Abstract
The current research work is about the synthesis of functionalized crystalline anilide, i.e., (Z)-4-((2-nitrophenyl)amino)-4-oxobut-2-enoic acid (NAOB) and its experimental and theoretical investigation. The single-crystal X-rays diffraction technique is employed for the crystal structure determination. The crystal structure of NAOB is compared with two closely related crystal structures of the literature that contain nitro group at the para position of the phenyl ring. The supramolecular assembly was thoroughly explored by Hirshfeld surface analysis. The most probable contact for the crystal packing interactions was determined by enrichment ratio analysis. The crystal packing environment was explored by the interaction energy between the molecular pairs. Energy frameworks were computed to understand the topology of the single crystal of the compound NAOB. Void analysis was performed to check the strength of the crystal packing. In order to visualize the relative polarity and active sites of crystal structure, molecular electrostatic potential (MEP) study was performed. The DFT-based calculated results were found in agreement with the experimental results.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anilides accompanied by the carboxylic acid and their derivatives denote significant class of important organic compounds. Currently, these versatile compounds have attracted substantial attention due to their interesting pharmacological medications including treatment of tuberculosis (Mycobacterium tuberculosis and tubercle bacillus that are accountable for the leading cause of death worldwide), cancer and ulcer [1,2,3]. It is important to mention that, an extreme decline has been observed in the number of TB cases, courtesy to the discovery of effective anti-mycobacterial agents including isoniazid, ethambutol, rifampicin, pyrazinamide, and streptomycin. Functionalized anilide such as NAOB having the nitro group and carboxylic acid functionality could be interesting chemical architecture as the presence of many hetero atoms might be responsible for non-covalent interactions and could also be used as potential ligand in coordination chemistry for the production of metal–organic framework (MOFs) [4,5,6]. For instance, the similar structural pattern can be found in many important biologically active compounds having potential activities like anticancer and antidepressant [7, 8], antimicrobial and analgesic. Similarly, carboxamides and analogs having structural similarity to the NAOB, could be used as activators of caspases and inducers of apoptosis [9]. Similar structural sequence could also be observed in the anti-inflammatory and antioxidant agents. Some of the chemical structures with potential anti-HIV-I activity has also a similar structural format (Fig. 1) [10].
The Hirshfeld surface analysis is an important tool that is used for the crystalline compounds to find the van der Waals interactions and hydrogen bonding [11,12,13,14,15]. These non-covalent interactions are considered to play the major role in the crystal packing [16,17,18,19]. Herein, we are presenting our findings regarding crystal structure, the supramolecular assembly exploration by Hirshfeld surface analysis and DFT inspection of the functionalized crystalline anilide (NAOB).
Experimental
Chemicals and instrumentation
The acetic acid and other chemical used for the synthesis and purification used were of highest grade and used as received without any further purification.
Synthetic procedure of NAOB
The compound NAOB (2E)-4-[((2-nitrophenyl)amino)]-4-oxobutanoic acid) was prepared using the general procedure of the literature [20]. Accordingly, equivalent molar quantities of 2-nitroaniline, i.e., C6H6N2O2 (1 mmol, 138 mg) and succinic anhydride, i.e., (CH2CO)2O (1 mmol, 100 mg) in 10 mL of glacial acetic acid was stirred at room temperature for 30 min. The solid form was separated out from the reaction mixture via filtration, washed with hexane and dried. The crude product was recrystallized using methanol (Scheme 1).
Single Crystal XRD analysis details of NAOB
The single crystal XRD data of NAOB is collected on Bruker Kappa Apex-II diffractometer with APEX-II software for data collection. The absorption correction is performed on SADABS software. The SHELXT-2014 [21] and SHELXL 2019/2 [22] software are employed for structure solution and refinement, respectively. Anisotropic displacement parameters are assigned to all non-hydrogen atoms whereas isotopic displacement parameters are assigned to H-atoms. H-atoms are placed at the calculated positions by using riding model. ORTEP-3 [23] PLATON [24] and Mercury 4.0 [25] are used for the graphical representation of SC-XRD results.
