Abstract
The review is devoted to current trends in the development of the chemistry of tricyclic diterpenoids, in particular maleated derivatives of resin acids of the abietane series. Among the derivatives of plant terpenes, maleopimaric acid has become a popular molecule in recent years and is attracting growing interest. In this review, the chemical properties of maleopimaric acid (MPA) and its methyl ester (MMP) was studied, data on the methods of synthesis of imides, amides, and amidoimides of acid are systematized. Oxidation reactions and ozonolysis with the participation of monomethyl ether MPA are presented. Synthesis of monomers and polymers based on MPA is described. The expediency of using the most interesting developments with the use of MPA in pharmacology, in the chemistry of macromolecular compounds, photolithography, stereochemistry, etc. is considered.
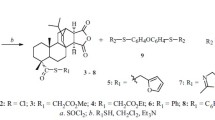
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The search, development of new approaches to the synthesis and study of the pharmacological, physicochemical properties of derivatives of polycarbocyclic compounds generated from plant raw materials is an important area of research in modern organic chemistry. Maleopimaric acid 1 (MPA) is a readily available compound obtained from levopimaric acid 2 and maleic anhydride by the Diels–Alder reaction [1] (Scheme 1). MPA is widely used in the technology of paints and varnishes and polymeric materials [2] and is considered as a promising starting material for the synthesis of biologically active compounds [3,4,5] and chiral ligands [6].
Resin acids, about ten of them are known in total, belong mainly to two structural types, pimaranic and abietanic, the ratio of which in natural sources is very different [7,8,9,10].
Abietic acid 3 is the most common resin acid and is found in all types of rosin. Levopimaric acid 2 was first found in French rosin [11]. Pimaric, isopimaric, etc. acids do not contain conjugated double bonds and therefore are resistant to heating in air. Acids with conjugated double bonds (levopimaric, abietic, etc.) are rapidly oxidized in air and easily isomerized into each other [12,13,14]. At elevated temperatures, they are in a state of mobile equilibrium, which is continuously shifting toward the formation of levopimaric acid. This is the basis for the possibility of synthesizing maleopimaric acid 1 on an industrial scale.
Among nitrogen-containing derivatives of maleopimaric acid, amides, imides, amidoimides with a complex of valuable properties, including biologically active ones, have been described.
To date, many methods have been proposed for modifying MPA with aliphatic, aromatic, and heterocyclic amines at the anhydride and carboxyl groups. The methods described in the literature for condensation of MPA with amines do not allow achieving complete conversion of MPA. Known catalytic systems and reaction conditions do not always provide high yields of target molecules. The main direction of the review is the analysis and generalization of materials that offer solutions to these problems, as well as the most interesting publications and promising areas of MPA chemistry.
Methods of preparation and properties of maleopimaric acid imides
Known methods for the preparation of MPA imides are divided into two groups: the first method is direct melting with various amines [15,16,17], the second method is the condensation with amines in various high-boiling solvents [18,19,20,21]. The main disadvantages of these methods are the duration of the process, limitations on the nature of amino compound, low conversion of MPA and low product yields.
The interaction of MPA with a twofold excess of liquid ammonia gave the simplest maleopimarimide 4 in 35% yield (Scheme 2). In order to simplify the technology, it is proposed to use maleopimaric acid in the form of a reaction mass obtained by the interaction of rosin and maleic anhydride in a turpentine media [22].
The development of an efficient approach to the synthesis of MPA N-arylimides without isolation of maleopimaric acid from rosin was reported (Scheme 3) [23, 24].
Imides 5a-l were obtained in 51–71% yields (with respect to the adduct of rosin with maleic anhydride) or 60–99% (with respect to maleopimaric acid contained in the adduct of rosin with maleic anhydride). The duration of the reaction depends on the type of substituent in the aromatic ring and increases in the series R = OH (4 h) < CH3 (6 h) < Br (10 h) < H (16 h). Diimide diacid 6a was isolated in 46% yield upon refluxing MPA with p-aminoimide in dichlorobenzene for 3–8 h (Scheme 4). Upon fusion of MPA with 5d for 2 h at 260–270 °C, the product yield was 37%. Diacid diimides were obtained in higher yields upon the interaction of MPA with phenylenediamines (6a—68% and 6b—48%).
Dimethyl and diallyl ethers 7a, 7b were obtained from 6a by treatment with dimethyl sulfate or allyl bromide (Scheme 5).
N-arylimides of MPA were successfully separated into diastereomeric atropisomers 8a and 8b, stable at room temperature (Scheme 6) [25, 26]. Compound 8a exhibited higher cytotoxicity compared to fluorouracil. It was also revealed that the cytotoxicity of the R-configuration is higher than that of S.
As epoxy hardeners, diacid imide MPA 9 and diimide 10 in DMF at 160 °C in an argon atmosphere for 4 h were obtained (Scheme 7) [27, 28]. Rosin-based diacid imides have a significantly higher glass transition temperature, elastic modulus, and better dynamic-mechanical properties than diacid imide 12 obtained from trimellitic acid anhydride 11.
Yuvchenko et al. [16, 17, 29] obtained MPA N–H-alkylimides 13a-d by the interaction of primary aliphatic amines with MPA in melt or in solution, and salts 14a-f with amines and triphenylphosphine were synthesized (yields higher than 94%) [30] (Scheme 8).
Since the rate of formation of maleopimarimides in solution decreased with an increase in the length of the alkyl radical in amines, the reaction of MPA with dodecyl and octadecylamines was carried out in the melt. The yields of imides reached 99% and the reaction time was reduced by 4 times.
Azomethines 17f-x, 18a-n were synthesized by condensation of substituted benzaldehydes 16 with 3-aminophenylene-N-imide of MPA 15 (MeOH-DMF, 1:1, 3–4 h.) [31]. Maleopimarimide 15 was obtained by condensation of MPA with 3-phenylenediamine (toluene, 6 h.) (Scheme 9).
The possibility of obtaining azomethines using 4-aminophenylene-N-maleopimarimide was also shown [32]. 2-Aminothiazole derivatives of MPA have been synthesized 19 as potential antioxidants (Fig. 1) [33].
Sodium N-dodecylmaleimidepimarcarboxylate 21 was prepared as a surfactant (Scheme 10) [34].
Worm-like annular, filamentous, and ordinary micelles with a diameter of 100–200 nm in an anisotropic homogeneous phase were formed without any additives [35].
Methods of preparation and properties of methyl maleopimarate imides
Methyl ester 22 is one of the available derivatives of MPA, is obtained in good yield by treating MPA with diazomethane in diethyl ether (Scheme 11) [1].
Since MMP has a higher cytotoxic activity than MPA [36] the synthesis of imides MMP and the study of their biological activity are of great interest.
