Abstract
In this study, liquid-phase microextraction using low-density extraction solvents with microfunnel was applied for the extraction and preconcentration of Bisphenol A and 4-Nonylphenol in aqueous samples. The goal of the present method is to develop a special device that allows organic solvent remain on the surface of aqueous phase as a thin layer during the extraction time. At the end of extraction period, organic phase containing the extracted analytes was collected easily and analyzed by HPLC–FLD. Toluene used as extraction solvent and some of experimental parameters were optimized by L16 Taguchi experimental design. According to the results, the volume of extraction solvent as well as ionic strength showed significant effect on the extraction recovery. Under the optimum conditions (sample volume: 320 mL; pH 8.0; ionic strength: 10% (w/v) NaCl, extraction time: 90 min and extractant: 600 μL toluene), limit of detection, limit of quantification and dynamic linear range of the proposed method for Bisphenol A were calculated as 0.05, 0.2 and 0.2–62.5 μg L−1, and for Nonylphenol were obtained as 3.1, 6.2 and 6.2–125 μg L−1, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bisphenol A (BPA) and Nonylphenol (NP) are carbon-based synthetic compounds with the chemical formula C15H16O2 and C15H24O, respectively. BPA is a monomer that employed to make certain plastic, epoxy resins, polyesters and polycarbonates such as baby and water bottle [1, 2], food cans, vegetable packaging, paints, coating and building materials [3,4,5,6].
NP can be produced industrially, naturally and by the environmental degradation of alkylphenols ethoxylates. It is used in the manufacturing of antioxidants, lubricants, emulsifiers, dish and laundry detergents [7, 8].
Materials containing BPA and NP can be hydrolyzed at high temperatures or in acidic and basic situations. They enter into the environmental water and food chain and finally contaminate them [2,3,4, 9]. Thus, these materials have been attended from many years ago due to their estrogenic effects and are known as endocrine disrupting chemicals [10]. They called xenoestrogens, and caused some male/female diseases occur in human/animal body that can produce sexual dysfunction [4], hormone biosynthesis, metabolism, homeostatic control problems, cancer, fertility problems thereupon as a result of decreasing count of sperm or reducing of sperm quality [11]. Finally, they can be found in placenta, breast milk, cord blood and amniotic fluid [3,4,5,6, 12].
Variety methods and techniques have been raised for determination of trace amount of BPA and NP including hollow fiber liquid-phase microextraction (HF-LPME) [10, 13], liquid-phase microextraction (LPME) [10], headspace solid-phase microextraction (HD-SPME) [14], molecular imprinted solid-phase extraction (MISPE) [15], micelle-mediated extraction [16], dispersive liquid–liquid microextraction (DLLME) [3, 7, 8, 11, 17,18,19] and ionic liquid-phase microextraction (ILPME) [8, 20].
In regard to hazard of BPA and NP, it is necessary to improve determination methods for preparation and preconcentration of them that be easy to do, have low consumption of sample and extraction solvent, to be environmental friendly, fast in time, selective and sensitive with high preconcentration factor.
In 2012, Cabala et al. introduced bell-shaped extraction device (BSED) for preconcentration of volatile and semivolatile compounds in water samples with use of low-density organic solvent prior to GC/MS [21].
Saleh et al. extracted antifoulant agents (Irgarol 1015, diuron and its metabolite 3, 4-dichloroaniline) by a modified microfunnel device based on liquid-phase microextraction (MF-LPME) combined with HPLC–UV in seawater samples [22].
In 2017, Saleh et al. optimized and applied MF-LPME method for the determination of 2,4-dichlorophenoxyacetic acid (2,4-d) and 4-chloro-2-methylphenoxyacetic acid (MCPA) from natural water samples and good results were obtained [23].
In another study in 2019, microfunnel magnetic stirring-assisted liquid–liquid microextraction (MF-MSA-LLME) in combination with flame atomic absorption spectrometry (FAAS) was successfully developed for the extraction and measurement of trace amounts of silver ion after chelate formation with 1-(2-Pyridylazo)-2-naphthol (PAN) into octanol solvent [24].
MF-LPME has some advantages including easy operation, non-exhausting extraction method, efficient, sensitive and low-cost method due to minimum consumption of extraction solvent and device, flexibility in extraction solvent volume (tens to hundreds µL) and use of less dense extraction solvent depend on the nature of the analyte.