Computational methodology
Density function theory (DFT) method was used to obtain the HOMO–LUMO energy values, using B3LYP method with the 6–311 + + G(d,p) basis set in Gaussian 16 and GaussView 6 software programs [26, 27]. First, after importing the experimental geometry, Gaussian optimization algorithm was used to obtain optimized geometry [28]. Next, Gaussian energy calculation algorithm was implemented to compute energy values for the frontier molecular orbitals, energy values of the whole structures, values of the dipole moment and visualization of the frontier molecular orbitals. Studies of the conceptual density functional theory (CDFT) were performed by using Multiwfn version 3.7 [29].
Result and discussion
The Cambridge structure database conformed that the crystal structure of NAOB is novel. The details related to single crystal X-Ray diffraction (SC-XRD) are listed in Table 1 whereas the important bond lengths and bond angles are listed in Table 2.
SC-XRD description of the crystal structure of NAOB.
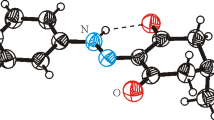
In NAOB (Fig. 2, Table 1) the (Z)-4-amino-4-oxobut-2-enoic acid group A (C1-C4/N1/O1-O3) and phenyl ring B (C5-C10) are planar with root mean square (r.m.s.) deviation of 0.0639 and 0.0056 Å, respectively, with dihedral angle A/B of 38.8 (6)°. The nitro group C (N2/O4/O5) is oriented at the dihedral angle of 42.8 (2)º relative to its parent group A. The deviation of O1-atom from plane of group A is larger than other atoms in the plane and deviation for O1-atom is 0.1157 (2) Å. The selected bond lengths and bond angles are listed in Table 2. The O1-C1and O2-C1 bond lengths are 1.304 (3) and 1.204 (3) Å, respectively, which showed there is single bond between O1 and C1 atoms and double bond between O2 and C1 atoms. The one of the O-atom of carboxylate group and one of O-atom of nitro group act as H-bond acceptor for NH group to form S (7) and S (6) H-bonded loops [30]. These intramolecular H-bonding are the main aspect of the molecular configuration for NAOB. The molecules are interlinked in the form of dimers through the combination of O–H \(\cdots\) O and C–H \(\cdots\) O bonding to form \({\mathrm{R}}_{2}^{2}(8)\) loop [31] (Fig. 3, Table 3). C7 and C5 infinite chains are formed by O–H \(\cdots\) O and C-H \(\cdots\) O bonding, respectively. The one-dimensional chain network of molecules is formed with base vector [10 \(\overline{1 }\)]. The crystal packing is further stabilized by the presence of off-set π \(\cdots\) π stacking interaction [32] between phenyl rings of symmetry related molecules. The inter-centroid separation between interacting rings is 3.751 (2) Å and ring off-set is 1.315 Å. Infinite chain of molecules is formed due to off-set π \(\cdots\) π stacking interaction that runs along a-axis (Fig. 4).
It is possible to recognize the atom or a group of atoms of a molecule to be involved in intermolecular interactions. For the said purpose, we calculate the full interaction map. The main focus behind the full interaction map calculations is to visualize H-bond donors, H-bond acceptors, and the interactions that involve aromatic ring. The result of the full interaction map is shown in Fig. 5. The dark red region around the hydroxyl group indicates that it acts as a strong H-bond donor whereas the red region around NH group is light red indicates that it is a weak H-bond donor. It is evident from Table 3 that NH group is not engaged in any convection H-bonding, but it is involved in N–H \(\cdots\) π interaction which is comparatively weaker than the convectional H-bonding. H-bond acceptors are indicted by blue regions around them. A pair light red region around aromatic ring indicated the involvement of the ring in π \(\cdots\) π stacking interactions. The simulated powder X-ray diffraction (PXRD) pattern of NAOB is formed which inferred that there are three most intense peaks at positions 10.8°, 25.1° and 26.1° and labeled with corresponding hkl values (02 \(\overline{1 }\)), (12 \(\overline{1 }\))and (11 \(\overline{2 }\)), respectively (Fig. S1). These sharp peaks of NAOB indicate the high crystallinity of as prepared single crystal compound with no other impurities. The cambridge structure database [33] search inferred that the crystal structures with reference code JAYGEW [34] and MNPMAL01 [35] that have close similarity with the crystal structure of NAOB. The crystal structure of JAYGEW and MNPMAL01 have nitro group at the para position of the phenyl ring and both have one molecule in the asymmetric unit, crystallized in same crystal system and space group. The crystal structure with reference code JAYGEW and MNPMAL01 looks similar at first look. Both were crystallized in the same crystal system and space group but these structures are polymorphous. The crystal packing of JAYGEW and MNPMAL01 was different from one another. The molecules of JAYGEW were connected by N–H \(\cdots\) O and C-H \(\cdots\) O bonding in the form of chains of rings whereas the molecules of MNPMAL01 were interlinked by same H-bonding in the form sheets parallel to (\(\overline{1 }\) 01) plane.