Studies of the inhibitory activity and cytotoxicity of maleopimarimide-substitutedphenylalanine show significant cytotoxicity against MGC-803 and Hct-116 cells (IC50 = 9.85 ± 1.24 and 8.47 ± 0.95 µM, respectively) [37].
The atropisomerism and kinetic data of N-arylimides MMP 23a-g were studied (Fig. 2) [38]. The compounds 23a-f with lower steric effects do not exhibit atropisomerism, whereas compounds 23 g undergo a slow cis–trans transition when dissolved in CDCl3 at room temperature.
A new class of atropisomericarylimides MMP 24, 25 with limited rotation around the C(SP2)–N bond was obtained and analyzed (Scheme 12) [39].
Effective method [40] for the synthesis of methyl maleopimarimides using 5-aminouracil under ultrasound exposure (with a frequency of 22 Hz) in DMSO has been developed (Table 1, Scheme 13).
Lossen's rearrangement of p-toluenesulfonate of N-hydroxymaleopimaric acid 28 in the presence of amines in methanol led to the formation of the corresponding ureidoesters 29, 30 with high regio- and stereoselectivity (Scheme 14) [41].
Treatment of the obtained ureidoesters 29 with bromine led to the product 31, and with phosphoryl chloride gave cyclic amidines by intramolecular cyclization 32. Dioxoimidazolidines 33 were obtained by the reaction of ureidoesters with glyoxal in an acidic medium, and treatment with sodium ethylate led to compounds of the naphtho [1,2-n] quinosaline series 34 (Scheme 15) [41].
Then 32 was converted to phenacyl derivative 35 (Scheme 16) [42].
The MMP-aspartic acid condensation product 36 was reacted with thionyl chloride in benzene, resulting in an anhydride 37. Then 37 reacted with a twofold excess of Ph3P = CH2 (Scheme 17), to form an isomeric mixture of products 38a-d [43].
An efficient method of condensation of MMP with amino acids under ultrasound exposure in DMSO was proposed (Scheme 18) [36, 40].
The resulting N-maleopimarimide-substituted amino acids were used to conjugate the diterpene block with important pharmacaphoric and functional groups: chloro-, bromomethyl ketones 41, 42 [44, 45], sulfide 43 [44], allenoates 44 [46,47,48,49], 1,2,3- triazoles 45 [49, 50], methanofullerenes 46 [51] and cyclopentenofullerenes 47 [52] C60 [53,54,55], adamantane derivatives 48 [56] (Schemes 19, 20).
The use of ultrasound made it possible to synthesize maleopimarimides even in the case of poorly soluble amines, significantly reduced the reaction time and increased the yield of target products [57]. A number of new maleopimarimides [36, 58] with fragments of: peptide 48a, b; aminoacridine 49, aminoguanidine 50, aminopyridines 51a, b; hydrazines 52a, b; ethanolamine 53, amantadine 54 have been obtained (Scheme 21).
Synthesis and properties of MPA amides
Syntheses of alkyl-, alkoxyphenylamides 56a-f and imidoamides 57a-h have been proposed through the maleopimaric acid chloride 55 [59]. Compounds 56a-f and 57a-h (Scheme 22) are capable of forming stable chiral liquid crystal compositions and can be used in various electro-optical systems for displaying and converting information, in particular, in displays with a matrix addressing system [59].
The synthesis of MPA N-aryl (aralkyl) imidoamides 59a-k was carried out by the reaction of the corresponding aromatic MPA amides with amines by refluxing in p-xylene (Scheme 23) [24, 60].
Boiling of MPA amides 58b-d in an aqueous-methanol solution of KOH for 2 h led to monoamides of trans-fumaropimaric acid 60a-c in 82–98% yields with an impurity of cis-isomers 61a-c, which were separated (Scheme 24) [61, 62].
The acylation of diamines 62–64 with 55 and benzotriazolylmaleopimarate 65 at a temperature of 20–65 ºC gave N- [3- (pimiridin-2-yl) aryl] amides MPA 66–69. Biologically active methanesulfonates 70, 71 were obtained from MPA N-arylamides 66, 68 (Scheme 25) [63].
New bioactive MPA amides 72 containing fragments of methyl esters of amino acids, aliphatic amines, imidazole, and N-methylpiperazine were synthesized (Fig. 3). The compounds act bi-directionally as anti-inflammatory and anti-ulcer agents and have no negative effects on the body [64].
Maleopimaric acid N-diethanolamide 73 was synthesized from maleopimaric acid and diethanolamine according to Scheme 26 [65]. Having better viscosity and stabilization indicators compared to a commercial dispersant—a condensate of sulfated naphthalene and formaldehyde, compound 73 can be used as a dispersant and a viscosity depressor in a water-coal suspension.
Compounds 75a-d exhibiting fungicidal and herbicidal activities and containing two thiosemicarbazidefragments were obtained (Scheme 27) [66].
Oxidation reactions and ozonolysis of MMP
The first modifications of MMP 22 are associated with the study of the ozonolysis of trimethyl ether 76 [67, 68], when the inertness of the double bond with regard to ozone was shown (Scheme 28). Ozone attack on the isopropyl group resulted in oxyether 77. This is followed by dehydration to diene 78 and ozonolysis to ketoester 79. Oxidation of ketone with KBrO led to acid 80, and with CF3CO3H to ketoester 81 [67, 68].
However, experiments [64, 69] show the possibility of the reaction proceeding at the olefin fragment. Ozonolysis of MMP followed by treatment with Me2S yielded diene 78 (10%), epoxide 82 (19%), alcohol 83 (18%), and keto acid 84 (32%) (Scheme 29). Ozonolysis of MMP in the CH2Cl2—MeOH system at 0 °C increased the yield of keto acid 84 [64].
Described ozonolysis of MMP in the presence of TCE, leading to epoxide 85 in 20% yield and stable ozonide 86 in 7% yield [65]. Compound 81 is oxidized under these conditions to oxylactone 87, the structure of which was confirmed by transformation into dilactone 88 and oxidation into ketolactone 89 (Scheme 30).
Triol 90 and imide 91 were oxidized to epoxides 92 and ketones 93. Triol 90, dioxy acid 94 and oxylactone 95 were formed by the reduction of MPA with LiAlH4 (Scheme 31) [70].
Selective oxidation of MPA with an excess of KMnO4 in an alkaline medium was carried out [1]. The yield of lactone 96 exceeds 90%. Upon oxidation with less than two equivalents of KMnO4, lactone 99 was obtained in 10% yield. Reduction of lactone 96 led to tetraol 98. The reaction of MPA with bromine in an alkaline medium gave bromlactone 99 (Scheme 32).
Regioselective oxidation of the double bond of MMP with dimethyldioxirane (DMD) led to 13(15)-ene-14S-hydroxy derivative 101 (Scheme 33) [71].
