Abstract
Conductivity, density and refractive index of 1-Benzyl 3- Methyl imidazolium chloride(BzMImCl)and1-Allyl 3-Methyl imidazolium Bromide(AMImBr) ionic liquids (ILs)At 298.15 K, Mixed solvents have been tested with different mole fractions of alcohols, containing aqueous and alcoholic-aqueous (methanol, ethanol and glycerol). The conductivity and surface tension and refractive index measurements were used to assess the critical micelle concentration (CMC) of (BzMImCl) and (AMImBr). The CMC was found to increase as the alcohol mole fraction increased in all solvents used. The results indicate that the CMC of (BzMImCl) and (AMImBr) methanol, ethanol, and glycerol, in that order. Micellization was discovered to be a naturally occurring process. The molar volume of the two surfactants was calculated and discussed based on the density information. The polarizability and molar refraction of BzMImCl and AMImBr were also measured and discussed using the refractive index results. For all calculations, a computer program was used.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ionic liquids (ILs) are new organic salts consist of a large organic cations and an inorganic polyatomic anions, that exist in the liquid state at relatively low temperatures. In the last few years, ILs have drawn the attention of the scientific community, and many studies that involve different aspects of ILs have been published in the scientific literature. From the scientific and industrial points of view, a fundamental understanding of the physico-chemical properties of ILs is needed before their application to several processes. For instance, knowledge of some basic properties can be useful for fluid property estimation, thermodynamic property calculations, and phase equilibrium [1, 2]. ILs have unique properties that can include ‘low melting point, negligible vapor pressure, good electrochemical and thermal stability, and tunable structures’, and so forth.
From this point, the field of ILs expanded rapidly, both in terms ofthe different ions used and in the range of applications being investigated. The applications include those on a research lab-scale and also an industrial scale; the commercial use of ILs has been under development since the late 1990s [3]. The low melting point of ILs drives interest in their use as pharmaceutical salts, where the cation or anion is an active pharmaceutical ingredient [4,5,6]. The low melting point removes the concern of a salt crystallizing into an alternative polymorph (crystal structure) from that which has been trialed and patented, as the formation of polymorphs has significant medical and legal implications. Having the drug in a liquid form may also make it easier to be administered top attaints.
The ionic nature of ILs also means that they provide quite unique solvation environments compared to conventional molecular solvents, and this is exploited in a variety of different synthetic reactions, materials processing/extraction, and gas separation. There is also extensive interest in their use for biomass processing. ILs are being investigated for both the dissolution of a variety of different biomaterials and for their processing into higher-value products [6, 7]. For biotechnological applications, the ability of ILs to dissolve and stabilize enzymes and proteins, DNA, and RNA is also extremely valuable. Unique solubilizing properties, coupled with good electrochemical stability, also underlie the use of ILs for rare-earth processing and recycling [8].
ILs are promising for two techniques–as anionic extracting for the separation of rare-earth salts, and as the medium for the subsequent electrodepositing of the pure rare-earth metal. Excellent electrochemical stability is arguably one of the most important characteristics of some ILs, as evidenced by their extensive use in electrochemical devices, for electrowinning, water splitting, and so on. In addition, ILs found application in a wide range of other synthetic reactions – organic, inorganic, biological, and so on [9]. In the field of energetic materials [10], the huge structural variability of ILs is a great advantage, as are their low vapor pressure, wide liquid range, and good thermal stability.
Most properties are identical to those of a basic electrolyte at low concentrations.
The surface tension, which drops rapidly as the concentration of ILs rises, is one notable exception. All of the properties (interfacial and bulk) shift suddenly at a certain concentration, which is consistent with the fact that surface-active ions or molecules in solution associate to form larger units at and above this concentration. Micelles (self-assembled structures) are the name for these related units, and the first aggregates formed are usually spherical.
