Abstract
The presence of some specific drugs in animal tissues may affect the time of minimal postmortem intervals estimated during forensic entomological investigations. To test the effects of a specific drug on decomposition, a field study was conducted at Fayoum University campus, Egypt, from March to May 2013, using tramadol, a synthetic analgesic opioid used to treat moderate to severe pain in humans. Albino rats were used as the animal model during this study. The duration of the fresh stage of tramadol treated rat (Ttr) carcasses was significantly shorter (2.4 ± 0.27 days) compared to tramadol free rat (Tfr) carcasses (6.4 ± 0.49 days). The dry carcass stage of Ttr lasted longer (10.3 ± 0.99 days) as compared to (7.4 ± 0.18 days) the Tfr carcass. The decomposition process of the (Ttr) carcass was not significantly faster (24.9 ± 1.58 days) as compared to (Tfr) carcasses (29.5 ± 1.69). Lucilia cuprina (Wiedemann), Chrysomya albiceps (Wiedemann), and Musca domestica L. were less attracted to Ttr carcass-baited traps than traps with Tfr carcasses. However, females of Sarcophaga spp. showed a greater attraction to Ttr carcasses. Females of another sarcophagid fly, Wohlfahrtia spp. exhibited similar attraction tendencies to both types of trap baits. Larvae of S. argyrostoma (Robineau-Desvoidy) collected from Ttr carcasses developed to a significantly longer total body length (10.4 ± 0.04 mm) as compared to the average length of the larvae collected from Tfr carcasses (8.9 ± 0.34 mm). During days 9–13 after rat death, the relative lengths of larvae from Ttr carcasses were not significantly different from Tfr carcasses. Larvae fed on Ttr carcasses pupated 2 days later than the control larvae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tramadol is a synthetic analgesic opioid used to treat moderate to severe pain in humans (Katz 1996). Tramadol was initially introduced as a medication for cancer patients (Sacerdote et al 2000). When taken in overdose amounts, significant morbidity and mortality can occur (Clarot et al 2003, Rossi 2013). Additionally, fatal intoxication with tramadol may occur unintentionally (Pothiawala & Ponampalam 2011). The WHO Uppsala Monitoring Centre reported 253 cases of death (1%) and 12 cases of sudden death (0.05%) out of 22,753 reported adverse effect cases over a 2-year period worldwide (WHO 2006). In Egypt, tramadol is generally affordable and readily provided inexpensively. The drug became a prescriptional only in 2004 (WHO 2006, Fawzi 2011). Egyptians use tramadol as a recreational drug, and also reported as an aphrodisiac but most commonly as energy boost (Salem et al 2008, Fawzi 2011). During 2010, a total of 325 out of 640 cases (50.8%) of tramadol abuse overdose were recorded. Moreover, 109 suicides were linked to tramadol overdoses (17%, Fawzi 2011).
After a corpse or a carcass is exposed to the environment, usually, a predictable pattern of insect succession occurs under certain air temperature conditions (Catts & Goff 1992, Matuszewski et al 2008, 2010, AbouZied 2014). Forensic entomologists can estimate the minimal postmortem intervals (PMI) based on the development age of fly larvae present on a corpse at time of discovery (Amendt et al 2005, Verma & Paul 2013). The rate of decomposition and the relative sequence of attraction of carrion entomofauna can vary when certain chemicals are present in the tissues of the corpse (Catts & Goff 1992, Bourel et al 1999, Goff & Lord 2001, Clark et al 2006).
The objectives of this study were to determine (1) if the presence of tramadol metabolites in animal tissues affected the rate of decomposition as mediated by insects, potentially causing errors in estimating PMI, (2) if the presence of tramadol in tissues of carcasses either repelled or attracted flies of forensic importance, and (3) if tramadol presence affected the relative rate of development of fly larvae.
Material and Methods
Study area
The current study was conducted at the campus of Fayoum University, Egypt (29.32°N, 30.84°E, 52 m a.s.l) during March–May 2013. A digital maximum minimum thermo-hygrometer was used to record daily temperature and the relative humidity (RH%).