MPA in the synthesis of monomers and polymers
Polymers obtained on the basis of MPA have high thermal stability [72, 73], show good mechanical properties. These compounds have great prospects for use for various purposes, as evidenced by the progressive interest of chemists in research in this direction. The biochemical part of this subject can also be noted, for example, it is reported about new reliable antimicrobial agents 102, 103 obtained from resin acid, which are effective against a wide range of bacteria and do not cause significant hemolysis of red blood cells in a wide concentration range [74, 75]. The reaction of ethyl bromide with imide 104 leads to the quaternary ammonium salt 105 (Scheme 34). Further, esterification of the latter with propargylalcohol gives 102. Compound 103 was obtained by the action of azide-substituted poly(Ɛ-caprolactone) in the presence of a catalytic amount of CuI/DBU [74, 75].
Antifungal [76, 77], antimicrobial [78,79,80,81] activities of the MPA ammonium salts were also investigated. The potential application of quaternary ammonium derivatives of MPA as corrosion inhibitors [82], dispersants for magnetite nanoparticles [83, 84] and as inhibitors of protein aggregation processes [85,86,87].
In the work [88] maleopimarimide segments were introduced into the polymer product (Scheme 35). As the amount of diterpene in the molecule increased, the melting point, crystallization temperature and degree of crystallinity gradually decreased, while the impact strength and stretching increased.
Allyl imides were obtained with the subsequent synthesis of allyl ethers (Scheme 36) [89]. Imides 13b,c,e can be used as components of adhesive materials, allyl ethers 106b,c,e are of interest as new monomers for the functionalization of olefin copolymers and mixtures thereof. Octadecylmaleopimarimide 13e is of interest as a surfactant for thin films.
Since MPA contains both anhydride and carboxyl groups, the selective esterification of the carboxyl group with alcohols is difficult. Allyl bromide and propargyl bromide reacted regioselectivelywithcarboxyl group of MPA upon treatment with K2CO3 in DMF to form 107 (Scheme 37) [90]. Compound 107a was also synthesized in 62% yield using oxalyl chloride and allyl alcohol in THF [91].
The reaction of esterification of the maleopimaric adduct with α,α,ω-trihydroperfluoroalkanols under catalysis with concentrated sulfuric acid at 150–220 °C led to new three substituted polyfluoroalkyl ethers 118 in 55–70% yields (Scheme 38) [92, 93].
A chain extender based on MPA 109 was synthesized and introduced. The polyurethane polymer with the shape memory effect at 100% deformation reshapes to 96% in 3 min at room temperature (Scheme 39) [19].
A method was proposed for the preparation of new epoxy resins 110, which are of interest as a hardener for polyester powder paints with a high glass transition temperature (~ 153.8 °C), a high storativity at room temperature (~ 2.4 hPa), and good thermal stability (Fig. 4) [19, 94].
To obtain bionanocomposites, an epoxy resin based on MPA 111 [95, 96] was synthesized as well as polyurethane based on castor oil and carbon nanotubes (Scheme 40). The impact strength of such a film is 15 kg/cm higher than that of a pure resin system; the cell survival rate for 48 and 72 h exceeds 90%, which indicates excellent biocompatibility. The synthesis of tetraglycidyl dimaleopimaryl ketone 112 has also been reported [97].
The epoxyacrylate derivative of MPA 113 (EEW = 199.68 g/eq.) is suitable for use as a resin cross-linked with styrene, methacrylated eugenol or methacrylated guaiacol and can be prepared according to scheme 41 [98] via maleopimaric acid triester, trimethylolpropane and epoxy derivative [99].
Diterpenoidethylene glycol acrylate 114 has been proposed as combinatorial cross-linking agents [100], a selective stationary phase in HPLC [101] and to increase the thermal stability of styrene-acrylate copolymers [102] (Scheme 42).
Polymer 115a was proposed as a base for two-component polyurethane water-dispersion coatings and paints [103]. The inclusion of a diterpene moiety improved such material properties as strength, gloss, hardness, water resistance and alcohol resistance [104]. The synthesis, modification and properties of nonisocyanate polyurethane coatings based on MPA 115b were also studied (Fig. 5) [105].
The authors [106] compared the rosin-modified phenolic resin with an environmentally friendly, phenol-free resin 116, which exhibited superior carrier properties in terms of gloss, yellowing, usability and storage stability (Scheme 43).
Dianhydride MPA 117 was obtained by boiling MPA with methyl sulfonic acid in toluene under nitrogen. Further, a polyamide-imide copolymer 118 was obtained, which can be used as a structural plastic and also as film materials (Scheme 44) [107].
Benzoxazines are a special type of aminophenol formaldehyde resins and high temperature polymer binders [108]. Diterpene benzoxazine monomer 119 was obtained via imidophenol (88% yield) using aniline or 4-aminobenzoic acid (Scheme 45) [109, 110].
Tetraglycyl epoxy ester resins 120a,b with high strength and high chemical resistance to solvents were investigated (Fig. 6) [111].
A trivinyl derivative of MPA 121 (Scheme 46) was synthesized and proposed as an alternative to some petroleum-based monomers [112]. It is considered as hard monomers for copolymerization with acrylic epoxidized soybean oil having improved glass transition temperature, tensile modulus, and curing modulus.
Further, a mechanism for the polymerization of tri-allylmaleopimarate was proposed (Scheme 47) [113].
The use of rosin-based polycaprolactones with flexible dianhydride part 122, obtained according to scheme 48 [114]—as a bio-based curing agent for epoxy resins.
By creating a biofunctional vinyl ester resin 123, an excellent alternative to styrene has been proposed [98]. The obtained samples showed better thermal stability and mechanical strength and better chemical and corrosion resistance and had comparable characteristics compared to oil based materials (Fig. 7).
Diterpenoid polyethylene glycol ethers (PEGs) have been investigated as microencapsulated materials for long-term drug delivery [115, 116]. Two approaches have been shown for the synthesis of oligomers 124,125 (Scheme 49). Compounds of this type can also be used as dental films for the treatment of periodontitis [117] and as anticorrosive materials for carbon steel [118].
Obtained by esterification of rosin with different molecular weights of polyethylene glycol (PEG 400, 600, 1000, 2000) (Scheme 50), surfactant derivatives of MPA126 were proposed as oil sludge dispersants [119, 120].
The introduction of MPA into a fluorosilicone rubber according to Scheme 51 can enhance its microphase separation, ultimate tensile strength, and heat resistance [121].
MPA in the synthesis of photoresists
The reaction of MPA with hydroxylamine gave N-hydroxymaleopimarimide 127, which was esterified with 2,1,4-DNQ-Cl to obtain N-hydroxymaleopimarimide sulfonate 128 (Scheme 52) [18]. The 2,1,4-DNQ group is easily photolyzed under light irradiation at 365 nm. Thus, new single-component glasses of molecular composition 129a-c were obtained with good yields, which showed great potential as high-performance photoresists [18].