This association phenomenon occurs at a critical micelle concentration occurs (CMC). The Critical Micelle Concentration indicates the usually narrow range of concentrations separating the limits, at below which most of the surfactant is in the monomeric state and above which virtually all additional ILs enter the micellar state [11]. The variation of the CMC with chemical and physical parameters provides good insights into the nature of the ILs self-association. Conductivity, solubility, viscosity, light scattering and surface tension measurement are some of the physical methods used to determine CMC measurement of ion activity, Gel filtration spectrophotometrically and counter ion magnetic resonance [12,13,14,15,16]. Many researchers have used conductivity measurements to investigate ionic liquid micellization [17,18,19,20,21,22] and surfactants [23,24,25,26,27,28,29,30,31,32]. The density measurements had been used to calculate the molar volume of some ionic liquids [33] and other substances in different solutions [33,34,35]. The refractive index measurements had been used to study the solvation of some substances in different solutions [36,37,38].
The aim of this research is to look into solvation BzMImCl and AMImBr at 298.15 K, using density, surface tension, refractive index and conductivity measurements in aqueous and alcoholic-aqueous solvents. The study aims to use the surface tension and, refractive index and conductivity measurements to estimate the CMC and the thermodynamic parameters of BzMImCl and AMImBr. The molar volumes, the molar refraction and the polarizability estimation of BzMImCl and AMImBr are also one of the study aims from the density and refractive index measurements.
Experimental
Chemicals and solutions
Chemicals used were all of the highest available purity as shown in Table 1.
Apparatus
The chemical structure of ILs under study,1-Benzyl 3-Methyl imidazolium chlorid (BzMImCl) and 1-Allyl 3-Methylimidazolum Bromide (AMImBr) is shown in (Scheme 1). Both of the solutions were rendered in glass volumetric flasks that had been washed. To make the solutions, the water was Bidistilled and had a conductivity of 0.05 to 0.5 S cm−1a readymade solution (0.1 mol L−1) of the BzMImCland AMImBr surfactant, respectively, was prepared.
Apparatus and methodology
A Jenway Conductivity Bridge was used to make the conductivity measurements. The Kcell, cell constant was determined various potassium chloride standard solutions were used to calibrate the conductivity bridge [39]. The conductivity was calculated as a function of the concentrations of BzMImCl and AMImBr ionic liquids. To prevent dilution errors when making different BzMImCl and AMImBr. The concentration of the sample solution was progressively increased with the addition of surfactant solutions increased by adding 0.1molL−1 of the previously prepared surfactant solution to the initial sample size is 20 mL pure water in a double jacket glass cell, i.e., followed by the addition of the surfactant. Using an ultra thermostate of sort, Within 0.1 K of a desired temperature, the temperature of the solution in the double jacket glass cell was kept constant (MLW 3230, Germany). After each addition, the solution was stirred to ensure uniform mixing, and the conductivity was measured. The conductivity test has a 0.025 S cm−1 uncertainty. The precise conductance was calculated twice, with the average of the results used for estimates as well as debate Surface tension measurements were taken with a wireless tensiometer K9. were made (ring method). The refractive index was measured for surfactants solution in both water and ethanol mole fraction solvent by putting one drop of the solution understudy into sample tray by using Digital Refractometer (DR101-60-A. KRÜSS Optronic GmbH – Germany).
Results and discussion
CMC determination
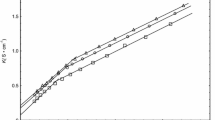
As stated in the experimental section, the conductivity of BzMImCl and AMImBr ILs at 298.15 K, different mole fractions of alcohols were tested in aqueous and alcoholic-aqueous mixed solvents (methanol, ethanol and glycerol). Relationship of measured conductivity, surface tension and refractive index versus the two ILs concentrations was used to CMC Estimation of BzMImCl and AMImBr in various solutions, as shown in Figs. 1, 2, 3, 4, 5, 6. Table 2 summarizes the CMC values of BzMImCl and AMImBr with various mole fractions of alcohols.
