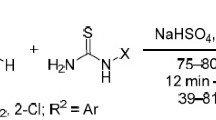
Abstract
The reaction of 1-methylpyrimidine-(1H,3H,5H)-2,4,6-trione (1-MBA 1a’) as an unsymmetrical barbituric acid with cyanogen bromide (BrCN) and various aldehydes in the presence of L-(+)-tartaric acid (L-(+)-TA) as an organocatalyst afforded heterocyclic stable 5-aryl-1,1′-dimethyl- and 5-aryl-3,1′-dimethyl-1H,1′H-spiro[furo[2,3-d]pyrimidine-6,5′-pyrimidine]2,2′,4,4′,6′(3H,3′H,5H)-pentaones which had the dimeric form of 1-methyl barbiturate at the range of 0 °C to room temperature. In this reaction, L-(+)-TA acted as a sieve to reduce the number of diastereomeric products. Knoevenagel condensation and Michael adduct were the main processes involved in this reaction. The structure of compounds was investigated by 1H and 13C-NMR, and FTIR spectroscopy techniques.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pharmaceutical and biological effects of fused uracils [1,2,3], furo[2,3-d]pyrimidines [4,5,6,7,8] and spirobarbituric acids [9,10,11,12] are well established. C-nucleophilic property of pyrimidine in barbituric acids (BA) and their 2-thio-analogues, both substituted and unsubstituted at nitrogen, have been studied. The reaction of these compounds with aliphatic or aromatic aldehydes yields 5-aryl or 5-alkylmethylenebarbituric acids [13, 14]. The nucleophilic reaction of barbituric acids with a variety of electrophiles such as carbodiimides, benzophenone derivatives, 2,2′-bipyridil, and erythrolactol obtains diaminomethylenebarbiturates [15], triphenylmethylium salt [16], 5,5′-(2-pyrilidine)bisbarbituric acid [17, 18], spirobarbituric deoxyribonucleoside [19], spiro-linked condensed [1,2-α]quinolones [20], and π-conjugated systems including BA and 1,3-dimethyl barbituric acid (DMBA) derivatives [21].
Chiral tartaric acid (TA) and its derivatives play a key role in various chemical processes such as epoxidation [22,23,24], unsymmetrical hydrogenation [25, 26] and Simmons–Smith cyclopropanation [27,28,29,30]. Additionally, L-(+)-TA has also been used in the synthesis of pharmaceutical and natural product compounds such as (+)-pinellic acid [31], 5,5′-bis(1,3-dioxolan-4-ones) of tartaric acids [32], and (−)-muricatacin [33]. It participates in critical processes including selective conjugate addition of nitromethane to enoates [34], enantioselective synthesis of (1R)-1-(hydroxymethyl)-2-acetyl-1,2,3,4-tetrahydro-β-carboline [35], novel chiral dithioethers [36], etc.
Despite the toxicity of cyanogen bromide (BrCN), it is commonly used in organic synthesis. It is an apt reagent for both bromination and cyanation of imidazoles [37], bromination of alkenes [38], and α-bromination of β-aminoenones [39]. von Braun is a well-known reaction in which tertiary amines react with BrCN to yield organocyanamides [40]. A selective synthesis of thiocyanates using BrCN has been reported by Chambert et al. [41]. This agent is currently used in the synthesis of various compounds, among which are (spiro)cyclopropanes [42,43,44,45], β-triketones [46], and spirodihydrofuranes [47, 48].
The reaction of barbituric acid (BA) with BrCN has been reported in the literature [49,50,51]. Recently, we have reported the racemic synthesis of 5-alkyl and/or 5-aryl-1H,1′H-spiro[furo[2,3-d]pyrimidine-6,5′-pyrimidine]-2,2′,4,4′,6′(3H,3′H,5H)-pentaones and their thio-analogues through the reaction of symmetric (thio)barbituric acids with aldehydes [47] and ketones [52, 53]. These reactions were conducted in the presence of BrCN and triethylamine (Et3N) and/or pyridine as basic media. We have also performed this reaction with 1-methyl barbituric acid (as an unsymmetric barbituric acid) under the same conditions. The product of this reaction was diastereomeric mixture of stable stereoisomers 1′,3-dimethyl-5-aryl-1H,1′H-spiro[furo[2,3-d]pyrimidine-6,5′-pyrimidine]-2,2′,4,4′,6′(3H,3′H,5H)-pentaone [54]. Acidic media can also provide a suitable environment. For instance, L-(+)-TA was used in similar reaction including symmetric BA [55]. All these recent investigations are shown in Fig. 1.