Animal model
The laboratory rat, Rattus norvegicus (Wistar albino strain), was used in this study. Fifty rats were used with an average weight of 203 ± 12.9 g and were kept at the animal house of Zoology Department, under controlled temperature (25–27°C) and relative humidity (30–40%). The Committee of Zoology Department, Faculty of Science, Fayoum University, on Animal Research and Ethics (FU-CARE) permitted the use of the experimental rats; approved and monitored the method of euthanizing the animals (permission # I25-013). FU-CARE follows the CITES no. 123 of 18 March 1986 and 2005 revision of the European convention for the protection of vertebrate animal used for experimental and other scientific purposes (http://conventions.coe.int/Treaty/EN/Reports/HTML/123.htm) and the Commission Recommendation of 18 June 2007 on guidelines for the accommodation and care of animals used for experimental and other scientific purposes (C (2007) 2525: http://ec.europa.eu/transparency/regdoc/rep/3/2007/EN/3-2007-2525-EN-1-0.Pdf).
Dose determination
Tramadol hydrochloride tablets (200 mg) (Zydol® SR Tabs; Grünenthal Ltd, UK) were provided by Prof Mohamed Abdel-Hakim (Faculty of Medicine, Al-Azhar University, Egypt). The maximum recommend adult human dose is two tablets/day (http://www.drugs.com/pro/Tramadol-tablets.html). Each tablet (weight 0.35 ± 0.03 g) was ground in a dry-sterilized porcelain mortar with pestle. The corresponding dosage for the albino rats was calculated according to Paget and Barnes (1964) as equal to 6.3 mg/200 g rat. An overdose of 12.6 mg/200 g dissolved in 0.2 ml distilled water was administered to rats twice a day. Each rat was injected intraperitoneal.
Stages of decomposition
Twenty albino rats were weighted and divided into two groups of 10 rats each. One group was injected twice a day with 0.2 ml of isotonic 0.9% saline solution as control (tramadol-free rat; Tfr). The second group was injected twice a day with 0.2 ml of 12.6 mg/200 g rat (Ttr) until the death of each individual. Tfr rats were killed by cervical dislocation (Cressey 2013) not damaging the external tissue including the head. This protocol is recommended by CITES No. 123. After the death of the Ttr rats (4–6 days), a whole rat was transferred to a metal box of 0.75 × 0.5 × 1 m with a metal mesh cover and base, and labeled with date of death. Boxes were placed 500 m from each other. Boxes were placed in five garden sites in Fayoum University Campus. Boxes were examined daily to observe the stages of decomposition of each carcass. The experiment lasted from 24 March to 20 April, 2013. The pattern of the stages of decomposition was determined according to Gennard (2007), Matuszewski et al (2008, 2010), and Wang et al (2008).
Field collection of the larvae
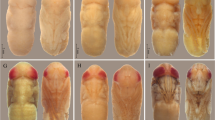
The previous experiment was repeated (during 24 April–20 May). Ten rats were used, one group (five rats) was used as the control (Tfr), and the other group of five rats was treated carcasses (Ttr) as described before. After the end of the bloat stage, larvae were detected invading the body cavity of the rat carcass. Collection of the fly larvae was carried out at random, using a metal scoop with a bowl of 5 cm3. Collected larvae were killed by immersion in boiling water and preserved in 75% ethanol until identification. Larvae were identified according to Zumpt (1965) and Szpila (2010). Sarcophaga argyrostoma (Robineau-Desvoidy) larvae were identified according to Greene (1925) and Draber-Mońko et al (2009). Two sets (20 larvae each) were collected from Tfr and Ttr carcasses. Collected larvae were reared in the laboratory to reach the adult stage, in order to confirm the identification.
Larval development
Inside the laboratory (24°C and 30–35% RH), two sets of five rats each were prepared. One set was used as the control (Tfr), the other set of five rats was injected with tramadol (Ttr). Since S. argyrostoma larvae were dominant among both sets of the rat carcasses in the field experiment, this species was used to study the effect of the tramadol on larval development. Each rat carcass was isolated inside (2000-ml) glass bottle, covered with muslin, and fastend with rubber band. Each glass bottle was supplied with 20 laboratory reared virgin, 10-day-old S. argyrostoma adults (10 males and 10 females). Adults were chosen at random as reared from each of the five rats. A small vial of wet cotton and a small vial of sugar crystals were, also, placed inside each glass bottle. Adult flies were removed 48 h later. Glass bottles were examined for larvae. Collection of the larvae was carried out during 5, 9, 13, and 16 days after rats were killed. Using a spoon with a bowl of 5 cm3, larvae were randomly chosen from each glass bottle. Larvae were fixed by immersion in boiling water, labeled as control or treated, and preserved in 75% ethanol. The maximum body lengths of S. argyrostoma larvae were determined according to Day and Wallman (2006). Statistical comparisons between the mean lengths of S. argyrostoma larvae collected from Tfr and Ttr groups were carried out using Student’s t test on SPSS software (version 15; SPSS, Chicago, Illinois, USA).
