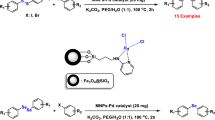
Abstract
Research on the preparation of diallyl sulfides and selenides is always an important challenge among chemists because these compounds are of high biological, pharmaceutical, industrial and chemical importance. For this purpose, in this attractive and highly efficient approach, we wish to report that copper (I) chloride immobilized on magnetic nanoparticles modified with benzothiazole–pyrimidine ligand (Fe3O4@BTH-Pyr-CuCl) is a novel and efficient magnetically recoverable catalyst for C–S and C–Se bonds formation through reaction of a category of heterocyclic compounds with aryl iodides, sulfur and selenium sources. The structure of Fe3O4@BTH-Pyr-CuCl nanocatalyst was well identified with FT-IR, SEM, TEM, EDX, elemental mapping, TGA, XRD, VSM and ICP-OES techniques. The recycling tests confirmed that the Fe3O4@BTH-Pyr-CuCl nanocatalyst was reused for 6 times without considerable reduction in its activity. This method is almost better than other methods reported in the literature for C–S and C–Se coupling of heterocycles for the following reasons, such as the use of an environmentally friendly solvent, high yields of products, the use of a catalyst that can be separated and reused and the performance of the reaction in a shorter time, presentation of well-analysis for catalyst and full NMR for products.
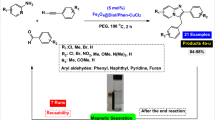
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The immobilization of catalyst in particular transition metals on the surface of solid supports is one of the popular and efficient strategies in catalysis field [1, 2]. Therefore, choosing a suitable and active solid support plays an important role in the performance of the catalyst [3]. An ideal catalyst from the point of view of green chemistry should have a large active surface and be separable [4]. The high active surface along with the ability to separate the catalyst at the end of the reaction has made nanocatalysts a bridge between homogeneous and heterogeneous catalysts [5,6,7,8]. In metallic nanocatalysts, the nanomaterial support and the metal catalyst together form a nanocomposite that is suitable for achieving the best performance [9,10,11,12,13]. Recently, magnetic nanoparticles are widely used as a catalyst support, because these nanoparticles have features such as catalytic capacity, high stability and strength, easy recovery, biocompatibility and low toxicity [14,15,16,17]. Magnetic nanoparticles with a core–shell structure form a new type of catalysts, whose shell contains catalytically active species, and the magnetic core acts as a holder that allows separation and recovery of the catalyst [18,19,20]. One of the most attractive and popular features of magnetic nanocatalysts is the easy separation of this catalyst from the reaction mixture [21, 22]. The catalyst fixed on the magnetic nanoparticles can be easily separated from the reaction medium by an external magnet and can be reused [23,24,25]. Among the magnetic nanoparticles used as a support for the immobilization of transitional metal complexes as catalyst, Fe3O4 nanoparticles are more popular because they are available, their preparation is easier, their surface modification is easily possible, and they have high stability and magnetic properties [25,26,27,28].
The formation of carbon–sulfur bonds is an essential step in the preparation of important organic compounds and intermediates [29, 30]. Diaryl sulfides are one of the most important sulfur-containing organic compounds that play a unique role in chemistry, biochemistry, and organic synthesis [31,32,33]. These compounds are used as multipurpose reagents in organic synthesis and are useful structural parts for the synthesis of sulfur-containing organic compounds [34,35,36]. Aryl sulfides have shown activities as anti-inflammatory agents, treatment of diabetes, Alzheimer’s disease and Parkinson’s disease, or as inhibitors for the treatment of human immunodeficiency virus, asthma and obstructive pulmonary disease [37,38,39]. On the other hand, the synthesis of diaryl selenides has recently become a very attractive and important research field in organic chemistry [40, 41]. Aryl selenides play an important role in medicinal and biological chemistry in particular as anticancer and antiviral drugs [42,43,44,45]. The utilization of sulfur and selenium sources for the C–S and C–Se coupling reactions is one of the most popular and efficient strategies for the preparation of diaryl sulfides and selenides [46]. In the past decades, reactions in which transition metals were used as catalysts have played an important role in the progress of organic chemistry. Copper is one of the most popular and widely used transition metals, which has been recently used for coupling reactions, due to its characteristics such as being cheap, environmentally friendly and having various oxidation states [47, 48].