In this study, we utilized MF-LPME for extraction and preconcentration of BPA and NP from aqueous samples. Due to special design of MF and the internal diameter of wide section of MF, extraction solvent can remain on the surface of water sample as a floating droplet during extraction time. So, the analytes are transferred from aqueous phase to organic phase. Also, it is possible to use a few to hundreds µL of organic solvent for extraction of analytes from sample volumes with hundreds of mL. At the end of the extraction period, organic phase containing analytes is collected easily, evaporated and then the extract is dissolved by proper solvent for next instrumental analysis.
Experimental
Reagents and materials
BPA and NP were purchased from Sigma-Aldrich (Milwaukee, WI, USA). Stock standard solutions of BPA and NP (1000 mg L−1) were prepared in HPLC grade methanol purchased from Samchun company (China). All standard solutions were stored in glasswares at 4 °C and far from light. The working standard solutions were prepared by diluting the stock solutions with double distilled water. Toluene, n-hexane, 1-hexanol and dichloromethane (DCM) were purchased from Merck (Darmstadt, Germany) and used as extraction solvents. Purified water with a Milli-Q ultra-pure water purification system (Millipere, Bedford, MA, USA) was used in all steps of study. All materials including toluene, n-hexane, 1-hexanol, DCM and sodium chloride were used with analytical reagent grade and without further purification.
Instrumentation
Chromatographic separations were carried out on an Agilent 1100 HPLC system (Wilmington, DE, USA) including a G 1379 A, micro vacuum degasser, a G 1312 A, binary pump, a G 1158 A, six-port two-position injection valve with a 100 µL sample loop and equipped with a fluorescence detector (FLD). The detection of BPA and NP was performed at λex = 230 to λem = 305 nm. Separations were carried out on a C18 column (250 mm × 4.6 mm × 5 µm) from Agilent company using mixture of water:acetonitrile (45:55) as mobile phase under flow rate of 1 mL min−1. The separations were occurred as gradient elution up to 3 min with water:acetonitrile (45:55) and after that the mobile phase composition change to water:acetonitrile (10:90). pH values of the solutions were measured using a Bante pH meter equipped to a combined glass electrode (China). The MF specification used in this study including size of wide part, narrow length and internal diameter of narrow part was selected as 27, 65 and 2 mm, respectively. An IKA magnetic stirrer (model C-MAG HS7, Wilmington, NC, USA) was used for agitation of samples.
MF-LPME procedure
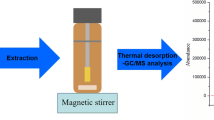
For MF-LPME, a 320 mL aqueous sample containing BPA and NP was introduced into a 350 mL glass vessel including a small magnetic rod (0.5 × 1 cm) for agitation of sample solution. The vessel was placed on the surface of the magnetic stirrer for agitation of sample. The opening of the vessel was closed with the stopper and a laboratory made MF was passed through the stopper, in a way that wide part of MF contacted with aqueous solution (Fig. 1). During the extraction, the MF was tangent to the sample surface and 600 µL toluene (as extraction solvent) was filled into the narrow part of MF using a 1 mL syringe (Hamilton, USA). After that the solution was stirred at 240 rpm for 90 min. Due to special design of wide part of MF, extraction solvent can remain on the surface of aqueous sample as a floating drop during extraction time. So, the analytes were transferred to the extraction phase. After the extraction, MF was pushed into the aqueous solution so that the extraction solvent moves toward narrow part of MF (Fig. 1). Extraction solvent containing analytes was collected using a 100 µL microsyringe (Hamilton, USA) and was poured into a glass micro-tube. The extraction solvent was evaporated by air purging. The residual in the bottom of the glass micro-tube was dissolved into 50 µL methanol and diluted by 100 µL distillated water. Finally, 100 µL of this solution was injected to HPLC–FLD for further analysis.