Collection of adult flies
Two groups of yellow plastic fly traps were used. Each group comprised three traps. The first group was baited with three Tfr carcasses, whereas the second was baited with three Ttr carcasses. The traps were hung 1.5 m from the soil surface, distributed 500 m apart from each other, labeled with death date, and type of bait. All the traps were exposed to a sunny unshaded area on the campus of Fayoum University. This experiment lasted for 10 days. The experiment was repeated twice, during March 19–29 and April 12–22 2013).
Traps were transported daily to the insectary of the Department of Zoology, Faculty of Science, Fayoum University. Traps containing live flies were placed in a freezer for 30 min to kill them. Adult flies were removed, counted, sorted to families, dried, mounted, and pinned for further identification. Calliphorids were identified according to Zumpt (1965) and Shaumar et al (1989). Adult sarcophagids were identified to the generic level according to Lehrer (2006). The identification of forensically important Sarcophagidae is based on examination of only males and required taxonomic expertise (Jordaens et al 2013). All counts of female sarcophagid species were listed as Sarcophaga spp. or Wohlfahrtia spp.
Since the data differences of the collected flies from the different traps were not normally distributed, fly catch was expressed as percentage of adults/trap/day. Statistical comparisons between fly catch of both Tfr and Ttr rat carcasses were examined with Z test in GraphPad® Prism 4 software (GraphPad Software, Inc. La Jolla, CA, USA).
Results
Average temperatures of 21.1 ± 0.6, 22.1 ± 0.6, and 24.7 ± 0.4°C were recorded during March, April, and May, respectively. The relative humidity varied over a narrow range, almost constant during March (30 ± 2.2%) and April (30 ± 2.1%), with slight increase during May (31 ± 1.96%). Wind velocity ranged from 28 ± 0.9 km/h during March up to 31 ± 0.7 km/h during May. No precipitation was reported during the study period (Egyptian Meteorological Authority, Cairo, Egypt with permission).
The duration of the fresh stage was significantly shorter for the Ttr carcasses (2.40 ± 0.27 days) than for the Tfr carcasses (6.4 ± 0.49 days, t = 7.07, df = 18, p = 0.0001) (Table 1). No significant difference was observed during the duration of the bloat stage of Ttr carcasses (4.7 ± 0.87 days) as compared to the bloat stage of Tfr (7.0 ± 0.73 days; p = 0.058). Also, the duration of the active decay stage of Trt carcasses was not significantly shorter (7.4 ± 0.91 days; t = 0.553, p = 0.587) as compared to 8.3 ± 1.35 days in case of Tfr carcasses. During the dry carcass stage, Ttr carcasses exhibited a significantly longer duration (10.3 ± 0.99 days; t = 2.83, df = 18, p = 0.017,), as compared to 7.4 ± 0.18 days in those of Tfr carcasses. The overall decomposition of the Ttr carcasses was not significantly faster (24.9 ± 1.58 days) as compared to Tfr carcasses (29.5 ± 1.69 days; t = 1.98, df = 18, p = 0.06).
During field collection, fly larvae of three species were detected after the end of the bloat stage. Larvae of Lucilia cuprina (Wiedemann) and Chrysomya albiceps (Wiedemann) were detected first in both the controlled and treated rat carcasses. Larvae of S. argyrostoma were collected subsequently after the two above calliphorid species. Once S. argyrostoma larvae appeared, larvae of L. cuprina and C. albiceps became uncommon.
Larvae of S. argyrostoma from Ttr rat carcasses developed to a significantly longer length by day 5 after rat death (10.4 ± 0.04 mm; t = 2.19, df = 21, 43, p = 0.03) as compared to the average length (8.9 ± 0.34 mm) of the larvae collected from Tfr carcasses (Table 2). On day 9, the average body length of the larvae collected from Ttr carcasses was not significantly different (11.6 ± 0.76 mm; t = 1.59, df = 30, 51, p = 0.11) from that of the Tfr carcasses (13.3 ± 0.71 mm). Thirteen days after rat death, the average length of the larvae (13.4 ± 0.43 mm) from Ttr carcasses was not significantly smaller (t = 1.08, df = 49, 69, p = 0.28) than those from Tfr carcasses (14 ± 0.34 mm). Larvae fed on the Tfr rat carcasses developed successfully on day 16 to pupae, whereas the larvae fed on Ttr rat carcasses developed into pupae on day 18.