Based on the above-mentioned concepts and in the continuation of our research line, we studied another route for similar reactions. In this work, L-(+)-TA was used as either organocatalyst or acidic media provider in the reaction of 1-methylbarbituric acid (as unsymmetrical BA) with some selected aldehydes (from our previous works) [47, 54, 55] in the presence of BrCN. The number of product diastereomers was reduced from diastereomeric mixture [54] to one or two diastereomers in which detected by means of 1H and 13C NMR spectroscopy.
Results and discussion
This paper describes a new one-pot reaction of 1-methylbarbituric acid (1a′ as an unsymmetrical barbituric acid) with BrCN and various aldehydes (containing electron-withdrawing and electron-donating substituents) (2a–p) in the presence of L-(+)-TA (as either a chiral catalyst or acidic condition) affording reduced diastereomeric mixtures of a new class of stable heterocyclic spiro barbiturates 6a–m through 9a–m, respectively (Scheme 1, path a). Moreover, the reaction of 1a′ with aldehydes 1-naphthaldehyde (2n), 9-antharanaldehyde (2o) and furfural (2p) were also afforded Knoevenagel adducts (a mixture of Z- and E-isomers) of 4n–4p through 5n–5p (Scheme 1, path b).
The structures of symmetrical (thio)barbituric acids (1b′–1e′) are shown in Fig. 2. The reaction of 1b′–1e′ with aldehydes (2a–p) and BrCN in the presence of triethylamine as a basic media have been accomplished [47]. In this reaction, the salts of triethylammonium-5-bromo-(thio)barbiturates (10b′–10e′) and the racemic mixture of spiro[furo[2,3-d]pyrimidine-6,5′-pyrimidine]-pentaones (11b′–11e′) have been obtained since their 1H-NMR spectra show one singlet peak for C5-H. We also performed the reaction of 1-methylbarbituric acid 1a′ (as an unsymmetric barbituric acid) with aldehydes 2 in the presence of BrCN and triethylamine under the same conditions and obtained the salt of triethylammonium-5-bromo-2,4,6-trioxohexahydro-1-methylpyrimidin-5-ide (3), diastereomeric mixture of four new class of heterocyclic stable compounds (5S,5′S)- (6), (5R,5′S)-1,1′-dimethyl-5-aryl-1H,1′H-spiro[furo[2,3-d]pyrimidine-6,5′-pyrimidine]-2,2′,4,4′,6′(3H,3′H,5H)-pentaone (7), (5S,5′S)- (8) and (5R,5′S)-1′,3-dimethyl-5-aryl-1H,1′H-spiro[furo[2,3-d]pyrimidine-6,5′-pyrimidine]-2,2′,4,4′,6′(3H,3′H,5H)-pentaone (9) in good yields, respectively (Scheme 1, path a) [54]. The attempt to separate four diastereomers was failed due to their almost same polarity.
Structures of symmetrical (thio)barbituric acids (1) and corresponding salts (10) and 11 [47]
5-Bromo-1-methyl barbituric acid (12) plays a significant role (either as a nucleophile or electrophile) in the synthesis of 8 and 9 through the reaction of 1a′ and 2 in the presence of L-(+)-TA. A proposed mechanism for the formation of 12 is shown in Scheme 2. On the basis of the well-established chemistry of barbituric acid [56] and according to the mechanism of the bromination of symmetric barbituric acids [47, 52, 53] and 1-alkyl imidazoles by cyanogen bromide [37], it is reasonable to assume that the enolic form of 1a′ reacted with cyanogen bromide forming intermediate (A). The intramolecular rearrangement of A afforded 5-bromo-1-methylbarbituric acid 12 followed by the loss of hydrogen cyanide (HCN). At the same time, as a competition reaction, Knoevenagel condensation between 1a′ and 2 also occurred (Scheme 2, path b).
The proposed mechanism of the formation of 8a and 9a as representatives is shown in Scheme 3. First, the Knoevenagel condensation of 2a with 1a′ afforded (Z)- (4a) and (E)-1-methyl-5-phenylmethylenepyrimidine-2,4,6(1H,3H,5H)-trione (5a) and then, Michael addition of the enolic form of 5-bromo-1-methyl barbituric acid 12 (act as a nucleophile) to the β-carbon position of both 4a and 5a as an α,β-unsaturated carbonyl compound gave intermediates B, C, B′ and C′. Unfortunately, all attempts failed to separate and characterize these intermediates. L-(+)-TA as a chiral catalyst can bind to 4 and/or 5 by intermolecular H-bond and controlled the Michael addition of 12 (Scheme 3). Finally, intramolecular nucleophilic attack of hydroxyl group to carbon atom to leave bromide ion (as an electrophile) afforded 8a and 9a in good yields (Scheme 3).