The CMC values obtained using different techniques (Conductivity, Surface Tension and Refractive Index) are close together, indicating good agreement between these techniques and correct value of CMC (Fig. 7 as example). In both solvents tested, the CMC of AMImBr and BzMImCl increased as the alcohol mole fraction increased. Both ILs’ CMC also increased in the following order: glycerol-water, methanol–water and ethanol–water. This, perhaps, attributed to the higher viscosity of the liquids in the same order: glycerol-water, methanol–water and ethanol–water. The lower the solvation of AMImBr and BzMImCl, the higher the viscosity, the higher the micellization, the higher the CMC values are found. The micelle’s degree of ionization (α) as well as the sum binding of counter ions, β = (1 − α) of (BzMImCl) and (AMImBr) in comparison to the mole fraction of alcohol was suggested as in the following equation:
S2/S1 is the ratio of the slopes of the post and pre micelle areas, and was determined as, (β = 1 − α). The slopes were calculated using linear conductivity versus IL concentration plots. In Table 3, the values of and are recorded. The thermodynamic parameters of micellization were obtained using the following equation.
where (ΔGmic) is the standard free energy change, α is the micelle's degree of ionization, R is the gas constant and T is the absolute temperature, the results were presented in Table 3.
From Table 3, the values of ΔGmic were found to be negative in all situations, indicating the spontaneity of the micellization process and indicating that the concentration of alcohols increases the spontaneity of the process.
The values of α and β were determined from the conductivity data only. In conductivity curves, the slope of the graph after CMC is seem to be small, so that, α is small value and β is high value. This depends on the nature of change in the measured properties which may be dependent on the type of ionic liquid.
Solution surface properties:
According to the measurement of surface tension of the ionic liquid under study in water and alcohol-water mixed solvent at 298.15 K, some surface properties such as maximum surface concentration, minimum area per molecules, and effectiveness of reduction of surface areas were calculated as follows:
The effectiveness of surface tension reduction was calculated by using the following equation [40]:
where γο is the surface tension of pure water at the appropriate temperature and γCMC is the surface tension of the solution at the CMC.
Maximum surface excess concentration (Γmax) [41] considered effective adsorption of the ionic liquid on the air − water interface. This is defined as the concentration of ionic liquid molecules in a surface plane, relative to that at a similar plane in the bulk which can be calculated by using the Gibbs adsorption (Eq. 4).
where R is the universal gas constant, T is the absolute temperature, and (∂γ/∂log C) is the ratio between surface tension values at CMC to concentration at CMC.
The minimum surface area of ionic liquid molecules at air−water solution interfaces (Amin) [42] can be calculated from the following:
where N is the Avogadro number. The values of effectiveness, excess surface concentration and minimum surface area are summarized in Tables 4 and 5. The results show no significant deference in minimum area per molecule of the two ionic liquids (BzMImCl) and (AMImBr). This may be related to no significant difference in the effectiveness of surface tension reduction.
The Walden product (Λ˚η˚), which is informative from the point of view of ion–solvent interaction [43], has constant value due to the molar conductance of an ion at infinite dilution depends only upon its speed and hence, the product of ion conductance by the viscosity of the medium should be independent of the solvent nature. Hence, the Walden product (Λ˚η˚) is expected to be constant for a given electrolyte in a series of solvent mixtures in which the ion solvent interactions are uniform.
The Walden product (Λ˚η˚) values were calculated for BzMImCl and AMImBrinAt 298.15 K, the mole fractions of alcohols. Similar mole fractions of alcohols were measured in aqueous and alcoholic-aqueous mixed solvents (ethanol, methanol, and glycerol) with different mole fractions of alcohols (ethanol, methanol, and glycerol), and the findings are described in Tables 6 and 7. The fluidity ratio (Rx) which is the ratio between the values of the Walden product of the two surfactants in alcohol-water solvent to that of water can be calculated.