Symmetry codes: (i) x + 1/2, − y + 1/2, z − 1/2; (ii) x − 1/2, − y + 1/2, z + 1/2.
Hirshfeld Surface Analysis
The intermolecular interactions in a single crystal of molecule were studied by Hirshfeld surface analysis on Crystal explorer 21.5 [36]. The concept of Hirshfeld surface (HS) arose when the researchers were trying to dissociate the crystal electron density into molecular fragments. Figure 6a is the Hirshfeld surface plotted over dnorm on which the red spots are the representatives of short contacts. White spots stand for the contacts for which the distance between the interacting atoms is equal to the sum of the Van der Waal radii of atoms whereas blue spots stand for the longer contacts [37,38,39]. Figure 6b, c shows the HS of NAOB along with de and di. Where de and di shows the distance from the Hirshfeld surface (HS) to the nearest nucleus external and internal to the surface, respectively. In order to visualize the π \(\cdots\) π stacking interactions in NAOB, HS is plotted over curvedness (Fig. 6d) shape index (Fig. 6e). The large flat green region around the aromatic ring on the HS plotted over curvedness indicates the involvement of the aromatic ring in π \(\cdots\) π stacking interactions. The presence of consecutive red and blue triangular regions around the aromatic ring also indicates the involvement of the aromatic ring in π \(\cdots\) π stacking interactions. Close contacts with neighbor atoms from inside to outside O \(\cdots\) H and H \(\cdots\) O are represented in Fig. 6f on which the red color circles revealed the hydrogen bonding contacts and blue regions show the longer contacts on the HS surface.
The 2D fingerprint plots (Fig. 7) were provided for additional information about the intermolecular interactions. It provides direct insight of the supramolecular assembly in the single crystals [40,41,42]. The analysis inferred that H \(\cdots\) O, H \(\cdots\) H and C \(\cdots\) C as major contributors for the crystal packing. The highest contribution was found 48.5% for H \(\cdots\) O to the crystal packing and 20.7% for H \(\cdots\) H followed by C \(\cdots\) C 9.9%. Other contacts or interactions were negligible and are given in supporting information.
Molecular Electrostatic Potential (MEP)
MEP is the method to visualize relative polarity and active sites of crystal structure, this is effective way to investigate reactivity and the relative polarity of molecule [43,44,45]. Figure 8 shows the 3D MEP maps of NAOB, the negative potential represented by red regions are mainly localized over the terminal oxygen atoms of NAOB anion. So red regions are most suitable positions for electrophilic attack in the NAOB. The blue region represents the positive potential region of NAOB that indicates possible sites for nucleophilic attack.
Void Analysis
Promolecular surfaces were used to visualize the size, shape and the space belong to molecule in crystal. Similarly, these surfaces can also be used to identify the region or space not related with a molecule or with molecules in a crystal. So, crystal voids can be visualized by simply building an isosurface of the procrystal electron density. Figure 9 demonstrates the correlation between isosurface of the procrystal electron density and 0.002 au promolecule isosurfaces for NAOB. All the atoms are assumed to be spherically symmetric and electron density of all the atoms are added up in order to find voids [46,47,48]. We found the void surface with volume of 79.77 Å3 per unit cell and total cell volume was found 999.4 Å3 which inferred that the molecules are strongly packed as the void occupy just 7.98% of the space of the unit cell. The promolecule isosurface for individual volume of NAOB was calculated 221.45 Å3 (Fig. 9a). Figure 9b shows the electron densities overlap in the procrystal, the sum of their individual volumes for two molecules per unit cell was found 442.90 Å3.