There are eight possible spiro stereoisomers (four or less diastereomers) that were synthesized and derived from the reaction of 1a′ with 2 in the presence of BrCN and Et3N (Figs. 3 and 4a). Instead, in the presence of BrCN and L-(+)-TA afforded only two diastereomers under the same conditions (Fig. 4b). These observations indicated that L-(+)-TA as a chiral catalyst controlled the progress direction of the reaction (see later). For instance, 1H and 13C NMR spectra of the reaction between 1a′ and 2c show exclusively one product of 6c–9c. 1H NMR spectrum of the product shows a singlet for C5-H at δ 5.26 ppm, and its 13C NMR spectrum shows fifteen distinct peaks (two distinct peaks for N-CH3 carbon atoms). These data show the diastereoselectivity of the reaction in the presence of L-(+)-TA. In contrast, 2c gives four mixtures of diastereomers (6c–9c) [54]. Four possible diastereomers were also obtained from the reaction of 1a′ and 2k (as a representative) in the presence of BrCN and triethylamine (under basic condition) [55]. Representatively, the existence of six overlapped singlets for C5-H proton of the diastereomeric mixture of 6k–9k revealed that presumably there was an equilibrium mixture of lactam and lactim forms (each diastereomer consisting of an equilibrium mixture of lactam and lactim forms). Instead, in the presence of L-(+)-TA, only two distereomers were obtained and each diastereomer had both lactam and lactim forms (Scheme 4).
Expanded C5-H proton’s peaks of an equilibrium mixture of lactam and lactim forms of diastereomeric mixture of 6k, 7k, 8k and 9k derived from the one-pot reaction of 1a′ with 2k in the presence of BrCN and Et3N (a) and diastereoselective formation of 6k and 7k (and/or 8k and 9k) in the presence of L-(+)-TA (b) in DMSO-d6
Another evidence for the diastereoselective formation of 6–9 by the reaction of 1a′ with 2 and BrCN in the presence of L-(+)-TA is shown in Fig. 5. For instance, there are four different chemical shifts for N-CH3 in 11kc′ [47] (Fig. 5a) while there are two different environments for N-CH3 of spiro compound derived from the reaction of 1a′ and 2k in the presence of L-(+)-TA (Fig. 5b). These observations also indicated the diastereoselective formation of 6k–9k. The number of diastereoselective reaction products from some aldehydes is summarized in Table 1.
In comparison, the aromatic aldehydes possessing electron-withdrawing substituents were more reactive than those containing electron-donating substituents [50]. Owing to the aromatic nature of 1b′ and 1d′, the nucleophilicity of these compounds was less than that of 1c′ and 1e′. Therefore, the reactivity of 1a′ was higher than that of 1b′ and 1d′ due to amide resonance dominates over the aromaticity in barbituric acids [41].
Reaction yield in the presence of L-(+)-tartaric acid was higher than that of the presence of triethylamine under the same conditions. As we previously reported for the reaction of symmetrical (thio)barbituric acids with BrCN in the presence of L-(+)-tartaric acid [55], the intermediate 5-bromo (thio)barbituric acid 12 was formed which was soluble in methanol and caused the progress of reaction. This intermediate was vital for the formation of spiro compounds. Instead, in the reactions in the presence of triethylamine, the salt of triethylammonium 5-bromo-1-methyl-2,4,6-trioxohexahydropyrimidin-5-ide (3 in Scheme 1) was formed, and a small portion of this salt was precipitated which reduced the reaction yield. Likewise, this salt was an intermediate for the formation of spiro compounds. In these types of reactions, a small fraction of triethylammonium hydrobromide salt (Et3NHBr) was also formed in the presence of BrCN and trimethylamine that decreased reaction yield [47].
Conclusion
In conclusion, in the reaction of symmetric barbituric acids with aromatic aldehydes and cyanogen bromide in the presence of triethylamine, the racemic mixture of 5-aryl-1H,1′H-spiro[furo[2,3-d]pyrimidine-6,5′-pyrimidine]2,2′,4,4′,6′(3H,3′H,5H)-pentaones was afforded. The reaction of 1-methylbarbituric acid 1a′ as an unsymmetric barbituric acid with the same aldehydes and cyanogen bromide in the presence of triethylamine afforded a mixture of eight stereoisomers (four diastereomers) under the same conditions. Instead, the reaction of 1a′ with the same aldehydes and cyanogen bromide in the presence of L-(+)-tartaric acid reduced the number of stereoisomers.