The Walden product (Λ˚η˚) of BzMImCl and AMImBr solutions was found to increase in the presence of alcohol. This is due to the fact that mixed alcohol-water has a higher viscosity than pure water. As the mole fraction of alcohol (ethanol, methanol, and glycerol) increases, the value of the limiting molar conductance decreases. This means that the viscosity of the solvent, not the limiting molar conductance, is the most important factor in changing the Walden substance.
Molal volumes
At 298.15 K, the density of various molal concentrations of (BzMImCl) and (AMImBr) surfactants in aqueous and alcoholic-aqueous mixed solvents (methanol, ethanol, and glycerol) with various mole fractions of alcohols was measured. The apparent molal volume, Vφ of BzMImCl, and AMImBr were determined using the following equations [41] based on the molal concentration and density values.
where M is the molecular weight of BzMImCl, and AMImBr, The molal concentrations of BzMImCl and AMImBr in solution are denoted by m, and the solution and solvent densities are denoted by ρ and ρo, respectively. Tables 4 and 5 show the measured apparent molal volumes, Vφ of BzMImCl and AMImBr in glycerol, methanol, andethanol with different alcohol mole fractions at 298.15 K for BzMImCl and AMImBr.
The packing density (the relation between the Van der Waals volume and the partial molal volume) of relatively large molecules is found to be constant [44, 45]. Therefore, it is possible to calculate the Van der Waals volumes (Vw) of the polymers under study by apply the following equation [46].
The electrostriction volume (Ve) which is the volume compressed by the solvent [44,45,46,47], can be calculated using the following equation.
The electrostriction rate, Van Der Waal volume, and solvated radius values are according to Tables 6 and 7.
In the case of methanol–water and ethanol–water solvents, the densities of BzMImCl and AMImBr solutions decreased as the mole fraction of alcohol increased, while they increased in the case of glycerol-water solvents. In the case of methanol–water and ethanol–water,VQ of BzMImCl solutions increases with increasing the alcohol mole fraction, but it decreases with increasing the alcohol mole fraction in the case of glycerol-water. This may be related to the density of the alcoholic solvent under investigation (glycerol has more density than methanol, water and ethanol). The change in the molal volume and Van der walls volume with the mola fraction of ethanol as example was shown in (Fig. 8).
Polarizability, refractive index, and molar refraction
At 298.15 K, the refractive indices of BzMImCl and AMImBr in water, glycerol, methanol and ethanol-water with various. The mole fractions of alcohol were determined, and the findings are shown in Tables 6 and 7. BzMImCl and AMImBr refractive indices in methanol, glycerol, methanol, and ethanol–water solutions. As the alcoholic mole fraction increases, different alcohol mole fractions increase.
The molar refraction of the two surfactants in glycerol, ethanol, methanol–water with different alcohol mole fractions can also be determined centered on the refractive indices that have been calculated (Rm) was calculated [48] using the following equation.
The apparent molal volume of the two surfactants in solution is given by Vφ, and n is the BzMImCl solution’s refractive index. The gross molar polarization, or distortion polarization, is equal to the percentage of both the electron polarization (PE) and the atomic polarization (PA) on Eq. (3). The following equation was used to determine the atomic polarization (PA) [49]
The optical refractive index (n) of a substance containing N molecules per unit volume can be used to measure the mean value of molecular dipole polarizability (α; dipole moment caused by electric field). The refractive index is related to the polarizability (α) of the molecules by the Lorenz-Lorenz formula [50]. As shown in the following equation
where \(\hat{n} = \frac{N}{{\varphi V}}\), (N) is the Avogadro’s number and (φV) is the apparent molal volume. From Eq. (7), the polarizability of BzMImCl and AMImBr in The alcohol mole fractions of glycerol, ethanol, and methanol–water were determined. Calculated molar refraction (Rm), atomic polarization and polarizability (α) were reported, in Tables 8 and 9.