The traps baited with Ttr carcasses attracted significantly lower percentage of adult flies (42.4%) as compared to those collected from the traps baited with Tfr carcasses (57.6%; Z test = 2.97, df = 380, p = 0.004) (Table 3). Both L. cuprina as well as the Musca domestica L. were not present in Ttr rat carcass-baited traps. The average number of adults of C. albiceps attracted to Tfr baited traps (26 adults/trap/day; 23.6%) was significantly higher than those attracted to Ttr baited traps (6 adults/trap/day, 7.4%; Z test = 2.97, df = 189, p = 0.004). Of the 480 adult sarcophagids, only six were males and these were identified as S. argyrostoma. The average number of female Sarcophaga spp. was significantly more abundant (32 adults, 39.5%; Z test = 3.93, df = 189, p = 0.0003) at Ttr rat carcasses as compared to those collected from Tfr-baited traps (16 adults, 14.5%) (Table 3). There was no significant difference (Z test = 1.04, df = 189, p = 0.298) between the number of adult females of Wohlfahrtia spp. attracted to Tfr carcasses (Tfr, 50 females, 45.4%) and Ttr carcasses (Ttr, 43 females, 53.7%).
Discussion
A normal dose of tramadol for humans is reported to be completely absorbed in the blood within 2 h. Meanwhile, tramadol and its metabolites are excreted by the kidneys within a mean half-life of 5 h (Karhu et al 2007 http://www.drugs.com/pro/Tramadol-tablets.html). During the current study, albino rats were injected with an overdose (double the normal therapeutic dose). According to Pothiawala & Ponampalam (2011), higher doses are usually associated with classic opioid toxicity including respiratory depression and cardiovascular collapse. Meanwhile, Clarot et al (2003), Marquardt et al (2005), and Rossi (2013) indicated that tramadol overdose is known to be associated with significant morbidity and mortality.
The duration of the fresh stage of tramadol treated rat carcasses lasted for 2.4 ± 0.27 days, as compared to Tfr carcasses (6.4 ± 0.49 days). However, the total decomposition rate of Ttr carcasses was not significantly faster than the Tfr carcasses during our study period (March–May, i.e., spring season). In contrast, Wyman et al (2011) reported that during exposure to summer temperatures, pig carcasses injected with four different cocktails of 16 drugs decomposed more rapidly. Each cocktail contained one of the following opioids morphine, methadone, propoxyphene, and oxycodone mixed with one of each of the following groups: tricyclic antidepressant (TCA; amitriptyline and doxepin), serotonin selective reuptake inhibitor (SSRI; citalopram, fluoxetine, and venlafaxine), and benzodiazepine (diazepam). The authors reported that after 2 days, blood, heart, kidneys, brain, and vitreous were no longer present, but drug residues in the liver and muscles persisted for 1 week.
The minimum PMI can be estimated by using the temperature data and the stage of development of the specific species of fly larvae at the time of collection (Parry et al 2011). PMI estimates need to be accurate during legal proceedings. The rate of fly larval development can be affected if tissues of the carcass include drug residues. Such effects depend on the type of the drug, its concentration, and the insect species (Parry et al 2011). Carvalho et al (2001) concluded that the presence of foreign substances such as cocaine in corpses can alter the developmental rates of fly larvae affecting PMI.
Five days after the death of the Ttr rats, larvae of S. argyrostoma had a significantly longer body length compared to that of the larvae fed on Tfr rat tissues. Goff et al (1991) reported a rapid development of the sarcophagid maggots of Boettcerisca pergrina (Robineau-Desvoidy) fed on heroin treated rabbit tissues as compared to the control, under laboratory conditions. El-Samad et al (2011) reported larger sized L. sericata larvae that fed on rabbit tissues treated with tramadol during 30, 97, and 152 h, compared with the control under laboratory conditions. Carvalho et al (2001) reported that larvae of C. albiceps and C. putoria exposed to cocaine-treated liver developed more rapidly than the corresponding larvae fed on non-treated liver, after 54 h of exposure. After 30–42 h, these authors reported that larvae of C. putoria fed on cocaine-treated liver developed more rapidly than larvae fed on untreated liver (control). However, after 30–42 h, there were no differences in the average weight of C. albiceps larvae fed on untreated or treated liver (Carvalho et al 2001).