Interaction energy and energy frameworks
Interaction energies of NAOB were calculated with model B3LYP/6-31G(d,p)by using Crystal explorer version 21.5. The interaction energy is the sum of four kinds of energies named as electrostatic, dispersion, polarization and repulsion [49,50,51]. The interaction energy is calculated for the cluster of molecules that lie within 3.8 Å3 volume of the reference molecule (Fig. 10a). The interaction energy calculations inferred that the interaction energy is greatest for the pair of molecules that are connected by symmetry (x + 1/2, -y + 1/2, z + 1/2) (Fig. 10e). Energy frameworks represents the strength of a particular kind of energy. The thickness of the cylinders is directly proportional to the strength of the particular kind of energy. The energy framework calculations inferred that the dispersion energy has greater contribution as compared to the coulomb energy in defining the total interaction energy (Fig. 10b, c, d).
Computational results
DFT inspection
The application of density functional theory (DFT) delivers an extensive foundation that can be used to characterize various properties of the compound, including physical and chemical properties that can be used to predict how the single crystal structure will behave [52]. We can achieve that by determining the energies associated with molecular orbitals and the energy gaps between them. Energy gap, i.e., a minimum required energy for an electron to reach an excited state, ΔE (ELUMO–EHOMO), represents the reactive ability of molecules to interact with other molecules, (electrophiles or nucleophiles). A hard, less polarizable, and less reactive molecule has larger energy gap, when a soft, highly polarizable and higher chemically reactive molecule has smaller energy gap.
As a first step of the DFT analysis, geometry optimization to minimum energy state was performed for the title compound by applying B3LYP/6–311 + + G(d,p) method (Fig. 11). DFT calculations were performed on both experimentally obtained from X-Ray geometry and optimized geometry. Energy values for the multiple molecular orbitals from the HOMO to the fourth HOMO (HOMO – HOMO-3) and from the LUMO to the fourth LUMO (LUMO – LUMO + 3) were evaluated (Tables 4 and 5).
The analysis of the energy gaps between molecular orbitals (Table 5) revealed higher energy gaps for experimentally obtained crystal structure of the title compound comparing to the DFT-optimized geometry. This finding suggests that the experimental geometry of the compound has restricted mobility of the electrons and is less reactive. Overall low values of the dipole moment (Table 5) represent the nearly symmetrical charge distribution, when at the same time experimentally obtained structure possesses a slightly asymmetrical distribution of charges.
Last, the conceptual density functional theory (CDFT) was used to calculate the chemical reactivity descriptors that were originally introduced by Robert Parr [53,54,55], to provide a quantitative analysis of the investigated compound, and to provide an intrinsic understanding of the chemical and biological activities. Previously derived computational data for the HOMO and LUMO energy levels were utilized to find the global chemical reactivity descriptors, such as ionization potential (IP), electron affinity (EA), electronegativity (χ), chemical potential (µ), global hardness (η), global softness (σ), electrophilicity index (ω) and nucleophilicity index (N) (Table 6). The title compound possesses high ionization potential, electronegativity, and global hardness and nucleophilicity index values, thus representing strong nucleophilic structure, that at the same time does not favor the change in the electron distribution. Supporting that, low escaping tendency of electrons is represented by low chemical potential value. In comparison with theoretically optimized geometry of the title compound, the experimentally obtained structure has slightly stronger pronounced properties of nucleophilic structure.
Conclusion
The crystalline anilide, i.e., (Z)-4-((2-nitrophenyl)amino)-4-oxobut-2-enoic acid (NAOB) was synthesized by using the reported method and experimental and theoretical investigation were performed. The structure of NAOB was unambiguously confirmed by the SC-XRD technique which showed that the molecular configuration was stabilized by intramolecular N–H \(\cdots\) O bonding. The crystal packing was stabilized by O–H \(\cdots\) O, C–H \(\cdots\) O and off-set π \(\cdots\) π stacking interactions. Hirshfeld surface analysis inferred that O \(\cdots\) H contact was the most significant contributor of the crystal packing. The crystal packing environment was further explored by the interaction energy between the molecular pairs which showed that the dispersion energy played dominant role for the stabilization of solid-state assembly. The DFT calculations performed to analyze the chemical and physical properties of the NAOB demonstrated the comparable energy states of the experimentally obtained crystal structure and the computationally optimized structure, but increased energy gaps between LUMO and HOMO molecular orbitals for the experimentally obtained crystal structure, revealing the lower mobility of the electrons and insignificant asymmetrical distribution of charges. The chemical reactivity descriptors obtained upon quantification analysis of the energy gaps between molecular orbitals demonstrated that NAOB possesses strong nucleophilic properties that are even stronger pronounced than those for the theoretically optimized structure.