The apparent molal volume is directly proportional to the molar refraction and polarizability. As the mole fraction of glycerol, methanol and ethanol increases, so does the polarizability and molar refraction BzMImCl and AMImBr in glycerol, methanol, and ethanol-water. This increase in the molar refraction and the polarizability of BzMImCl and AMImBrwith the mole fraction ofglycerol, methanol and ethanol may be related to the increase in apparent molar volume of the two surfactants with glycerol, methanol and ethanol mole fractions, respectively, in the apparent molar volume the two surfactants with the mole fraction of ethanol, methanol and glycerol, respectively. This increase in the apparent molar volume of BzMImCl and AMImBr with the mole fractions of ethanol, methanol and glycerol can be due to the increase in the molar refraction and polarizability of the two surfactants with the mole fractions of ethanol, methanol and glycerol. The change in the refractive index, polarizability and atomic polarization with the mola fraction of ethanol as example was shown in (Fig. 9).
Relation between different studied properties
With respect to the measured and the calculated properties, we can note that there is a relation between these properties. It was noted that as the mole fraction of methanol and ethanol in alcohol-water mixed solvent increase, the density of the solution decreased and so the molal volume increased. As a result of molal volume increase, the CMC, refractive index, polarizability and molar refraction increase. Also it was noted that as the mole fraction of glycerol in alcohol-water mixed solvent increase, the density of the solution increased and so the molal volume decreased. As a result of molal volume decrease, the CMC, refractive index, polarizability and molar refraction decrease.
Conclusion
The CMC of 1-Benzyl 3- Methyl imidazolium chloride (BzMImCl) and 1-Allyl 3-Methyl imidazolium Bromide(AMImBr) experimentally, In aqueous and alcoholic-aqueous mixed solvents, surfactants are used (glycerol, methanol, and ethanol) with various mole percentages of alcohols have been calculated at 298.15.Kusing the refractive index, surface tension and conductivity measurements. The CMC of (BzMImCl) and (AMImBr) was found to increase in all solvents used, the alcohol mole fraction increased. The CMC value from conductivity, surface tension and refractive index measurements is found to be in good agreement.
The temperature dependence of micellization constants was used to measure the thermodynamic parameters (ΔG˚) of the micellization processes. It was also discovered that the CMC of (BzMImCl) and (AMImBr) increases in the following order: methanol ethanol glycerol. Micellization was discovered to be a normal process. At 298.15 K, the refractive index and density of BzMImCl and AMImBr in aqueous and alcoholic-aqueous mixed solvents (ethanol, glycerol and glycerol methanol) with various mole fractions of alcohols were determined experimentally. The molar volume of the two surfactants was calculated using density data. The molar refraction and polarizability of BzMImCl and AMImBr were also calculated using the refractive index data.
References
H. Ohno, Electrochemical Aspects of Ionic Liquids (Wiley, New Jersey, 2005)
J.A. Lazzús, Estimation of density as a function of temperature and pressure for imidazolium-based lonic liquids using a multilayer net with particle swarm optimization. Int. J. Thermophys. 30(3), 883–909 (2009). https://doi.org/10.1007/s10765-009-0591-5