The polyaddition reaction of N-hydroxymaleopimarimide 127 with divinyl ethers made it possible to obtain new acetoester polymers 130a-c according to Scheme 53 [122]. The ester bond in the polymer chain can be hydrolyzed in the presence of a strong acid with mild heating. The obtained polymer films have excellent UV light transmission (above 230 nm) and great potential for use as high-performance photoresists in lithography technology.
Sulfonate derivatives of N-hydroxymaleopimarimides 131a-c, promising as photoacid generators of a new type, were obtained (Fig. 8) [123]. Sulfonate compounds have good solubility in typical organic solvents and high thermal stability, transparency within 193 nm, and can be used as polyalkylene glycol (PaGs) photoresists.
MPA in the synthesis of chiral ligands
Phosphorus-containing ligands and chiral complexes of rhodium (I) were synthesized for enantioselective reactions [124]. MPA 1 was converted to triol 132 (Scheme 54). Further protection and benzylation resulted in the desired diol 133 in 40% yield and the tetrahydrofuran derivative 134 in 50% yield. And the use of a weaker acid, pyridinium p-toluenesulfonate, increased the diol 133 yield to 80%. Next, the diol 133 was converted to the chiral ligand of bisphosphine 135 via ditosylate. The asymmetric hydrogenation of (Z)-N-acetylaminocinnamic acid and its derivative with the catalytic amount of the obtained cationic complex Rh (I) 136 led to 137a in 27% optical yield and 137b- 37% [124].
The synthesis of chiral alcohols and phosphorus derivatives based on MPA was reported. Their use in31P-NMR analysis for the determination of the enantiomeric excess of alcohols and amines was proposed [125].
Also the synthesis of crown ether 138 according to Scheme 55 was reported [126]. The MMP derivative is capable of recognizing the enantiomers of amines.
Some rosin-modified materials
A simple method for making rosin-modified superhydrophobic wood surfaces by impregnation has recently been reported (Scheme 56) [127].
Similar materials based on rosin and starch were also obtained [128].
Based on the results of the absorption experiments, it was shown that nano-micelles 139 obtained from MPA and tetraethylenepentamine in aqueous solution have an outstanding ability to absorb metal (Fig. 9). Moreover, the adsorption of metal ions did not depend on pH, and the materials had a higher affinity for Ni (II) than for Cu (II) and Cd (II) [129].
The interaction of microcrystalline cellulose with maleopimaric acid chloride gave ether, which is promising as thermoplastic materials, hot melt adhesives, superhydrophobizing agents for paper, or as biodegradable polymers (Fig. 10) [130].
Conclusion
Analysis of literature data indicates that maleopimaric acid and its monomethyl ether have unique properties and attract many researchers from all over the world to create affordable and valuable materials based on them. A wide range of biological activity of diterpenoid derivatives is associated with the structural features of maleopimaric acid, namely the similarity of the structure of the A, B and C rings with the structure of steroid hormones.
To date, mainly aromatic and heterocyclic amides, aliphatic and aromatic imides of MPA have been obtained. The target products are obtained both from the individually isolated MPA in the reaction with amines, and from the resin or rosin by the reaction of diene synthesis with N-substituted maleimides. Various high-boiling solvents have been proposed to accelerate the process (glacial acetic acid, toluene, xylene, DMF, dichlorobenzene, etc.). An efficient method was developed for the synthesis of imides MMP upon condensation with amino acids and various amines under ultrasonic action in a DMSO medium [40, 57]. The method made it possible to increase the yields of target products, shorten the reaction time by an order of magnitude, and synthesize maleopimarimides even in the case of poorly soluble amines by dispersing adducts. Diacid diimides were obtained in the reaction of diamines with a twofold excess of MPA [23, 27].
The chemistry of diterpenoids has developed greatly in recent years [131]. The interest in these compounds is due to their availability, low cost, low toxicity, and easy modifiability. To date, data have been obtained on the most probable fields of application of MPA derivatives in medicine, etc., including in the form of polymer systems. These compounds can be successfully used as biodegradable polymers, high-performance photoresists and chiral ligands, engineering plastics, film materials, curing agents, absorbents, thermoplastic materials, hot melt adhesives, surfactants, paints and coatings, superhydrophobizators and sealing agents for paper, photoacid generators of a new type, corrosion inhibitors, dispersants for magnetite nanoparticles (Fe3O4), inhibitors of protein aggregation processes, as microencapsulated materials for drug delivery, etc.
Thus, maleopimaric acid and substances based on it can be confidently considered excellent objects of innovative research, and the expansion the scope of application of these compounds in the synthesis of various materials with valuable properties is of interest both from a scientific point of view and from a practical point of view.
References
L.H. Zalkov, R.A. Ford, J.P. Kutney, J. Org. Chem. 27(10), 3535–3537 (1962)
C. Ling, T. Gongye, Paint Coating Ind. 35(2), 12–15 (2006)
E.V. Tretyakova, I.E. Smirnova, O.B. Kazakova, G.A. Tolstikov, Bioorg. Med. Chem. 22, 6481 (2014). https://doi.org/10.1016/j.bmc.2014.09.030
E. Haslinger, D. Hofner, Monatsh. Chem. 129, 297–308 (1998). https://doi.org/10.1007/PL00000088
D. Hofner, E. Haslinger, Monatsh. Chem. 129, 393–407 (1998). https://doi.org/10.1007/PL00000096
A. Tolstikov, N. Karpyshev, O. Tolstikova, T. Khlebnikova, G. Sal’nikov, V. Mamatyuk, Y. Gatilov, I. Bagryanskaya, Russ. J. Org. Chem. 37(8), 1134–1148 (2001). https://doi.org/10.1023/A:1013144431609
V.V. Plemenkov, S.A. Appolonova, D.A. Kirlitsa, ‘To the question of the native content of resin acids in the resin of conifers’, Khimiya I komp'yuternoye modelirovaniye, Butlerovskiye soobshcheniya [Chemistry and Computational Simulation. Butlerov Communs.] 5(1), 30 (2004) (in Russian).
V.E. Medyantsev, V.M. Fomin, Vestnik Nizhegorodskogo universiteta im. N.I. Lobachevskogo. Seriya: Khimiya [Bulletin N.I. Lobachevsky University of the Nizhny Novgorod. Series: Chemistry.] 1, 37–42 (2004) (in Russian).