In this attractive and highly efficient approach, we fabricated copper (I) chloride immobilized on magnetic nanoparticles modified with benzothiazole–pyrimidine ligand (Fe3O4@BTH-Pyr-CuCl) and evaluated its catalytic activity for the preparation of heteroaryl-aryl sulfides and selenides through reaction of a category of heterocyclic compounds with aryl iodides, sulfur and selenium sources.
Result and discussion
Details of fabrication of Fe3O4@BTH-Pyr-CuCl nanocatalyst are shown in Scheme 1. First, magnetic Fe3O4 nanoparticles were coated with 3-amino-4-mercaptobenzoic acid in order to prepare the Fe3O4@AMBA nanocomposite. Next, Fe3O4@BTH-Pyr nanomaterial as ligand was prepared through treatment of Fe3O4@AMBA nanocomposite with pyrimidine-2-carbaldehyde in ethanol under reflux conditions. Finally, CuCl was successfully immobilized on Fe3O4@BTH-Pyr ligand in order to fabricate the Fe3O4@BTH-Pyr-CuCl nanocatalyst.
Characterization of Fe3O4@BTH-Pyr-CuCl nanocatalyst
The structure of Fe3O4@BTH-Pyr-CuCl nanocatalyst was well identified with FT-IR, SEM, TEM, EDX, elemental mapping, TGA, XRD, VSM and ICP-OES techniques.
XRD and TGA analysis
In order to study the structure and nature of Fe3O4@BTH-Pyr-CuCl nanocatalyst, XRD analysis was used. XRD spectra of Fe3O4@BTH-Pyr-CuCl nanocatalyst are shown in Fig. 1. The X-ray analysis of the catalyst is in full agreement with the magnetic analysis of the nanoparticles reported in the references, which indicates that the nature of the Fe3O4@BTH-Pyr-CuCl catalyst has not changed despite the immobilization of the functional groups and CuCl on the surface of magnetic Fe3O4 nanoparticles [49]. TGA analysis of Fe3O4 NPs and Fe3O4@BTH-Pyr-CuCl nanocatalyst is shown in Fig. 1. The weight reduction of about 7% to the removal of surface hydroxyl groups and solvents does not affect the surface of the particles. Also, a weight loss of about 15% was observed at the temperature of 225–600 °C, which is related to the decomposition and removal of functional groups and copper complex immobilized on magnetic nanoparticles.
FT-IR spectroscopy
FT-IR spectra of Fe3O4@BTH-Pyr ligand and Fe3O4@BTH-Pyr-CuCl nanocatalyst are shown in Fig. 2. The formation of Fe–O bond is confirmed by an obvious peak at about 570 cm−1 in both spectra. The broad peaks at about 3400 cm−1 are related to O–H groups on the surface of magnetic nanoparticles. C–H aromatic bonds were also confirmed by several small peaks at about 2800–3000 cm−1. The C–N bond was also confirmed by a characteristic peak at about 1630 cm−1. The peak related to the C–N bond in the Fe3O4@BTH-Pyr ligand appeared in the region of 1662 cm−1, while the same peak in the Fe3O4@BTH-Pyr-CuCl nanocatalyst appeared in the region of 1632 cm−1. The shift of the peak location to the lower region is due to the presence of the ligand bond with the Cu metal.
EDX, elemental mapping and ICP-OES spectroscopic techniques
EDX and elemental mapping techniques were used to determine the elements in the structure of the Fe3O4@BTH-Pyr-CuCl catalyst (Fig. 3). The presence of Fe, O, C, N, S and Cu elements in the structure of the Fe3O4@BTH-Pyr-CuCl catalyst was well confirmed by these techniques. ICP-OES analysis was used to find out the amount of copper in the structure of the Fe3O4@BTH-Pyr-CuCl nanocatalyst, and the results showed that the amount of Cu in the structure of the nanocatalyst is 14.25 × 10–5 mol g−1.