References
M.A. El-Hashash, S.A. Rizk, S.R. Atta-Allah, Molecules 20, 22069 (2015). https://doi.org/10.3390/molecules201219827
M. Bianchi, A. Butti, Y. Christidis, J. Perronnet, F. Barzaghi, R. Cesana, A. Nencioni, Eur. J. Med. Chem. 23, 45 (1988). https://doi.org/10.1016/0223-5234(88)90166-3
M.D. Vitorović-Todorović, A. Erić-Nikolić, B. Kolundžija, E. Hamel, S. Ristić, I.O. Juranić, B.J. Drakulić, Eur. J. Med. Chem. 62, 40 (2013). https://doi.org/10.1016/j.ejmech.2013.01.006
M. Bazargan, F. Ghaemi, A. Amiri, M. Mirzaei, Coord. Chem. Rev. 445, 214107 (2021). https://doi.org/10.1016/j.ccr.2021.214107
M. Bazargan, M. Mirzaei, A. Franconetti, A. Frontera, Dalton Trans. 48, 5476 (2019). https://doi.org/10.1039/C9DT00542K
R. Khajavian, M. Mirzaei, H. Alizadeh, Dalton Trans. 49, 13936 (2020). https://doi.org/10.1039/D0DT02798G
P. Theivendren, A. Subramanian, I. Murugan, S.D. Joshi, U.A. More, Chem. Biol. Drug Des. 89, 714 (2017). https://doi.org/10.1111/cbdd.12894
T. Panneerselvam, S. Arumugam, M.A. Ali, K. Selvaraj, M. Indhumathy, A. Sivakumar, S.D. Joshi, ChemistrySelect 2, 2341 (2017). https://doi.org/10.1002/slct.201601763
W. Kemnitzer. S. Cai. J. Drewe, N. Sirisoma, Google Patents2008.
M.J. Kukla, H.J. Breslin, C.J. Diamond, P.P. Grous, C.Y. Ho, M. Miranda, J.D. Rodgers, R.G. Sherrill, E. De Clercq, R. Pauwels, K. Andries, L.J. Moens, M.A.C. Janssen, P.A.J. Janssen, J. Med. Chem. 34, 3187 (1991). https://doi.org/10.1021/jm00115a007
A. Hassanpoor, M. Mirzaei, H. Eshtiagh-Hosseini, A. Majcher, CrystEngComm 20, 3711 (2018). https://doi.org/10.1039/C8CE00573G
H. Eshtiagh-Hosseini, M. Mirzaei, M. Biabani, V. Lippolis, M. Chahkandi, C. Bazzicalupi, CrystEngComm 15, 6752 (2013). https://doi.org/10.1039/C3CE40743H
A. Ali, M. Khalid, M.F.U. Rehman, S. Haq, A. Ali, M.N. Tahir, A.A.C. Braga, ACS Omega 5, 15115 (2020). https://doi.org/10.1021/acsomega.0c00975
A. Ali, Z.U. Din, M. Ibrahim, M. Ashfaq, S. Muhammad, D. Gull, M.N. Tahir, E. Rodrigues-Filho, A.G. Al-Sehemi, M. Suleman, RSC Adv. 13, 4476 (2023). https://doi.org/10.1039/D2RA07681K
A. Ali, M. Ashfaq, Z.U. Din, M. Ibrahim, M. Khalid, M.A. Assiri, A. Riaz, M.N. Tahir, E. Rodrigues-Filho, M. Imran, A. Kuznetsov, ACS Omega 7, 39294 (2022). https://doi.org/10.1021/acsomega.2c05441
O. Simsek, M. Ashfaq, M.N. Tahir, S. Ozturk, E. Agar, J. Struct. Chem. 64, 942 (2023). https://doi.org/10.1134/S0022476623050128
S.L. Rubab, A.R. Raza, B. Nisar, M. Ashfaq, Y. Altaf, R. Hussain, H.M. Ali, Molecules 28, 4375 (2023). https://doi.org/10.3390/molecules28114375
R.K. Askerov, M. Ashfaq, E.V. Chipinsky, V.K. Osmanov, M.N. Tahir, E.V. Baranov, G.K. Fukin, V.N. Khrustalev, R.H. Nazarov, G.N. Borisova, Z.V. Matsulevich, A.M. Maharramov, A.V. Borisov, Results Chem. 4, 100600 (2022). https://doi.org/10.1016/j.rechem.2022.100600