D.O. Foster, D.F. Zinkel, J. Chromatogr. 248(1), 89–98 (1982)
A.Yu. Klyuev, E.D. Skakovsky, N.G. Kozlov, N.R. Prokopchuk, N.D. Gorscharik, et al., ‘Study of the composition of terpenoid-maleic adducts’, Trudy BGTU. Khimiya, tekhnologiya organicheskikh veshchestv I biotekhnologiya [Proceedings of BSTU.Chemistry, organic matter technology and biotechnology] 4, 154–164 (2015) (in Russian).
F. Z. Galin, O. B. Flekhter, E. V. Tretyakova, Khimiya I komp'yuternoye modelirovaniye. Butlerovskiye soobshcheniya [Chemistry and Computational Simulation. Butlerov communs.] 5(2), 1–21 (2004) (in Russian).
I. Portugal, J. Vital, L.S. Lobo, Chem. Eng. Sci. 51(11), 2577–2582 (1996)
E.D. Skakovsky, L.Yu. Tychinskaya, O.A. Gaidukevich, A.Yu. Klyuev, N.G. Kozlov, A.V. Baranovsky, S.V. Rykov, [Structure and dynamics of molecular systems] 1, 545–548 (2007) (in Russian).
E. D. Skakovsky, L. Yu. Tychinskaya, S. V. Matveichuk, A. Yu. Klyuev, et al. Trudy BGTU. Khimiya, tekhnologiya organicheskikh veshchestv I biotekhnologiya [Proceedings of BSTU. Chemistry, organic matter technology and biotechnology] 1(241), 74–81 (2021) (in Russian).
M.P. Bei, A.P. Yuvchenko, Russ. J. Gen. Chem. 80(2), 228–232 (2010). https://doi.org/10.1134/S107036321002012X
M. P. Bei, Vestsi Natsyyanal’nai akademii navuk Belarusi. Seryya khimichnykh navuk [Proceedings of the National Academy of Sciences of Belarus, chemical series] 5, 13–14 (2006) (in Russian).
M. P. Bei, Abstract ... Cand. Chem. Sciences, Minsk, 6 (2015) http://iboch.bas-net.by/files-iboch/k_Bei.pdf (in Russian).
J. Yu, N. Xu, Zh. Liu, L. Wang, A.C.S. Appl, Mater. Interfaces 4, 2591–2596 (2012). https://doi.org/10.1021/am300259g
L. Zhang, Y. Jiang, Zh. Xiong, X. Liu, H. Na, R. Zhang, J. Zhu, Mater. Chem. A. 1, 3263–3267 (2013). https://doi.org/10.1039/C3TA01655B
C. Huani, Y. Manyi, Y. Guiyang, L. Yajin, Zh. Yongtao, W. Hengshan, Chem. J. Chin. Univer. Chin. 35(4), 839–846 (2014). https://doi.org/10.7503/cjcu20130796
X. Liu, J. Zhu, Patent CN102329309A, 25.01.2012. https://patents.google.com/patent/CN102329309A/en
D.YA. Svikle, R.A. Rasinya, A.YA. Prikule, YA.G. Zandersons, A.I. Zhurin'sh, G.K. Baumane, A.A. Rupays, A.YA. Kul'kevits, Patent USSR SU1157834A1. 15.07.1994. https://patents.google.com/patent/SU1157834A1/en?oq=1157834
M.P. Bei, A.P. Yuvchenko, N.V. Puchkova, Russ. J. Gen. Chem. 85(5), 1034–1039 (2015). https://doi.org/10.1134/S1070363215050047
M. P. Bei, A. P. Yuvchenko, Vestsi Natsyyanal’nai akademii navuk Belarusi. Seryya khimichnykh navuk [Proceedings of the National Academy of Sciences of Belarus, chemical series] 1, 74–78 (2010) ISSN: 1561–8331.
G. Yao, M. Ye, R. Huang, Y. Li, Y. Zhu, Y. Pan, Z.-X. Liao, H. Wang, Bioorg. Med. Chem. Lett. 23, 6755–6758 (2013). https://doi.org/10.1016/j.bmcl.2013.10.028
H. Wang, H. Wang, G. Zhou, Polym. Int. 60, 557–563 (2011). https://doi.org/10.1002/pi.2978
X. Liu, W. Xin, J. Zhang, Biores. Technol. 101, 2520–2524 (2010). https://doi.org/10.1016/j.biortech.2009.11.028
F.R. Mustata, N. Tudorachi, Ind. Eng. Chem. Res. 49, 12414–12422 (2010). https://doi.org/10.1021/ie101746v
A.P. Yuvchenko, M.P. Bei, Vestsi Natsyyanal’nai akademii navuk Belarusi. Seryya khimichnykh navuk [Proceedings of the National Academy of Sciences of Belarus, chemical series] 4, 68−74 (2013)
E.A. Dikusar, M.P. Bei, Khimiya rastitel'nogo syr'ya [Chemistry of plant raw materials] 1, 105–109 (2011) (in Russian).