SEM and TEM spectroscopic techniques
The morphology and structure of particles in Fe3O4@BTH-Pyr-CuCl nanocatalyst were studied by SEM and TEM photographs (Fig. 4). The SEM and TEM images clearly showed that the formed particles are spherical and uniform and their size is in the range of nanometers. TEM images showed that that the particles have a size in the range of 20 nm.
VSM analysis
The magnetic property of all fabricated-nanocomposites for the preparation of the Fe3O4@BTH-Pyr-CuCl nanocatalyst was measured by vibrating-sample magnetometer (VSM) analysis (Fig. 5). Magnetic Fe3O4 nanoparticles with a magnetic property of 61.85 (emu g−1) were prepared, and its amount gradually decreased by modifying its surface with 3-amino-4-mercaptobenzoic acid and pyrimidine-2-carbaldehyde. As you can see in Fig. 5, the results showed that the catalyst was prepared with an amount of 45.875 (emu g−1), which is a high amount and indicates the high magnetic property of this synthesized nanocatalyst.
Catalytic investigation of Fe3O4@BTH-Pyr-CuCl in C–H arylation
First the reaction of benzo[d]thiazole, iodobenzene and S8 as sulfur source for the preparation of 2-(phenylthio)benzo[d]thiazole (product 4a) was considered as the sample reaction for the optimization of conditions. In the absence of catalyst, the product 4a was not formed in the presence of KOH in DMF (Table 1, Entry 1). By using Fe3O4@BTH-Pyr-CuCl catalyst, the progress of the reaction increased significantly. By increasing the amount of the Fe3O4@BTH-Pyr-CuCl catalyst up to 8 mol%, the yield of the desired product also increased, but no change in the yield was seen at amounts higher than 8 mol% (9 and 10 mol% of the catalyst). Therefore, the amount of 8 mol% of the Fe3O4@BTH-Pyr-CuCl catalyst was chosen as the optimal amount (Table 2, Entry 5). The presence of base in the reaction had a great effect on the reaction because in the absence of base the desired reaction did not take place (Table 2, Entry 1). Among tested bases, the best results were seen in the presence of KOAc as base (Table 2, Entry 9). Finally, in order to select the best medium reaction, a number of solvents were tested under the optimized amount of Fe3O4@BTH-Pyr-CuCl nanocatalyst in the presence of KOAc as base. The results confirmed that PEG is the best solvent for the preparation of 2-(phenylthio)benzo[d]thiazole (product 4a) (Table 3, Entry 6). The yield of the obtained product 4a decreased at a temperature lower than 120 °C (Table 3, Entry 7), while temperatures above 120 °C did not affect the efficiency and progress of the reaction (Table 3, Entries 8–9). Only, 10% of the model product was obtained in the absence of solvent after 12 h (Table 3, Entry 10).
In next stage of experimental works, we decide to study the scope of various heterocyclic compounds and aryl or heteroaryl iodides for synthesis of heteroaryl-aryl and di-heteroaryl sulfides and selenides catalyzed by Fe3O4@BTH-Pyr-CuCl nanomaterial (8 mol%) in the presence of KOAc in PEG at 120 °C for 4 h (Table 4). The obtained results clearly showed that this catalytic system is very efficient because different derivatives of the heteroaryl-aryl and di-heteroaryl sulfide and selenide products were synthesized with high yields under the described conditions. One of the attractions of this method is that various derivatives of heterocyclic sulfides and selenides can be easily synthesized by this catalytic system. Although all the obtained heteroaryl-aryl and di-heteroaryl sulfide and selenides products are known and previously reported, synthesis of this number of heteroaryl-aryl and di-heteroaryl sulfides has never been reported by any methods.