A. Hassanpoor, M. Mirzaei, M. Niknam Shahrak, A.M. Majcher, Dalton Trans. 47, 13849 (2018). https://doi.org/10.1039/C8DT02986E
P. Praveen Kumar, Y. Dathu Reddy, C.V.R. Reddy, B.R. Devi, P.K. Dubey, Org. Chem. Int. 2014, 1 (2014). https://doi.org/10.1155/2014/576715
G.M. Sheldrick, Acta Crystallogr. Sect. A: Found. Adv. 71, 3 (2015). https://doi.org/10.1107/S2053273314026370
G.M. Sheldrick, Acta Crystallogr. C Struct. Chem. 71, 3 (2015). https://doi.org/10.1107/S2053229614024218
L.J. Farrugia, J. Appl. Crystallogr. 45, 849 (2012). https://doi.org/10.1107/S0021889812029111
A.L. Spek, Acta Crystallogr. D Biol. Crystallogr. 65, 148 (2009). https://doi.org/10.1107/S090744490804362X
C.F. Macrae, I. Sovago, S.J. Cottrell, P.T. Galek, P. McCabe, E. Pidcock, M. Platings, G.P. Shields, J.S. Stevens, M. Towler, J. Appl. Crystallogr. 53, 226 (2020). https://doi.org/10.1107/S1600576719014092
M. Frisch, G. Trucks, H. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, G. Scalmani, V. Barone, G. Petersson, H. Nakatsuji, Revision A 3, 310 (2016)
R. Dennington. T. A. Keith, J. M. Millam, Semichem Inc.: Shawnee Mission, KS, USA (2016).
A. Ali, M. Khalid, Z.U. Din, H.M. Asif, M. Imran, M.N. Tahir, M. Ashfaq, E. Rodrigues-Filho, J. Mol. Struct. 1241, 130685 (2021). https://doi.org/10.1016/j.molstruc.2021.130685
T. Lu, F. Chen, J. Comput. Chem. 33, 580 (2012). https://doi.org/10.1002/jcc.22885
J. Bernstein, R.E. Davis, L. Shimoni, N.L. Chang, Angew. Chem. Int. Ed. Engl. 34, 1555 (1995). https://doi.org/10.1002/anie.199515551
M. Mirzaei, H. Eshtiagh-Hosseini, Z. Karrabi, K. Molčanov, E. Eydizadeh, J.T. Mague, A. Bauzá, A. Frontera, CrystEngComm 16, 5352 (2014). https://doi.org/10.1039/C4CE00325J
H. Eshtiagh-Hosseini, M. Mirzaei, S. Zarghami, A. Bauzá, A. Frontera, J.T. Mague, M. Habibi, M. Shamsipur, CrystEngComm 16, 1359 (2014). https://doi.org/10.1039/C3CE41730A
C.R. Groom, I.J. Bruno, M.P. Lightfoot, S.C. Ward, Acta Crystallogr Sect. B: Struct. Sci. Cryst. Eng. Mater. 72, 171 (2016). https://doi.org/10.1107/S2052520616003954
J.L. Wardell, J.M. Skakle, J.N. Low, C. Glidewell, Acta Crystallogr Sect. E: Struct. Rep. Online 61, o3849 (2005). https://doi.org/10.1107/S160053680503374X
B.T. Gowda, M. Tokarčík, K. Shakuntala, J. Kožíšek, H. Fuess, Acta Crystallogr Sect. E: Struct. Rep. Online 66, o1671 (2010). https://doi.org/10.1107/S1600536810022245
P.R. Spackman, M.J. Turner, J.J. McKinnon, S.K. Wolff, D.J. Grimwood, D. Jayatilaka, M.A. Spackman, J. Appl. Crystallogr. 54, 1006 (2021). https://doi.org/10.1107/S1600576721002910
M.A. Spackman, D. Jayatilaka, CrystEngComm 11, 19 (2009). https://doi.org/10.1039/B818330A
S.A. Al-Jibori, G.H.H. Al-Jibori, M. Ashfaq, T. Khalil, M. Laguna, C. Wagner, M.N. Tahir, A.S.M. Al-Janabi, J. Mol. Struct. (2023). https://doi.org/10.1016/j.molstruc.2023.135803