Results and discussion
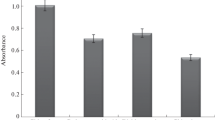
In the MF-LPME, several factors can influence the extraction efficiency. To obtain a sensitive and highly reproducible extraction method, it is necessary to optimize each of these factors. The type of extraction solvent is one of the most important parameters that should be considered. The proper extraction solvent for the MF-LPME procedure should have less specific gravity than water to float on the surface of sample. Also, it must be immiscible with water and show high solubility for analytes as well as it should be compatible with the analytical instruments that is used for analyte determination. To find the best extraction solvent, toluene (d = 0.87 g mL−1), n-hexane (d = 0.65 g mL−1), 1-hexanol (d = 0.81 g mL−1) and mixture of DCM/n-hexane (30:70) were examined as extraction solvent (Fig. 2). For this purpose, 320 mL of sample containing 31.25 µg L−1 of spiked analytes was extracted by 400 µL of each of the extraction solvent for 60 min. According to the results, toluene was the best solvent for this MF-LPME. Hexanol showed no extraction and n-hexane showed a little extraction respect to toluene (6.4%). Use of DCM improved the extraction efficiency and the 30:70 mixture of DCM/n-hexane showed lower extraction respect to toluene (97%).
In order to obtain the optimum conditions and to achieve the maximum extraction efficiency, Taguchi method as an orthogonal array design (OAD) was employed. In this study, an L16 (44) orthogonal array design was used to investigate the effects of four controllable factors including sample pH (2–8), ionic strength (0–20% w/v NaCl), extraction time (30–120 min) and solvent volume (100–600 μL) at four levels (Table 1). After obtaining the peak area for each experiment (each run), the average area (S/N value) was calculated for each factor and level.
In all optimization experiments, 320 mL sample containing 31.25 µg L−1 BPA and NP and toluene as extraction solvent were applied.
Effect of the extractant volume on the extraction efficiency
To study the effect of the extraction solvent volume on the MF-LPME method, four levels of toluene (100, 200, 400 and 600 µL) was investigated. As the results have been shown in Figs. 3 and 4, by increasing the volume of extractant, more analytes were extracted. In higher volumes of extractant, the usage of microfunnel is difficult and evaporation of organic solvent takes more times. So, 600 μL of toluene was chosen as the optimum volume of extractant.
Effect of the extraction time on the extraction efficiency
Often more time helps to more transfer of analytes from aqueous phase to organic phase. Extraction times were investigated at the range of 30, 60, 90 and 120 min (Figs. 3, 4). According to the results, the best extraction has been occurred at 120 min for BPA and at 90 min for NP. With increasing the extraction time, the equilibrium between the aqueous and organic phases can be achieved. To avoid long time of extraction, 90 min was selected as the extraction time for further experiments.
Effect of the ionic strength on the extraction efficiency
The influence of the ionic strength is important since the presence of ionic species in solution can affect the mass transfer of analytes from the aqueous phase to the extractant phase. So, the ionic strength was studied at 0, 5, 10 and 20% (w/v) levels by addition of NaCl to the solution. As results show (Figs. 3, 4), presence of 10% NaCl was better for the extraction of NP and there is no egregious difference between responses of 10% and 20% for BPA. This observation can be related to the engagement of more water molecules in the hydration layer around the ionic species that reduces the amount of water molecules available to dissolve the analytes. This salting out effect will derive additional extractable analytes into the extractant. Higher concentrations of ionic species may reduce the extraction efficiency due to the enhancement of viscosity and density of the aqueous phase that reduces mass transfer into the organic phase [25]. So, 10% NaCl was selected as the optimum ionic strength.
Effect of the sample pH on the extraction efficiency
The efficiency of the extraction process is directly related to the pH of the sample solution. BPA (pKa = 9.6–10.2) and NP (pKa = 10.7) should mainly exist in neutral form at pHs below 9.0 and they have nonpolar character [26]. Therefore, they can be extracted into the organic extractants at pHs < 9.0. At pHs higher than 10, they exists as ionic forms that have greater solubility in sample. In order to study the effect of solution pH on the extraction efficiency, pH of sample was studied in the range of 2.0–8.0. According to the results (Figs. 3, 4), pH 2 and 8 are better than other levels for BPA and pH 8 is best for the NP. So, pH 8.0 was selected as optimum value for further studies.
The analysis of variance (ANOVA) which is useful statistical test in comparing three or more means were carried out. The results showed the order of percent contributions as 36.64% (salt effect) > 33.02% (solvent volume) > 19.82% (extraction time) > 6.47% (pH).