In order to study reusability of Fe3O4@BTH-Pyr-CuCl nanocatalyst in this methodology, the reaction of benzo[d]thiazole, iodobenzene and S8 as sulfur source for the preparation of 2-(phenylthio)benzo[d]thiazole (product 4a) was considered as the sample reaction. After the compilation of the reaction, Fe3O4@BTH-Pyr-CuCl nanocatalyst was easily separated with an external magnet (washed several times with ethyl acetate and reused for next runs. As shown in Fig. 6, the Fe3O4@BTH-Pyr-CuCl nanocatalyst was reused for 6 times without considerable reduction in its activity. As shown in Fig. 6, VSM analysis of the reused Fe3O4@BTH-Pyr-CuCl nanocatalyst after 6 times has still high magnetic nature (43.524 emu g–1). ICP-OES analysis was also used to find out the amount of copper in the structure of the reused Fe3O4@BTH-Pyr-CuCl nanocatalyst after 6 times, and the results showed that the amount of Cu in the structure of the nanocatalyst is 14.19 × 10–5 mol g–1.
In order to investigate the efficiency of Fe3O4@BTH-Pyr-CuCl nanocatalyst for C–S coupling heterocycles, we compared the results of this methodology with other reported methods in the literature for the preparation of 2-(phenylthio)benzo[d]thiazole (product 4a) as the template reaction. As shown in Table 5, this method is more preferable than other methods, such as the use of an environmentally friendly solvent, high product yield, the use of a catalyst that can be separated and reused, the use of milder conditions and the performance of the reaction in a shorter time.
Conclusion
In summary, we constructed a novel magnetically recoverable copper nanocatalyst through copper (I) chloride immobilized on magnetic nanoparticles modified with benzothiazole–pyrimidine ligand (Fe3O4@BTH-Pyr-CuCl) and studied its catalytic activities in preparation of heteroaryl-aryl sulfides and selenides. One-pot three-component reactions of reaction of a category of heterocyclic compounds with aryl iodides, sulfur and selenium sources were successfully catalyzed by Fe3O4@BTH-Pyr-CuCl nanocomposite and the desired aryl sulfide and selenide products were afforded with high yields. The recycling tests confirmed that the Fe3O4@BTH-Pyr-CuCl nanocatalyst was reused for 6 times without considerable reduction in its activity.
Experimental
General procedure for preparation of C–S and C–Se bonds formation catalyzed by Fe3O4@BTH-Pyr-CuCl nanocomposite
In a round bottomed flask, a mixture of aryl iodides (0.5 mmol), sulfur or selenium source (0.4 mmol), KOAc (2 equiv) and Fe3O4@BTH-Pyr-CuCl catalyst (8 mol%) was stirred in PEG-400 at 120 °C. After 30 min, heteroaryls (0.3 mmol) were added to reaction mixture and stirred for 4 h. (The progress of the reaction was monitored by thin-layer chromatography (TLC).) After completion of the reaction, the Fe3O4@BTH-Pyr-CuCl was magnetically separated and reaction mixture was cooled to room temperature and H2O (4 mL) was added. The product was extracted with EtOAc (3 × 4 mL) and dried over anhydrous Na2SO4. The crude material was purified with chromatography column on silica gel (EtOAc/n-hexane) which gives the heteroaryl-aryl and di-heteroaryl sulfides products with 84–97%. All heteroaryl-aryl and di-heteroaryl sulfide and selenide products are previously reported and known [35, 55,56,57,58,59,60,61,62,63,64]. HNMR and CNMR were used in order to identify the structure of the heteroaryl-aryl and di-heteroaryl sulfide products.