N.V. Plechkova, K.R. Seddon, Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 37(1), 123–150 (2008). https://doi.org/10.1039/B006677J
J. Stoimenovski, D.R. MacFarlane, K. Bica, R.D. Rogers, Crystalline versus ionic liquid salt forms of active pharmaceutical ingredients: a position paper. Pharm. Res. 27(4), 521–526 (2010)
J.L. Shamshina, S.P. Kelley, G. Gurau, R.D. Rogers, Chemistry: develop ionic liquid drugs. Nature. 528(7581), 188–189 (2015). https://doi.org/10.1038/528188a
M. Smiglak, J. Pringle, X. Lu et al., Ionic liquids for energy, materials, and medicine. Chem. Commun. 50(66), 9228–9250 (2014). https://doi.org/10.1039/C4CC02021A
K. Dong, Q. Wang, X. Lu, Q. Zhou, S. Zhang, Structure, interaction and hydrogen bond. Struct. Bond. 151, 1–38 (2013). https://doi.org/10.1007/978-3-642-38619-0-1
Q. Zhang, Y. Hua, C. Xu, Y. Li, J. Li, P. Dong, Non-haloaluminate ionic liquids for low-temperature electrodeposition of rare-earth metals—A review. J. Rare Earths. 33(10), 1017–1025 (2015). https://doi.org/10.1016/S1002-0721(14)60520-2
T. Payagala, D.W. Armstrong, Chiral ionic liquids: a compendium of syntheses and applications (2005–2012). Chirality 24(1), 17–53 (2012). https://doi.org/10.1002/chir.21975
Q. Zhang, J.M. Shreeve, Energetic ionic liquids as explosives and propellant fuels: a new journey of ionic liquid chemistry. Chem. Rev. 114(20), 10527–10574 (2014). https://doi.org/10.1021/cr500364t
S. Chen, S. Zhang, X. Liu et al., Ionic liquid clusters: structure, formation mechanism, and effect on the behavior of ionic liquids. Phys. Chem. Chem. Phys. 16(13), 5893–5906 (2014). https://doi.org/10.1039/c3cp53116c
Y. Zhao, H. Wang, Y. Pei, Z. Liu, J. Wang, Understanding the mechanism of LCST phase separation of mixed ionic liquids in water by MD simulations. Phys. Chem. Chem. Phys. 18(33), 23238–23245 (2016). https://doi.org/10.1039/c6cp03439j
B. Dong, N. Li, L. Zheng, L. Yu, T. Inoue, Surface adsorption and micelle formation of surface active ionic liquids in aqueous solution. Langmuir 23(8), 4178–4182 (2007). https://doi.org/10.1021/la0633029
T. Inoue, H. Ebina, B. Dong, L. Zheng, Electrical conductivity study on micelle formation of long-chain imidazolium ionic liquids in aqueous solution. J. Colloid Interface Sci. 314(1), 236–241 (2007). https://doi.org/10.1016/j.jcis.2007.05.052
J. Sirieix-Plénet, L. Gaillon, P. Letellier, Behaviour of a binary solvent mixture constituted by an amphiphilic ionic liquid, 1-decyl-3-methylimidazolium bromide and water. Talanta 63(4), 979–986 (2004). https://doi.org/10.1016/j.talanta.2004.01.001
M. Blesic, M.H. Marques, N.V. Plechkova, K.R. Seddon, L.P.N. Rebelo, A. Lopes, Self-aggregation of ionic liquids: micelle formation in aqueous solution. Green Chem. 9(5), 481–549 (2007). https://doi.org/10.1039/b615406a
F. El-Dossoki, Micellization thermodynamics of some imidazolium ionic liquids in aqueous solutions–conductometric study. J. Solut. Chem. 42(1), 125–135 (2013). https://doi.org/10.1007/s10953-012-9947-8
A. Bhattarai, G. Shrivastav, C. Adhikari, Study of critical micelle concentration of cetyltrimethylammonium bromide (CTAB) in pure water in presence and absence of magnesium sulphate and sodium sulphate by measuring conductivity meter. Bibechana J. 11(1), 123–127 (2014)