On days 9–13 after the death of the rats, the average lengths of the larvae fed on treated rat carcasses were not significantly smaller than those fed on free rat carcasses. Similarly, George et al (2009) revealed that the length and the width of C. stygia larvae fed on pet minced food treated with three different morphine concentrations showed insignificant differences compared to the length and the width of the larvae fed on pet minced free food. In contrast, Goff et al (1993) found that larvae fed on rabbit tissues treated with methamphetamine were smaller in length but reached the maximum length earlier than those fed on the control tissues.
On day 16, larvae fed on Ttr rat carcasses reached its maximum length of 14.5 ± 0.2 mm. However, on day 16, the larvae fed on Tfr carcasses formed pupae more rapidly. It appears that larvae of S. argyrostoma fed on Ttr rat carcasses had delayed pupal development. The presence of drugs including tramadol and toxins can alter the developmental rates of carrion feeding insects on tissues from cadavers (Catts & Goff 1992, Goff et al 1992, Bourel et al 1999, Goff & Lord 2001, Byrd & Castner 2010, O’Brien and Turner 2004, Tabor et al 2004).
Adults of L. cuprina, C. albiceps, and M. domestica were less attracted to Ttr carcasses. Since the fresh stage duration was longer (6.4 ± 0.49 days) in the Tfr carcasses, it apparently indicated that adult calliphorid females had a longer opportunity to locate these carcasses. Similarly, Amendt et al (2005), Smith (1989), and Anderson & VanLaerhoven (1996) confirmed that female calliphorids visit carrion often within a few hours after carrion exposure, i.e., during the fresh stage. Also, Anderson & VanLaerhoven (1996) reported that the closely related L. illustris are known as primary colonizers of the exposed fresh stage of a carcass. Voss et al (2009) mentioned that adult C. albiceps was considered also as a first colonizer. Voss et al (2009) found that species of Sarcophagidae were secondary colonizers of carcasses during the bloat stage in all seasons except summer.
In this study, at the beginning of the bloat stage (2.4 ± 0.27 days), a distinctive necrotic odor was apparent. The prevalence of the necrotic odor seemed to be a key point for Sarcophaga spp. attraction, which increased the chance for the female sarcophagid flies to invade the Ttr baited traps earlier (about 4 days) before being oriented toward the Tfr-baited traps, since the bloat stage began after (6.4 ± 0.49 days). The total catch of sarcophagid flies was higher in the Ttr-baited traps. Possibly, tramadol metabolites accumulated inside the tissues of the rat carcasses increased the necrotic odor of the carcass’s tissues, more than in the case of normal control tissues, leading to more attraction of Sarcophaga spp. females. But, this hypothesis requires further investigation and chemical analysis in the future. Adult females of Wohlfahrtia spp. were nearly equally distributed between both traps either untreated or treated rat carcasses.
Adult and larvae of S. argyrostoma could be used as a possible taxon for determination of minimal PMI in Fayoum Province, Egypt. In the case of drug (tramadol) abused corpses, the accurate determination of the death should be corrected (2 days more) when S. argyrostoma larvae are present. Additionally, the calliphorids L. cuprina and C. albiceps also are useful taxa for the determining PMI estimates.