References
A.K. Sharma, H. Joshi, A.K. Singh, RSC Adv. 10, 6452 (2020)
Y. Zhang, N. Song, Biol. Mol. Chem. 1, 53 (2023)
V.G. Pandya, S.B. Mhaske, Org. Lett. 16, 3836 (2014)
R. Chawla, L.D.S. Yadav, Org. Biomol. Chem. 17, 4761 (2019)
S. Gupta, J. Synth. Chem. 1, 16 (2022)
M. Kazemi, Nanomater. Chem. 1, 1 (2023)
S. Vajar, M. Mokhtary, Polycycl. Aromat. Compd. 39, 111 (2019)
E. Doustkhah, S. Rostamnia, M. Imura, Y. Ide, S. Mohammadi, C.J.T. Hyland, J. You, N. Tsunoji, B. Zeynizadeh, Y. Yamauchi, RSC Adv. 7, 56306 (2017)
R. Deilam, F. Moeinpour, F.S. Mohseni-Shahri, Monatshefte Für Chemie - Chem. Mon. 151, 1153 (2020)
M. Ghobadi, J. Synth. Chem. 1, 84 (2022)
M. Ghobadi, M. Kargar Razi, R. Javahershenas, M. Kazemi, Synth. Commun. 51, 647 (2021)
S. Rostamnia, E. Doustkhah, R. Bulgar, B. Zeynizadeh, Microporous Mesoporous Mater. 225, 272 (2016)
S. Rostamnia, K. Lamei, F. Pourhassan, RSC Adv. 4, 59626 (2014)
P. Ghamari Kargar, C. Len, R. Luque, Sustain. Chem. Pharm. 27, 100672 (2022)
R. Arundhathi, D. Damodara, P.R. Likhar, M.L. Kantam, P. Saravanan, T. Magdaleno, S.H. Kwon, Adv. Synth. Catal. 353, 1591 (2011)
L.S. Ardakani, A. Arabmarkadeh, M. Kazemi, Synth. Commun. 51(6), 856–879 (2021)
R. Taghavi, S. Rostamnia, M. Farajzadeh, H. Karimi-Maleh, J. Wang, D. Kim, H.W. Jang, R. Luque, R.S. Varma, M. Shokouhimehr, Inorg. Chem. 61, 15747 (2022)
F.M. Moghaddam, M. Eslami, Appl. Organomet. Chem. 32, e4463 (2018)
M.R. Abdi, Biol. Mol. Chem. 1, 1 (2023)
A. Baghban, M. Heidarizadeh, E. Doustkhah, S. Rostamnia, P.F. Rezaei, Int. J. Biol. Macromol. 103, 1194 (2017)
A.R. Sardarian, F. Mohammadi, M. Esmaeilpour, Res. Chem. Intermed. 45, 1437 (2019)
E. Doustkhah, M. Heidarizadeh, S. Rostamnia, A. Hassankhani, B. Kazemi, X. Liu, Mater. Lett. 216, 139 (2018)
R. Eisavi, A. Karimi, RSC Adv. 9, 29873 (2019)
M. Kazemi, Synth. Commun. 50, 1899 (2020)
J. Hou, M. Kazemi, Res. Chem. Intermed. 50, 1845–1872 (2024)
A. Noory Fajer, H. Khabt Aboud, H.A. Al-Bahrani, M. Kazemi, Polycycl. Aromat. Compd. 43, 1–47 (2023). https://doi.org/10.1080/10406638.2023.2255723