M.P. Bei, A.P. Yuvchenko, E.A. Dikusar, V.I. Potkin, Russ. J. Gen. Chem. 81(7), 562–565 (2011). https://doi.org/10.1134/S1070363211030200
E.A. Dikusar, M.P. Bei, A.P. Yuvchenko, V.I. Potkin, Russ. J. Gen. Chem. 80(7), 1320–1323 (2010). https://doi.org/10.1134/S1070363210070170
L.R. Yakupova, R.A. Nasibullina, V.A. Shamukaev, R.M. Sultanova, R.L. Safiullin, Kinet. Catal. 61(2), 232–237 (2020). https://doi.org/10.1134/S0023158420020123
Zh. Zhai, X. Yan, Zh. Song, Sh. Shang, X. Rao, Soft Matter 14, 499–507 (2018). https://doi.org/10.1039/C7SM02163A
Z. Zhai, X. Yan, J. Xu, Z. Song, S. Shang, X. Rao, Chem. Eur. J. 24, 9033–9040 (2018). https://doi.org/10.1002/chem.201800628
I.M. Sakhautdinov, R.N. Malikova, D.V. Khasanova, L.F. Zainullina, V.A. Vakhitov, A.N. Lobov, Y.V. Vakhitova, M.S. Yunusov, Lett. Org. Chem. 15(10), 854–862 (2018). https://doi.org/10.2174/1570178615666180212154722
G. Yao, M. Ye, Y. Zhu, Zh. Liao, H. Wang, Anti-Cancer Agents Med. Chem. 16(6), 755–762 (2016). https://doi.org/10.2174/1871520616666151116121628
H. Chen, M. Ye, G. Yao, Y. Li, Yo. Zhu, H. Wang, Anal. Chem. J. Chin. Univ. 35(4), 839–846 (2014). https://doi.org/10.7503/cjcu20130796
G. Yao, Y. Li, Yo. Zhu, Y. Pan, F. Huang, H. Wang, Zh. Liao, New J. Chem. 38, 693–699 (2014). https://doi.org/10.1039/c3nj01194a
I.M. Sakhautdinov, R.N. Malikova, S.M. Ishbaeva, A.N. Lobov, L.V. Spirikhin, M.S. Yunusov, Chem. Nat. Compd. 54(2), 365–367 (2018). https://doi.org/10.1007/s10600-018-2348-5
S.V. Chernov, E.E. Shul’ts, M.M. Shakirov, V.Yu. Gatilov, G.A. Tolstikov, Russ. J. Org. chem. 46(8), 1140–1150 (2010). https://doi.org/10.1134/S1070428010080051
S.V. Chernov, E.E. Shul’ts, M.M. Shakirov, V.Yu. Gatilov, G.A. Tolstikov, Doklady Chimii 423(1), 299–304 (2008). https://doi.org/10.1134/S0012500808110098
N. A. Sergeeva, dis. … Cand. chem. Science: Ufa, 69 (2012) https://dlib.rsl.ru/viewer/01005046919#?page=1
I.M. Sakhautdinov, R.N. Malikova, O.V. Zakir’yanova, M.F. Abdullin, M.S. Yunusov, Chem. Nat. Compd. 52(1), 73–76 (2016). https://doi.org/10.1007/s10600-016-1551-5
I.M. Sakhautdinov, R.N. Malikova, O.V. Akchurina, S.F. Petrova, M.S. Yunusov, Lett. Org. Chem. 14(8), 575–584 (2017). https://doi.org/10.2174/1570178614666170614091621
R.N. Malikova, I.M. Sakhautdinov, M.A. Maksimova, USh. Kuzmina, Yu.V. Vakhitova, M.S. Yunusov, Russ. J. Bioorg. Chem. 46(1), 115–119 (2020). https://doi.org/10.1134/S1068162020010057
I.M. Sakhautdinov, A.M. Gumerov, R.N. Malikova, A.A. Fatykhov, M.S. Yunusov, Chem. Nat. Compd. 52(4), 651–655 (2016). https://doi.org/10.1007/s10600-016-1731-3
I.M. Sakhautdinov, A.M. Gumerov, R.N. Malikova, A.A. Fatykhov, M.S. Yunusov, J. Indian. Chem. Soc. 94, 327–330 (2017)
R.N. Malikova, I.M. Sakhautdinov, M.F. Abdullin, A.F. Mukhametyanova, M.S. Yunusov, Chem. Nat. Compd. 53(2), 341–344 (2017). https://doi.org/10.1007/s10600-017-1984-5
R.N. Malikova, I.M. Sakhautdinov, M.S. Yunusov, Chem. Nat. Compd. 55(1), 60–65 (2019). https://doi.org/10.1007/s10600-019-02614-w
Yu.N. Biglova, R.N. Malikova, S.F. Petrova, S.P. Ivanov, I.M. Sakhautdinov, A.G. Mustafin, Int. J. Chem. Kinet. 51, 311–320 (2019). https://doi.org/10.1002/kin.21254
I.M. Sakhautdinov, R.N. Malikova, Yu.N. Biglova, R.A. Khusnutdinov, A.M. Gumerov, E.M. Khamitov, S.P. Ivanov, M.S. Yunusov, J. Iran. Chem. Soc. 15(9), 1975–1985 (2018). https://doi.org/10.1007/s13738-018-1395-y
I.M. Sakhautdinov, R.N. Malikova, T.R. Nugumanov, Yu.N. Biglova, A.B. Atangulov, M.S. Yunusov, Chem. Nat. Compd. 54(3), 481–486 (2018). https://doi.org/10.1007/s10600-018-2384-1
R.N. Malikova, I.M. Sakhautdinov, S.M. Ishbaeva, M.S. Yunusov, Russ. J. Gen. Chem. 87(10), 2497–2499 (2017). https://doi.org/10.1134/S1070363217100371
L.R. Yakupova, I.M. Sakhautdinov, R.N. Malikova, R.L. Safiullin, Kinet. Catal. 60(1), 21–27 (2019). https://doi.org/10.1134/S0023158419010130
I.M. Sakhautdinov, R.N. Malikova, M.S. Yunusov, Chem. Nat. Compd. 54(1), 102–105 (2018). https://doi.org/10.1007/s10600-018-2269-3
I. M. Sakhautdinov, R. N. Malikova, Yu.V. Vakhitova, O.V. Zakir'yanova, M.S. Yunusov, Patent RU2591193, 10.07.2016, https://patents.google.com/patent/RU2591193C1/en?oq=RU2591193
R.N. Malikova, I.M. Sakhautdinov, E.O. Terenteva, Z.S. Khashimova, M.S. Yunusov, Sh.S. Azimova, Chem. Nat. Compd. 56(1), 101–104 (2020). https://doi.org/10.1007/s10600-020-02953-z
M. P. Bei, A. P. Yuvchenko, Al. An. Muravskii, and An. Al. Muravskii, ‘New Amides and Imidoamides of Maleopimaric Acid as a Chiral Dopants for Nematic Liquid Crystal Compositions’, Russ. J. Gen. Chem. 88(2), 251–256 (2018) DOI:https://doi.org/10.1134/S107036321802010X
M.P. Bei, A.P. Yuvchenko, O.V. Sokol, N.V. Puchkova, Russ. J. Gen. Chem. 86(4), 821–825 (2016). https://doi.org/10.1134/S1070363216040101
M.P. Bei, A.P. Yuvchenko, Bulletin of NAS of Belarus. Chem. sciences ser. 3, 84–87 (2010). (in Russian)
M. P. Bei, A. P. Yuvchenko; S. A. Makhnach, XXV Jubilee Intern. scientific and technical conf. – Ufa, 74–75 (2011) (in Russian).