References
AbouZied E (2014) Insect colonization and succession on rabbit carcasses in Southwestern Mountains of the Kingdom of Saudi Arabia. J Med Entomol 51:1168–1174
Amendt J, Krettek R, Zehner R (2005) Insekten auf Leichen: Forensische Entomologie. Biol Unserer Zeit 35:232–240
Anderson G, VanLaerhoven S (1996) Initial studies on insect succession on carrion in Southwestern British Columbia. J Forensic Sci 41:617–625
Bourel B, Martin-Bouyer L, Hedouin V, Cailliez J, Derout D, Gosset D (1999) Necrophilous insect succession on rabbit carrion in sand dune habitats in Northern France. J Med Entomol 36:420–425
Byrd J, Castner J (2010) Insects of forensic importance. 2nd Edn. pp. 39–79. In: Byrd JH & Castner JL (ed.) Forensic entomology: The Utility of Arthropods in Legal Investigations, p 681
Carvalho L, Linhares X, Trigo J (2001) Determination of drug levels and the effect of diazepam on the growth of necrophagous flies of forensic importance in Southern Brazil. Forensic Sci Int 120:140–144
Clarot F, Goullé J, Vaz E, Proust B (2003) Fatal overdoses of Tramadol: is benzodiazepine a risk factor of lethality? Forensic Sci Int 134:57–61
Catts E, Goff L (1992) Forensic entomology in criminal investigations. Annu Rev Entomol 37:253–272
Clark K, Evans L, Wall R (2006) Growth rates of the blowfly, Lucilia sericata, on different body tissues. Forensic Sci Int 156:145–149
Cressey D (2013) Best way to kill lab animals sought. Nature 500:130–131
Day M, Wallman J (2006) Width as an alternative measurement to length for post-mortem interval estimations using Calliphora augar (Diptera: Calliphoridae) larvae. Forensic Sci Int 159:158–167
Draber-Mońko A, Malewski T, Pomorski J, Slipinski LM (2009) On the morphology and mitochondrial barcoding of the flesh fly Sarcophaga (Liopygia) argyostoma (Robineau-Desvoidy, 1830) (Diptera: Sarcophagidae) an important species in forensic entomology. Ann Zool 59:465–493
El-Samad M, El-Moaty A, Makemer M (2011) Effects of Tramadol on the development of Lucilia sericata (Diptera: Calliphoridae) and detection of the drug concentration in postmortem rabbit tissues and larvae. J Entomol 8:353–364
Fawzi M (2011) Some medicolegal aspects concerning Tramadol abuse: The new Middle East youth plague 2010. An Egyptian overview. Egypt J Forensic Sci 1:99–102
Gennard D (2007) Forensic entomology: an introduction 2nd Edition. John Wiley-Blackwell, Hoboken, New Jersey, p 272
George K, Archer M, Green L, Conlan X, Toop T (2009) Effect of morphine on the growth rate of Calliphora stygia (Fabricius) (Diptera: Calliphoridae) and possible implications for forensic entomology. Forensic Sci Int 193:21–25
Goff M, Brown W, Hewadikaram K, Omori A (1991) Effect of heroin in decomposing tissues on the developmental rate of Boettcherisca peregrina (Diptera: Sarcophagidae) and implications of this effect on estimation of postmortem intervals using arthropod development patterns. J Forensic Sci 36:537–542
Goff M, Lord W (2001) Entomotoxicology: insects as toxicological indicators and the impact of drugs and toxins on insect development. In: Byrd, J.H. & Castner, J.L. (Ed.) Forensic Entomology: The Utility of Arthropods in Legal Investigations, pp 331–340
Goff M, Brown W, Omri A (1992) Preliminary observations of the effect of methamphetamine in decomposing tissues on the development of Parasarcophaga ruficornis (Diptera: Sarcophagidae) and implications of this effect on the estimations of postmortem intervals. J Forensic Sci 37:867–872
Goff M, Brown W, Omori A, La Pointe D (1993) Preliminary observations of the effect of amitriptyline in decomposing tissues on the development of Parasarcophaga ruficornis (Diptera: Sarcophagidae) and implications of this effect on the estimations of postmortem intervals. J Forensic Sci 38:316–322
Greene C (1925) The puparia and larvae of sarcophagid flies. Proc U S Nat Mus 66:1–26
Jordaens K, Sonet G, Richet R, Dupont E, Braet Y, Desmyter S (2013) Identification of forensically important Sarcophaga species (Diptera: Sarcophagidae) using the mitochondrial COI gene. Int J Legal Med 127:491–504
Karhu D, El-Jammal A, Dupain T, Gaulin D, Bouchard S (2007) Pharmacokinetics and dose proportionality of three Tramadol Contramid OAD tablet strengths. Biopharm Drug Dispos 28:323–30
Katz W (1996) Pharmacology and clinical experience with Tramadol in osteoarthritis. Drugs 52(Suppl 3):39–47