These results showed the importance of controlling the experimental parameters as their percent contribution.
Evaluation of figures of merit
Using figures of merit of each analytical method, it is possible to compare the efficiency of various methods with each other and also evaluate the ability of an analytical method for particular applications. To investigate the quantitative parameters of the proposed MF-LPME method for determination of BPA and NP, the figures of merit of this method was studied under the optimized experimental conditions (sample volume: 320 mL; pH 8.0; ionic strength: 10% (w/v) NaCl, extraction time: 90 min and extractant: 600 μL toluene) and the results were summarized in Table 2. The calibration curves of BPA and NP were obtained without and after preconcentration by the proposed MF-LPME method. Direct calibration curves were obtained via 100 μL injection of 1.25–25 mg L−1 of mixed standards of BPA and NP into the HPLC–FLD system.
For evaluation of dynamic linear range (DLR), ten spiked standard samples of BPA and NP in the range of 0.1–150 µg L−1 were prepared. Each standard sample was extracted by the proposed MF-LPME method under the optimized conditions, and the extractant was measured by HPLC. The limit of detection (LOD) and limit of quantification (LOQ) of the proposed method were calculated from CLOD = 3Sb/m and CLOQ = 10Sb/m that Sb is the standard deviation of five replicate blank preconcentration and m is the slope of calibration graph after preconcentration. For blank measurements, distilled water was extracted by MF-LPME under optimum conditions.
Preconcentration factors were calculated with MF-LPME of mixed solutions of BPA and NP (62.5 µg L−1of each analyte) under optimum conditions and were obtained via PF = Cf/Ci, where, Ci and Cf represent the initial and final concentrations of each analyte in aqueous and organic phases, respectively.
Also, extraction recoveries were calculated using Eq. 1 with obtained PF values at 62.5 µg L−1 of each analyte.
where Vorg and Vaq represent the volumes of organic and sample phases.
A comparison between the figures of merit of the proposed MF-LPME method with other methods reported in the literature for quantitative determination of BPA and NP in aqueous samples was summarized in Table 3. It clearly shows that our proposed method has good sensitivity and precision, wide linear dynamic range and low LOD in comparison with some of the other techniques.
Real sample analysis
To evaluate the applicability of the proposed MF-LPME method for the extraction of BPA and NP from aqueous real samples, Gachsar river water (Chalus, Iran) was investigated as a real sample. Firstly, the sample was extracted using proposed method and the results showed absence of these analytes in it. Secondly, in order to study the matrix effect, the sample of river water was spiked with 31.25 µg L−1 of each analyte. The accuracy and precision of the method were examined by extracting from 320 mL of river water under the optimum conditions and expressed as relative recovery percent. The results showed 30.0 and 26.1 µg L−1 for BPA and NP, respectively, which corresponded to 96.0 and 84.0% relative recovery.
Conclusion
In the present study, a liquid-phase microextraction method based on microfunnel device in combination with HPLC–FLD analysis was developed for extraction and preconcentration of Bisphenol A and 4-Nonylphenol from aqueous samples. This developed method was reasonable and safe due to the volume of organic extraction solvent, from tens to hundreds µL and it was convenient for the usage of low-density extraction solvent to extract analytes from the large volume of aqueous samples. Simplicity, low cost of the extraction device, minimum carryover and cross-contamination are the benefits of the proposed method.