E.V. Koroleva, K.N. Gusak, Zh.V. Ignatovich, A.L. Ermolinskaya, M.P. Bei, A.P. Yuvchenko, Russ. J. Org. Chem. 48(8), 1121–1125 (2012). https://doi.org/10.1134/S1070428012080143
O.B. Kazakova, E.V. Tret’yakova, O.S. Kukovinets, G.A. Tolstikov, T.I. Nazyrova, I.V. Chudov, A.F. Ismagilov, Russ. J. Bioorg. Chem. 36(6), 762–770 (2010). https://doi.org/10.1134/S1068162010060130
J. Li, G. Zhang, T. Shang, J. Zhu, Int. J. Min. Sci. Technol. 24, 695–699 (2014). https://doi.org/10.1016/j.ijmst.2014.03.025
G.-S. Lin, S.-Q. Dong, W.-G. Duan, B. Cen, X.-T. Xu, Z.-Q. Yang, Holzforschung 68, 549–554 (2013). https://doi.org/10.1515/hf-2013-0124
E.V. Tretyakova, I.E. Smirnova, E.V. Salimova, V.N. Odinokov, Bioorg. Med. Chem. 23(20), 6543–6550 (2015). https://doi.org/10.1016/j.bmc.2015.09.006 (PMID: 26372075)
L.H. Zalkow, N.N. Girotra, J. Org. Chem. 28, 2033 (1963)
S.C. Ness, M.I. Farah, S.Y. Eguhib, I.P. Mamura, J. Braz. Chem. Soc. 11(1), 59 (2000). https://doi.org/10.1590/S0103-50532000000100011
W. Seebacher, A. Hufner, E. Haslinger, R. Weis, Monatsh. Chem. 129, 697 (1998)
T.I. Nazyrov, E.V. Tret’yakova, O.B. Kazakova, L.V. Spirikhin, O.S. Kukovinets, Chem. Nat. Compd. 48(6), 1002–1003 (2013). https://doi.org/10.1007/s10600-013-0449-8
S.S. Ray, A.K. Kundu, S. Maiti, J. Appl. Polym. Sci. 36(6), 1283–1293 (1988)
L. Rosu, F. Mustata, D. Rosu, C.-D. Varganici, I. Rosca, T. Rusu, Prog. Org. Coat. 151, 106008 (2021). https://doi.org/10.1016/j.porgcoat.2020.106008
J. Wang, Y.P. Chen, K. Yao, P.A. Wilbon, W. Zhang, Chem. Commun. 48, 916–918 (2012). https://doi.org/10.1039/c1cc16432e
Ch. Tang, J. Wang, A. W. Decho, Y. P. Chen Patent US9357775. 2016.06.07. https://patents.google.com/patent/US9357775B2
H. Wang, T.T.H. Nguyen, S. Li, T. Liang, Y. Zhang, J. Li, Bioorg. Med. Chem. Lett. 25, 347–354 (2015). https://doi.org/10.1016/j.bmcl.2014.11.034
Z. Chen, S. Li, B. Tian, T. Liang, Y. Jin, Environ. Eng. Sci. 29, 606–610 (2011). https://doi.org/10.1089/ees.2011.0043
G. Liu, C. Chen, G. Wu, Z. Kong, BioResources 8, 4218–4226 (2013). https://doi.org/10.15376/biores.8.3.4218-4226
Zh. Li, X. Yang, H. Liu, X. Yang, Y. Shan, X. Xu, Sh. Shang, Zh. Song, Chem. Eng. J. 374, 564–575 (2019). https://doi.org/10.1016/j.cej.2019.05.208
Zh. Li, H. Liu, X. Xu, L. Ma, Sh. Shang, Zh. Song, Mater. Des. 189, 108493 (2020). https://doi.org/10.1016/j.matdes.2020.108493
H. Lin, M. Yang, C. Tian, C. Han, J. Song, J. Duan, J. Jiang, Colloid Surf. B 165, 191–198 (2018). https://doi.org/10.1016/j.colsurfb.2018.01.049
M.A. Atta, A.G. El-Mahdy, K.F. Dyab, A.H. Allohedan, Int. J. Electrochem. Sci. 8, 9629–9643 (2013)
A. Atta, G. El-Mahdy, H. Al-Lohedan, S. Al-Hussain, A.M. Atta, G.A. El-Mahdy, H.A. Al-Lohedan, S.A. Al-Hussain, Int. J. Mol. Sci. 15, 6974–6989 (2014). https://doi.org/10.3390/ijms15046974
A.M. Atta, A.M. El-Saeed, G.M. El-Mahdy, H.A. Al-Lohedan, RSC Adv. 5, 101923–101931 (2015). https://doi.org/10.1039/C5RA20730D
M. Ishtikhar, R.M.V. Khan, R.H. Khan, Int. J. Biol. Macromol. 93, 1174–1182 (2016). https://doi.org/10.1016/j.ijbiomac.2016.09.089
M. Ishtikhar, T.I. Chandel, A. Ahmad, M.S. Ali, H.A. Al-lohadan, A.M. Atta, R.H. Khan, PLoS ONE 10, e0139027 (2015). https://doi.org/10.1371/journal.pone.0139027
M. Ishtikhar, S.S. Usmani, N. Gull, G. Badr, M.H. Mahmoud, R.H. Khan, Int. J. Biol. Macromol. 78, 379–388 (2015). https://doi.org/10.1016/j.ijbiomac.2015.03.069
X. Liu, Ch. Li, D. Zhang, Y. Xiao, G. Guan, Polym Int. 55, 545–551 (2006). https://doi.org/10.1002/pi.2006
M.P. Bei, A.P. Yuvchenko, Russ. J. Gen. Chem. 80(2), 253–257 (2010). https://doi.org/10.1134/S107036321002012X
M.P. Bei, V.A. Azarko, A.P. Yuvchenko, Russ. J. Gen. Chem. 80(5), 940–944 (2010). https://doi.org/10.1134/S1070363210050130
J. Wang, J. Yu, Y. Liu, Y. Chen, C. Wang, C. Tang, F. Chu, Green Mater 1, 105–113 (2013). https://doi.org/10.1680/gmat.12.00013
L.M. Popova, S.V. Vershilov, A.U. Selivanova, Khimiya rastitel'nogo syr'ya [Chemistry of plant raw materials] 1, 73–76 (2014) (in Russian). DOI: https://doi.org/10.14258/jcprm.1401073
L.M. Popova, V.A. Ivanova, S.V. Vershilov, Khimiya rastitel'nogo syr'ya [Chemistry of plant raw materials] 2, 205–211 (2019) https://doi.org/10.14258/jcprm.2019023999
X. Liu, J. Zhang, Polym. Int. 59, 607–609 (2010). https://doi.org/10.1002/pi.2781
X.Q. Liu, W. Huang, Y.H. Jiang, J. Zhu, C.Z. Zhang, Express Polym. Lett. 6, 293–298 (2012). https://doi.org/10.3144/expresspolymlett.2012.32
L. Huo, D. Wang, H. Liu, P. Jia, J. Gao, J. Biomater. Sci. Polym. Ed. 27, 1100–1114 (2016). https://doi.org/10.1080/09205063.2016.1183332
R.A. El-Ghazawy, A.M. El-Saeed, H.I. Al-Shafey, A.-R.M. Abdul-Raheim, M.A. El-Sockary, Eur. Polym. J. 69, 403–415 (2015). https://doi.org/10.1016/j.eurpolymj.2015.06.025