Lehrer A (2006) Sarcophaginae et Paramacronychiinae du Proche Orient (Insecta, Diptera, Sarcophagidae). Pensoft, Sofia-Moscow, p 264
Marquardt K, Alsop J, Albertson T (2005) Tramadol exposures reported to statewide poison control system. Ann Pharmacother 39:1039–1044
Matuszewski S, Bajerlein D, Konwerski S, Szpila K (2010) Insect succession and carrion decomposition in selected forests of Central Europe. Part I: Pattern and rate of decomposition. Forensic Sci Int 194:85–93
Matuszewski S, Bajerlein D, Konwerski S, Szpila K (2008) An initial study of insect succession and carrion decomposition in various forest habitats of Central Europe. Forensic Sci Int 180:61–69
O’Brien C, Turner B (2004) Impact of paracetamol on Calliphora vicina larval development. Int J Legal Med 118:188–189
Paget G, Barnes J (1964) Evaluation of drug activities. In: Lawrence DR, Bacharacg (eds) Pharmacometrics (vol. 1). Academic, New York, p 161
Parry S, Linton SM, Francis S, O’Donnell M, Toop T (2011) Accumulation and excretion of morphine by Calliphora stygia, an Australian blow fly species of forensic importance. J Insect Physiol 57:62–73
Pothiawala S, Ponampalam R (2011) Tramadol overdose: a case report. Proc Singapore Healthc 20:219–223
Rossi S (2013) Australian Medicines Handbook, 13th edn. The Australian Medicines Handbook Unit Trust, Adelaide, p 965
Sacerdote P, Bianchi M, Gaspani L, Manfredi B, Maucione A, Terno G, Ammatuna M, Panerai A (2000) The effects of Tramadol and morphine on immune responses and pain after surgery in cancer patients. Anesth Analg 90:1411–1414
Salem E, Wilson S, Bissada N, Delk J, Hellstrom W, Cleves M (2008) Tramadol HCL has promise in on demand use to treat premature ejaculation. J Sex Med 5:188–193
Shaumar N, Mohammad SK, Mohammad SA (1989) Keys for the identification of species of Family Calliphoridae (Diptera: Egypt). J Egypt Soc Parasitol 19:669–681
Smith KGV (1989) An introduction to the immature stages of British flies 10 (14). Royal Entomological Society of London, p 259
Szpila K (2010) Key for identification of third instars of European blowflies (Diptera, Calliphoridae) of forensic importance. In: Amendt, J., Goff, M.L., Campobasso, C.P, Grassberger, M. (ed.) Current concepts in forensic entomology, pp 43–56
Tabor K, Brewster C, Fell R (2004) Analysis of the successional patterns of insects on carrion in southwest Virginia. J Med Entomol 41:785–95
Verma K, Paul MP (2013) Assessment of post mortem interval, (PMI) from forensic entomotoxicological studies of larvae and flies. Entomol Ornithol Herpetol 2:104. doi:10.4172/216-0983.1000104
Voss S, Spafford H, Dadour I (2009) Annual and seasonal patterns of insect succession on decomposing remains at two locations in Western Australia. Forensic Sci Int 193:26–36
Wang J, Zhignang L, Chen Y, Qiangsheng C, Yin X (2008) The succession and development of insects on pig carcass and their significant PMI in south China. Sci Int 179:11–18
WHO (2006) 34th ECDD 2006/4.5: assessment of tramadol. Available from: http://www.who.int/medicines/areas/quality_safety/5.2TramadolCritReview.pdf. Last retrieved at December 09, 2014
Wyman J, Dean D, Yinger R, Simmons A, Brobst D, Bissell M, Silveira F, Kelly N, Shott R, Ohr J, Howard R, Lewis B (2011) The temporal fate of drugs in decomposing porcine tissue. J Forensic Sci 56:694–699
Zumpt F (1965) Myiasis in man and animals in the Old World. Butterworths, London, p 267
Acknowledgments
Prof. Mohamed Abdel-Hakim (Fac. Med., Al-Azhar Univ., Egypt) is sincerely thanked for the statistical analyses. Many thanks are due to Dr. Magdi El-Hawagry (Entomology Dept., CU, Egypt), Prof. Thomas Pape (The Natural History Museum of Denmark), and Dr. John Deeming and Prof. Hasan Dawah (National Museum of Wales, Cardiff, UK) for helping in adult fly identification. Prof. Thomas Miller (UCR, USA) and Prof. Boris Kondratieff (CSU, USA) are wholeheartedly thanked for their valuable critical reading of the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Patrícia J Thyssen – UNICAMP
Rights and permissions
About this article
Cite this article
AbouZied, E.M. Postmortem Attraction of Sarcosaprophagous Diptera to Tramadol-Treated Rats and Morphometric Aspects of the Developed Larvae. Neotrop Entomol 45, 326–332 (2016). https://doi.org/10.1007/s13744-016-0375-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-016-0375-0