A.S. Faihan, R.H. AlShammari, M. Ashfaq, S. Muhammad, S.A. Al-Jibori, M.N. Tahir, M.R. Hatshan, A.S. Al-Janabi, S.M. Al-Moayid, J. Mol. Struct. 1286, 135633 (2023). https://doi.org/10.1016/j.molstruc.2023.135633
J.J. McKinnon, D. Jayatilaka, M.A. Spackman, ChemComm. (2007). https://doi.org/10.1039/B704980C
M. Haroon, M.W. Baig, T. Akhtar, M.N. Tahir, M. Ashfaq, J. Mol. Struct. 1287, 135692 (2023). https://doi.org/10.1016/j.molstruc.2023.135692
M. Kurbanova, M. Ashfaq, M.N. Tahir, A. Maharramov, N. Dege, N. Ramazanzade, E.B. Cinar, J. Struct. Chem. 64, 437 (2023). https://doi.org/10.1134/S0022476623030095
M. Mirzaei, H. Eshtiagh-Hosseini, A. Bauzá, S. Zarghami, P. Ballester, J.T. Mague, A. Frontera, CrystEngComm 16, 6149 (2014). https://doi.org/10.1039/C4CE00003J
Z. Hosseini-Hashemi, M. Mirzaei, A. Jafari, P. Hosseinpour, M. Yousefi, A. Frontera, M.L. Dashtbayaz, M. Shamsipur, M. Ardalani, RSC Adv. 9, 25382 (2019). https://doi.org/10.1039/C9RA05143K
M. Mirzaei, H. Eshtiagh-Hosseini, M. Shamsipur, M. Saeedi, M. Ardalani, A. Bauzá, J.T. Mague, A. Frontera, M. Habibi, RSC Adv. 5, 72923 (2015). https://doi.org/10.1039/C5RA09526C
M.J. Turner, J.J. McKinnon, D. Jayatilaka, M.A. Spackman, CrystEngComm 13, 1804 (2011). https://doi.org/10.1039/C0CE00683A
M.N. Tahir, A. Ali, M. Khalid, M. Ashfaq, M. Naveed, S. Murtaza, I. Shafiq, M.A. Asghar, R. Orfali, S. Perveen, Molecules 28, 2967 (2023). https://doi.org/10.3390/molecules28072967
W.A. Shehnaz, A. Siddiqui, M. Ashraf, M.N. Ashfaq, S.N. Tahir, Appl. Organomet. Chem. 37, e7077 (2023). https://doi.org/10.1002/aoc.7077
C.F. Mackenzie, P.R. Spackman, D. Jayatilaka, M.A. Spackman, IUCrJ 4, 575 (2017). https://doi.org/10.1107/S205225251700848X
M.J. Turner, S. Grabowsky, D. Jayatilaka, M.A. Spackman, J. Phys. Chem. Lett. 5, 4249 (2014). https://doi.org/10.1021/jz502271c
M. Kurbanova, M. Ashfaq, M.N. Tahir, A. Maharramov, N. Dege, A. Koroglu, J. Struct. Chem. 64, 302 (2023). https://doi.org/10.1134/S0022476623020142
A. Ali, M. Khalid, S. Abid, M.N. Tahir, J. Iqbal, M. Ashfaq, F. Kanwal, C. Lu, M. F. u. Rehman, Crystals 10, 778 (2020). https://doi.org/10.3390/cryst10090778
R.G. Parr, in Horizons of Quantum Chemistry. ed. by K. Fukui, B. Pullman (Springer, Netherlands, Dordrecht, 1980)
R.G. Parr, R.A. Donnelly, M. Levy, W.E. Palke, J. Chem. Phys. 68, 3801 (2008). https://doi.org/10.1063/1.436185
R.G. Parr, R.G. Pearson, J. Am. Chem. Soc. 105, 7512 (1983). https://doi.org/10.1021/ja00364a005
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ashfaq, M., Tahir, M.N., Bogdanov, G. et al. Crystal structure, supramolecular assembly exploration by Hirshfeld surface analysis and DFT inspection of the synthesized functionalized crystalline anilide. J IRAN CHEM SOC 20, 3147–3159 (2023). https://doi.org/10.1007/s13738-023-02904-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-023-02904-9