S. Jaswal, B. Gaur, Polym. Sci. Ser. B 57, 417–433 (2015). https://doi.org/10.1134/S1560090415050048
T. Thakur, S. Jaswal, S. Parihar, B. Gaur, A.S. Singha, Express Polym Lett 14(6), 512–529 (2020). https://doi.org/10.3144/expresspolymlett.2020.42
P. Li, T. Wang, F. Lei, P. Tang, X. Tan, Z. Liua, L. Shena, Polym Int 63, 1699–1706 (2014). https://doi.org/10.1002/pi.4694
X. Li, M. Li, J. Li, F. Lei, X. Su, M. Liu, P. Li, X. Tan, Anal. Methods 6, 6397–6406 (2014). https://doi.org/10.1039/C4AY00810C
C.L. Yu, F.A. Zhang, Q.H. Gong, Adv. Mater. Res. 236–238, 728–731 (2011). https://doi.org/10.4028/www.scientific.net/AMR.236-238.728
H. Si, H. Liu, Sh. Shang, J. Song, Sh. Liao, D. Wang, Zh. Song, Progr. Org. Coat. 90, 309–316 (2016). https://doi.org/10.1016/j.porgcoat.2015.11.003
H. Si, H. Liu, S. Shang, J. Song, S. Liao, D. Wang, Z. Song, J. Appl. Polym. Sci. 133, 43292 (2016). https://doi.org/10.1002/app.43292
G. Liu, G. Wu, J. Chen, Z. Kong, Prog. Org. Coat. 101, 461–467 (2016). https://doi.org/10.1016/j.porgcoat.2016.09.019
Y.B. Ha, M.Y. Jin, S.S. Oh, D.H. Ryu, Bull. Korean Chem. Soc. 33, 3413–3416 (2012). https://doi.org/10.5012/bkcs.2012.33.10.3413
H. Zhu, Zh. Wang, Patent CN102702525. 02.04.2014. https://patents.google.com/patent/CN102702525A/ru
X. Qingyu, Z. Ming, C. Jiangbing et al., React. Funct. Polym. 122, 158–166 (2018). https://doi.org/10.1016/j.reactfunctpolym.2017.12.001
S. Li, T. Zou, X. Liu, M. Tao, Des. Monomers Polym. 17, 40–46 (2014). https://doi.org/10.1080/15685551.2013.771317
J. Liu, T. Agag, H. Ishida, Polymer 51, 5688–5694 (2010). https://doi.org/10.1016/j.polymer.2010.08.059
A.M. Atta, R. Mansour, M.I. Abdou, A.M. El-Sayed, J. Polymer Res. 12, 127–138 (2005). https://doi.org/10.1007/s10965-004-2936-x
Q. Ma, X. Liu, R. Zhang, J. Zhu, Y. Jiang, Green Chem. 15, 1300–1310 (2013). https://doi.org/10.1039/c3gc00095h
Y. Lu, Z. Zhao, L. Bi, Y. Chen, J. Wang, S. Xu, Sci. Rep. 8, 2399 (2018). https://doi.org/10.1038/s41598-018-20695-5
H. Wang, X. Liu, B. Liu, J. Zhang, M. Xian, Polym. Int. 58, 1435–1441 (2009). https://doi.org/10.1002/pi.2680
D.M. Morkhade, V.S. Nande, U.V. Barabde, A.T. Patil, S.B. Joshi, AAPS Pharm. Sci. Tech. 8, 134 (2007). https://doi.org/10.1208/pt0802047
D.M. Morkhade, V.S. Nande, U.V. Barabde, S.B. Joshi, J. Bioact. Compat. Polym. 32, 628–640 (2017). https://doi.org/10.1177/0883911517705404
D.M. Morkhade, V.S. Nande, U.V. Barabde, A.T. Patil, S.B. Joshi, Drug Dev. Ind. Pharm. 44, 914–922 (2018). https://doi.org/10.1080/03639045.2017.1421660
G.A. El-Mahdy, A.M. Atta, H.A. Al-Lohedan, Int. J. Electrochem. Sci. 8, 5052–5066 (2013)
A.M. Atta, A.M. Elsaeed, J. Appl. Polym. Sci. 122, 183–192 (2011). https://doi.org/10.1002/app.34052
A.M. Atta, A.M. Ramadan, K.A. Shaffei, A.M. Nassar, N.S. Ahmed, M. Fekry, J. Disper. Sci. Technol. 30, 1100–1110 (2009). https://doi.org/10.1080/01932690802597806
T. Xu, H. Liu, J. Song, S. Shang, Z. Song, K. Zou, C. Yang, J. Appl. Polym. Sci. 132, 41888 (2015). https://doi.org/10.1002/app.41888
J. Yu, N. Xu, Q. Wei, L. Wang, J. Mater. Chem. C. 1, 1160–1167 (2013). https://doi.org/10.1039/c2tc00670g
L. Wang, W. Wang, X. Guo, Proc. of SPIE 5376, 608–615 (2004). https://doi.org/10.1117/12.534513
T.B. Khlebnikova, N.N. Karpyshev, O.V. Tolstikova, A.G. Tolstikov, Chirality 16, 40–50 (2004). https://doi.org/10.1002/chir.20045
Q. Wu, G. Yao, Y. Zhang, H. Wang, L. Yang, Y. Zhu, Y. Pan, Chem. Res. Chin. Univ. 29, 894–899 (2013). https://doi.org/10.1007/s40242-013-3009-7
H. Wang, C. He, Y. Pan, G. Yao, Q. Wu, H. Deng, J. Incl. Phenom. Macrocycl. Chem. 73, 177–183 (2012). https://doi.org/10.1007/s10847-011-0040-5
M. Yang, X. Chen, H. Lin, C. Han, S. Zhang, Eur. J. Wood Prod. 76, 1417–1425 (2018). https://doi.org/10.1007/s00107-018-1319-7
H. Li, R. Lin, J. He, H. Long, W. Liao, Q. Chen, New J. Chem. 40, 2856–2862 (2016). https://doi.org/10.1039/C5NJ02757H
W.-X. Tan, Z.-T. Lin, H.-T. Bu, Y. Tian, G.-B. Jiang, RSC Adv. 2, 7279–7289 (2012). https://doi.org/10.1039/C2RA20767B
I. Bicu, F. Mustata, Cellulose 24, 2029–2048 (2017). https://doi.org/10.1007/s10570-017-1243-8
S. Kugler, P. Ossowicz, K. Malarczyk-Matusiak, E. Wierzbicka, Molecules 24, 1651 (2019). https://doi.org/10.3390/molecules2409165
Acknowledgements
The review was prepared in accordance with the research plan of the Ufa Institute of Chemistry UFRC RAS on the topic "Synthesis of biologically active heterocyclic and terpenoid compounds" State Registration No. AAAA-A20-120012090026-9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malikova, R., Sakhautdinov, I. Maleated rosin-derived advanced materials: preparation, properties and application. J IRAN CHEM SOC 19, 3229–3248 (2022). https://doi.org/10.1007/s13738-022-02542-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-022-02542-7