References
M. Ash, I. Ash, Handbook of Plastic and Rubber Additives (Gower, Hampshire, 1995)
J.E. Biles, T.P. McNeal, T.H. Begley, H.C. Hollifield, J. Agric. Food Chem. 45, 3541 (1997)
N. Salgueiro-González, E. Concha-Graña, I. Turnes-Carou, S. Muniategui-Lorenzo, P. López-Mahía, D. Prada-Rodríguez, J. Chromatogr. A 1223, 1 (2012)
L. Peyre, P. Rouimi, G. Sousa, C. Héliès-Toussaint, B. Carré, S. Barcellini, M.C. Chagnon, R. Rahmani, Food Chem. Toxicol. 70, 9 (2014)
M. Kawaguchi, R. Ito, N. Endo, N. Okanouchi, N. Sakui, K. Saito, H. Nakazawa, J. Chromatogr. A 1110(1–2), 1 (2006)
C. Guo, C. Zhou, N. Sai, B.A. Ning, M. Liu, H. Chen, Z. Gao, Sens. Actuators B Chem. 166–167, 17 (2012)
S. Luo, L. Fang, X. Wang, H. Liu, G. Ouyang, C. Lan, T. Luan, J. Chromatogr. A 1217, 6762 (2010)
Q. Zhou, Y. Gao, G. Xie, Talanta 85(3), 1598 (2011)
J.A. Padilla-Sánchez, P. Plaza-Bolaños, R. Romero-González, N. Barco-Bonilla, J.L. Martínez-Vidal, A. Garrido-Frenich, Talanta 85(5), 2397 (2011)
M. Kawaguchi, R. Ito, N. Okanouchi, K. Saito, H. Nakazawa, J. Chromatogr. B 870(1), 98 (2008)
S.C. Cunha, J.O. Fernandes, Talanta 83(1), 117 (2010)
A.M. Morgan, S.S. El-Ballal, B.E. El-Bialy, N.B. El-Borai, Toxicol. Rep. 1, 92 (2014)
X.-W. Tan, Y.-X. Song, R.-P. Wei, G.-Y. Yi, Chin. J. Anal. Chem. 40(9), 1409 (2012)
L. Xiangli, L. Li, Z. Shichun, L. Chongyu, L. Tiangang, Chin. J. Anal. Chem. 34(3), 325 (2006)
F. Tan, H. Zhao, X. Li, X. Quan, J. Chen, X. Xiang, X. Zhang, J. Chromatogr. A 1216(30), 5647 (2009)
N. Saadatjou, Sh Shariati, M. Golshekan, ISRN Anal. Chem. 2013, 1 (2013)
A. Zgoła-Grzeskowiak, J. Chromatogr. A 1217(11), 1761 (2010)
P. Cabarcos, J.Á. Cocho, A. Moreda, M. Míguez, M.J. Tabernero, P. Fernández, A.M. Bermejo, Talanta 111, 189 (2013)
M. Rezaee, Y. Yamini, Sh Shariati, A. Esrafili, M. Shamsipur, J. Chromatogr. A 1216(9), 1511 (2009)
J.-N. Sun, J. Chen, Y.-P. Shi, Talanta 125, 329 (2014)
R. Čabala, M. Bursová, J. Chromatogr. A 1230, 24 (2012)
A. Saleh, N. Sheijooni Fumani, S. Molaei, J. Chromatogr. A 1356, 32 (2014)
A. Saleh, S. Abedi, Sh Shariati, S. Molaei, J. Percian Gulf 8(27), 21 (2017)
N. Bayat, M. Ramezani, J. App. Chem. Res. 13(3), 8 (2019)
M. Ganjikhah, Sh Shariati, E. Bozorgzadeh, J. Iran. Chem. Soc. 14, 763 (2017)
F. De Araujo, G.F. Bauerfeldt, Y.P. Cid, An. Acad. Bras. Cienc. 90(2), 1903 (2018)
M. Kawaguchi, K. Inoue, M. Yoshimura, R. Ito, N. Sakui, N. Okanouchi, H. Nakazawa, J. Chromatogr. B 805(1), 41 (2004)
L. Deng, Y.X. Liu, P.Y. Chen, L. Wang, N.S. Deng, Anal. Lett. 39(2), 395 (2006)
M. Kawaguchi, R. Ito, N. Endo, N. Okanouchi, N. Sakui, K. Saito, H. Nakazawa, J. Chromatogr. A 1110, 1 (2006)
S. Luo, L. Fang, X. Wang, H. Liu, G. Ouyang, C. Lan, T. Luan, J. Chromatogr. A 1217(43), 6762 (2010)
A. Sun, Q. Xu, X. Yu, Pol. J. Environ. Stud. 22(3), 899 (2013)
Acknowledgments
The authors are grateful to Rasht Branch, Islamic Azad University for supporting this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shariati, S., Yekeh Falah, K., Saleh, A. et al. Extraction and preconcentration of Bisphenol A and 4-Nonylphenol in aqueous solutions using microfunnel supported liquid-phase microextraction prior to high performance liquid chromatography. J IRAN CHEM SOC 18, 887–892 (2021). https://doi.org/10.1007/s13738-020-02077-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-020-02077-9