
Romero A, Hogsette JA, Coronado A (2010) Distribution and abundance of natural parasitoid (Hymenoptera: Pteromalidae) populations of house flies and stable flies (Diptera: Muscidae) at the University of Florida Dairy Research Unit. Neotrop Entomol 39:424–429
Rueda LM, Axtell RC (1985) Comparison of hymenopterous parasites of house-fly Musca domestica (Diptera, Muscidae), pupae in different livestock and poultry production systems. Environ Entomol 14:217–222
Rutz DA, Patterson RS (1990) Biocontrol of arthropods affecting livestock and poultry. Westview Press, 20 p
Schwinghammer KA, Knapp FW, Boling JA, Schillo KK (1986) Physiological and nutritional response of beef steers to infestations of the stable fly (Diptera: Muscidae). J Econ Entomol 79:1294–1298
Smith L, Rutz DA (1991) Relationship of microhabitat to incidence of house fly (Diptera: Muscidae) immatures and their parasitoids at dairy farms in Central New York. Environ Entomol 20:669–674
Spier SJ, Leutenegger CM, Carroll SP, Love JE, Pusteria JB, Carpenter TE, Mibalyi JE, Madigan JE (2004) Use of a real-time polymerase chain reaction-based fluorogenic 5′ nuclease assay to evaluate insect vectors of Corynebacterium pseudotuberculosis infections in horses. Am J Vet Res 65:829–834
(USDA) United States Department of Agriculture (2006) Equine 2005, part II: changes in the U.S. Equine Industry,1998–2005 USDA-APHIS-VS, CEAH. Fort Collins, CO #N452-0307
Zumpt F (1973) The Stomoxyinae biting flies of the world. Taxonomy, biology, economic importance and control measures. Gustav Fischer Verlag, Stuttgart, 175 pp
Acknowledgments
We thank Dr. Chris Geden for his review of this manuscript. This work was supported in part by funding from the USDA, NIFA, Extension Integrated Pest Management Coordination and Support Program (EIPM-CS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Patrícia Thyssen – UNICAMP
Rights and permissions
About this article
Cite this article
Machtinger, E.T., Leppla, N.C. & Hogsette, J.A. House and Stable Fly Seasonal Abundance, Larval Development Substrates, and Natural Parasitism on Small Equine Farms in Florida. Neotrop Entomol 45, 433–440 (2016). https://doi.org/10.1007/s13744-016-0376-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-016-0376-z