O. Esan, O. Olubunmi, A. Olumuyiwa, O. Olarenwaju, Effects of temperature and tetramethylammonium bromide salt on the micellization of cetyltrimethylammonium bromide in aqueous medium: A conductometric studies. Int. J. Thermodyn. 18(4), 246–252 (2015). https://doi.org/10.5541/ijot.5000130524
F. Corradini, G. Franchini, A. Marchetti, M. Tagliazucchi, Conductivity of tetraphenylphosphonium bromide in 2-methoxyethanol-water. J. Chem. Soc. Faraday Trans. 89(16), 3043–3047 (1993). https://doi.org/10.1039/FT9938903043
Z. Haq, N. Rehman, F. Ali, N. Khan, H. Ullah, Physico-chemical properties of cationic surfactant cetyltrimethylammonium bromide in the presence of electrolyte. J. Mater. Environ. Sci. 8(3), 1029–1038 (2017). https://doi.org/10.1038/451414a
F.I. El-Dossoki, S.A. Abd El-Maksoud, M.A. Migahed, M.M. Gouda, Micellization and solvation properties of newly synthesized imidazolium-and aminium-based surfactants. ACS Omega 5(16), 9429–9441 (2020). https://doi.org/10.1021/acsomega.0c00603
L. Zhang, Z. Xu, Y. Wang, H. Li, Prediction of the solvation and structural properties of ionic liquids in water by two-dimensional correlation spectroscopy. J. Phys. Chem. B 112(20), 6411–6419 (2008). https://doi.org/10.1021/jp8001349
G. Kumar, M. Chauhan, A. Kumar, S. Chauhan, R. Kumar, A study on solution behaviour of sodiumdodecyl sulphate and cetyltrimethylammonium bromide in water-alcohol mixed media. Der. Chem. Sin. 3(3), 628–635 (2012)
L. Tennouga, A. Mansri, K. Medjahed, A. Chetouani, I. Warad, The micelle formation of cationic and anionic surfactants in aqueous medium: determination of CMC and thermodynamic parameters at different temperatures. J. Mater. Environ. Sci. 6(10), 2711–2716 (2015)
A. Halpern, Colloidal system. In: A Laboratory Textbook, 2nd ed. Upper Saddle River, Prentice Hall, pp 469–477 (1997)
M. Motin, M. Mia, K. Reza, A. Islam, Effect of sodium dodecyl sulfate on volumetric properties of methanol ethanol n-propanol and iso-propanol at (298.15–323.15)K. Dhaka Univ. J. Sci. 60, 129–136 (2012). https://doi.org/10.3329/dujs.v60i1.10351
S. Shirzad, R. Sadeghi, Micellization properties and related thermodynamic parameters of aqueous sodium dodecyl sulfate and sodium dodecyl sulfonate solutions in the presence of 1-propanol. Fluid Phase Equilibria J. 377, 1–8 (2014). https://doi.org/10.1016/j.fluid.2014.06.009
E. Kolesnikova, N. Glukhareva, The influence of an electrolyte on micelle formation in aqueous solutions of sodium monoalkyl sulfosuccinates. Russ. J. Phys. Chem. A 83(12), 2119–2121 (2009). https://doi.org/10.1134/S0036024409120206
S. Kumar, K. Parikh, Influence of temperature and salt on association and thermodynamic parameters of micellization of a cationic gemini surfactant. J. Appl. Solut. Chem. Model. 1(1), 65–73 (2012). https://doi.org/10.6000/1929-5030.2012.01.01.7
F. El-Dossoki, E. Gomaa, O. Hamza, Solvation thermodynamic parameters for alkyl benzyl dimethyl ammonium chloride and cetyl trimethyl ammonium chloride surfactants in water and alcoholic-water solvents. J. Chem. Eng. Data. 64(10), 4482–4492 (2019). https://doi.org/10.1021/acs.jced.9b00527
F.I. El-Dossoki, E.A. Gomaa, O.K. Hamza, Solvation thermodynamic parameters for sodium dodecyl sulfate (SDS) and sodium lauryl ether sulfate (SLES) surfactants in aqueous and alcoholic-aqueous solvents. SN Appl. Sci. 1(8), 0974–0976 (2019)
G.R. Vakili-Nezhaad, A.M. AlAisaee, M.A. AlJahwari, S.S. AlBarwani, Z.K. AlJahwari, Density calculation of ionic liquids. Mater. Phys. Mech. 32(1), 8–13 (2017)