M. Lakshman, J. Synth. Chem. 1, 48 (2022)
S. Sajjadifar, M.A. Zolfigol, F. Tami, J. Chinese Chem. Soc. 66, 307 (2019)
K. Takagi, Chem. Lett. 16, 2221 (1987)
L. Shiri, A. Ghorbani-Choghamarani, M. Kazemi, Aust. J. Chem. 69, 585 (2016)
M. Kazemi, Synth. Commun. 50, (2020).
I.M. Yonova, C.A. Osborne, N.S. Morrissette, E.R. Jarvo, J. Org. Chem. 79, 1947 (2014)
P. Anbarasan, H. Neumann, M. Beller, Chem. Commun. 47, 3233 (2011)
X. Li, T. Yuan, Y. Yang, J. Chen, Tetrahedron 70, 9652 (2014)
L.-F. Niu, Y. Cai, C. Liang, X.-P. Hui, P.-F. Xu, Tetrahedron 67, 2878 (2011)
R. Zhang, H. Ding, X. Pu, Z. Qian, Y. Xiao, Catalysts 10, 1339 (2020)
M. Vaddamanu, K. Velappan, G. Prabusankar, New J. Chem. 44, 129 (2020)
M. Arisawa, T. Ichikawa, M. Yamaguchi, Org. Lett. 14, 5318 (2012)
C.C. Eichman, J.P. Stambuli, Molecules 16, 590 (2011)
X. Xu, W. Wang, L. Lu, J. Zhang, J. Luo, Catal. Letters 152, 3031 (2022)
V. Rathore, S. Kumar, Green Chem. 21, 2670 (2019)
Y. Kobiki, S. Kawaguchi, T. Ohe, A. Ogawa, Beilstein J. Org. Chem. 9, 1141 (2013)
A.R. Rosario, K.K. Casola, C.E.S. Oliveira, G. Zeni, Adv. Synth. Catal. 355, 2960 (2013)
G. Kumaraswamy, V. Ramesh, M. Gangadhar, S. Vijaykumar, Asian J. Org. Chem. 7, 1689 (2018)
H. Chuai, S.-Q. Zhang, H. Bai, J. Li, Y. Wang, J. Sun, E. Wen, J. Zhang, M. Xin, Eur. J. Med. Chem. 223, 113621 (2021)
D. Hu, M. Liu, H. Wu, W. Gao, G. Wu, Org. Chem. Front. 5, 1352 (2018)
X. Liu, S.-B. Zhang, H. Zhu, Z.-B. Dong, J. Org. Chem. 83, 11703 (2018)
L. Chen, A. Noory Fajer, Z. Yessimbekov, M. Kazemi, M. Mohammadi, J. Sulfur Chem. 40, 451 (2019)
M.M. Khodaei, A. Alizadeh, M. Haghipour, Res. Chem. Intermed. 45, 2727 (2019)
G. Balakishan, G. Kumaraswamy, V. Narayanarao, P. Shankaraiah, Heterocycl. Commun. 27, 17 (2021)
D. Kumar, B.B. Mishra, V.K. Tiwari, J. Org. Chem. 79, 251 (2014)
X. Hao, D. Feng, H. Chen, P. Huang, F. Guo, Chem.—A Eur. J. 29(60), e202302119 (2023)
V.N. Bochatay, P.J. Boissarie, J.A. Murphy, C.J. Suckling, S. Lang, J. Org. Chem. 78, 1471 (2013)
S. Ranjit, R. Lee, D. Heryadi, C. Shen, J. Wu, P. Zhang, K.-W. Huang, X. Liu, J. Org. Chem. 76, 8999 (2011)
P. Gandeepan, J. Mo, L. Ackermann, Chem. Commun. 53, 5906 (2017)
J. Rafique, G. Farias, S. Saba, E. Zapp, I.C. Bellettini, C.A. Momoli Salla, I.H. Bechtold, M.R. Scheide, J.S. Santos Neto, D. Monteiro de Souza Junior, H. de Campos Braga, L.F.B. Ribeiro, F. Gastaldon, C.T. Pich, T.E.A. Frizon, Dye. Pigment. 180, 108519 (2020)
R. Wang, H. Xu, Y. Zhang, Y. Hu, Y. Wei, X. Du, H. Zhao, Org. Biomol. Chem. 19, 5899 (2021)
X. Ren, Q. Liu, Z. Yang, Z. Wang, X. Chen, Chinese Chem. Lett. 34, 107821 (2023)
C. Ravi, D. Chandra Mohan, S. Adimurthy, Org. Biomol. Chem. 14, 2282 (2016)
S. Kundu, B. Basu, RSC Adv. 5, 50178 (2015)
Y. An, G. Xu, M. Cai, S. Wang, X. Zhong Wang, Y. Chen, L. Dai, Tetrahedron 79, 131829 (2021)
Y.-S. Zhu, Y. Xue, W. Liu, X. Zhu, X.-Q. Hao, M.-P. Song, J. Org. Chem. 85, 9106 (2020)
C. Gao, G. Wu, L. Min, M. Liu, W. Gao, J. Ding, J. Chen, X. Huang, H. Wu, J. Org. Chem. 82, 250 (2017)
J.M. Anghinoni, S.S. Ferreira, R.F. Schumacher, B.A. Iglesias, G. Perin, F. Penteado, E.J. Lenardão, New J. Chem. 47, 6066 (2023)
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, K., Chang, LY. Copper complex supported on the surface of magnetic nanoparticles: an ecofriendly catalyst for C–S and C–Se coupling reactions. J IRAN CHEM SOC 21, 1547–1560 (2024). https://doi.org/10.1007/s13738-024-03015-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-024-03015-9