F. El-Dossoki, Volumetric and solvation properties of glycyl-glycine and glycyl-l-leucine in aqueous acetate solutions. J. Solut. Chem. 44(2), 264–279 (2015). https://doi.org/10.1007/s10953-015-0314-4
F. El-Dossoki, Volumetric thermodynamic properties of aqueous binary mixtures of some alkanols at different temperatures. Int. Res. J. Pure Appl. Chem. 10(3), 1–18 (2016). https://doi.org/10.9734/IRJPAC/2016/21863
F. El-Dossoki, E. Gomaa, Excess refractive index, polarizability, polarization and the molar volume of some mixed solvents. J. Indian Chem. Soc. 82(3), 219–224 (2005)
F.I. El-dossoki, refractive index and density measurements for selected binary protic-protic, aprotic-aprotic, and aprotic-protic systems. J. Chinese Chem. Soc. 549, 1129–1137 (2007)
N. Hosny, M. Badr, F. El-Dossoki, Doped poly(m-phenylenediamine-co-aniline) (P(mPD-co-ANI)): synthesis, characterization, physical properties, and precursor for CuO nanoparticles. Polym. Plast. Technol. Eng. 57(14), 1485–1495 (2018)
D. Lide, CRC Handbook of Chemistry and Physics, 76th edn. (CRC Press, Boca Raton, FL, 1995)
M.A. Abdul-Raheim, M.E.-S. Abdel-Raouf, N.E.-S. Maysour, A.F. El-Kafrawy, A.Z. Mehany et al., Some sugar fatty ester ethoxylates as demulsifiers for petroleum sludge. J. Surf. Deterg. 16, 377–387 (2013)
R. Wadi, R. Kakkar, Partial molar volumes and viscosities of some monovalent ions in ethanolamine and water-ethanolamine mixtures at 29815 K. Indian J. Chem. Sect. A Inorg. Bio-inorg. Phys. 39, 598–602 (2000)
Z. Ul Haq, N. Rehman, F. Ali, N. Khan, H. Ullah, Physicochemical properties of cationic surfactant cetyltrImethylammonium bromide in the presence of electrolyte. J. Mater. Environ. Sci. 8, 1029–1038 (2017)
P. Walden, Über Den ZusammenhangZwischen Dem Grenzleitvermögen λ∞ Der BinärenElektrolyteInNichtwässerigenLösungsmitteln Und Der Viskosität η∞ Der Letzteren λ ∞• η ∞= Konst. Zeitschrift für Anorg und Allg Chemie. 113(1), 85–97 (1920)
F. Millero, Apparent molal expansibilities of some divalent chlorides in aqueous solution at 25.deg. J. Phys. Chem. 72(13), 4589–4593 (1968)
E. King, Volume changes for ionization of formic, acetic, and butyric acids and the glycinium ion in aqueous solution at 25.deg. J. Phys. Chem. 73(5), 1220–1232 (1969)
J. Millero, A. Surdo, C. Shin, The apparent molal volumes and adiabatic compressibilities of aqueous amino acids at 25 C. J. Phys. Chem. 82(7), 784–792 (1978)
R. Gopal, M. Siddiqi, A Study of Ion-Solvent. J. Phys. Chem. 73(10), 3390–3394 (1968)
A. El-Harakany, M. El-Dessouky, A. Taha, A. Bassiony, Solubilities and thermodynamic functions of transfer of substituted benzoic acids and aliphatic amine derivatives from water to water-sulpholane mixtures at different temperatures. Egypt J. Chem. 45(1), 1–32 (2002)
E. Mognaschi, L. Laboranti, Association of pure polar liquids: dielectric properties of docosanoic acid. J. Chem. Soc. Faraday Trans. 92(18), 3367–3369 (1996)
J. Hasted, Aqueous Dielectrics (Chapman and Hall, New York, 1993)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Dossoki, F.I., Gomaa, E.A. & Abdelzaher, M.A. Micellization, molal volume and polarizability of benzyl and allyl-methyl imidazolium ionic liquids in aqueous and alcoholic-aqueous solvents. J IRAN CHEM SOC 19, 729–739 (2022). https://doi.org/10.1007/s13738-021-02336-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-021-02336-3