Experimental section
General procedures
The drawing and nomenclature of compounds were performed by ChemBioDraw Ultra version 12.0 software. Melting points were measured with a digital melting point apparatus (Electrothermal) and were uncorrected. IR spectra were recorded in the region of 4000–400 cm−1 on a NEXUS 670 FT IR spectrometer by preparing KBr pellets (Urmia University, Urmia, Iran). 1H and 13C NMR spectra were recorded on Bruker 300 FT-NMR at 300 and 75 MHz, respectively (Urmia University, Urmia, Iran). 1H and 13C NMR spectra were obtained in the solution of DMSO-d6 as solvent using TMS as internal standard. The data were reported as s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet or unresolved, bs = broad singlet, coupling constant(s) in Hz, integration. All reactions were monitored by TLC with silica gel-coated plates (AcOEt:AcOH/80:20/v:v). The compound 1a′ was synthesized and purified in our laboratory as previously described in the literature [55, 57]. Cyanogen bromide was synthesized based on the method reported in Ref. [58]. Triethylamine and used solvents were purchased from Merck Company and were employed without further purification.
General procedures for the preparation of 6–9 (c, d, e, f, l)
The physical and spectral data of compounds 6c–9c are described as representatives.
In a 10-mL Teflon-faced screw cap tube equipped with a magnetic stirrer, 0.06-g (0.48 mmol) cyanogen bromide (BrCN), 0.15-g (0.96 mmol) 1-methyl barbituric acid, and 0.23-g (0.57 mmol) 2c were dissolved in 10-mL methanol, and then 0.06-g (0.6 mmol) L-(+)-tartaric acid was added into the solution at 0 °C. The reaction mixture was stirred for 3 h while heating from 0 °C to room temperature (Caution! Cyanogen Bromide is highly toxic. Reactions should be carried out under a well-ventilated hood). Teflon-faced screw cap tube prevented the vaporization of cyanogen bromide during the reaction. Reaction progress was monitored by thin layer chromatography (TLC). After the completion of reaction, the crystalline white solid was precipitated, filtered off, washed with a few mL methanol and dried (70% yield).
1′,3-Dimethyl-5-(3-nitrophenyl)-1,5-dihydro-2H,2′H-spiro[furo[2,3-d]pyrimidine-6,5′-pyrimidine]-2,2′,4,4′,6′(1′H,3H,3′H)-pentaone (6c–9c)
White solid; m.p. 261–265 °C (decomps); FT IR (KBr) 3427, 3023, 2813, 1736, 1707, 1655, 1528, 1441, 1355 cm−1; 1H NMR (DMSO-d6, 300 MHz) δ 2.34, 2.45, 3.14, 3.29 (4 s, 6H, 2Me), 5.26 (s, 1H, CH-aliph), 7.61 (m, 2H, CH-ar.), 8.11 (m, 2H, CH-ar.), 11.18 (s, 1H, NH), 11.96 (s, 1H, NH); 13C NMR (DMSO-d6, 75 MHz) 165.7, 164.8, 164.1, 159.1, 151.0, 149.8, 147.9, 137.6, 136.0, 130.1, 124.0, 123.8, 90.0, 85.9, 54.8, 29.3, 27.4.
5-(4-Bromophenyl)-1′,3-dimethyl-1,5-dihydro-2H,2′H-spiro[furo[2,3-d]pyrimidine-6,5′-pyrimidine]-2,2′,4,4′,6′(1′H,3H,3′H)-pentaone (6e–9e)
White solid; m.p. 292–296 °C (decomps.); FT IR (KBr) 3422, 3251, 3070, 2925, 2853, 1719, 1659, 1544, 1402, 1374 cm−1; 1H NMR (DMSO-d6, 300 MHz) δ 2.37–3.30 (6H, 2Me), 4.93 – 5.00 (3 s, 1H, CH-aliph., a mixture of three diastereomers), 7.05–7.17 (m, 2H, CH ar.), 7.48 (d, 2H, J = 8.1 Hz, CH-ar.), 11.1 (s, 1H, NH), 11.9 (s,1H, NH); 13C NMR (DMSO-d6, 75 MHz) 166.1, 166.0, 164.5, 164.1, 159.1, 151.0, 149.9, 149.9, 134.5, 131.4, 131.3, 122.2, 122.2, 90.2, 86.2, 55.6, 29.2, 27.4, 27.2.
5-(4-Hydroxyphenyl)-1′,3-dimethyl-1,5-dihydro-2H,2′H-spiro[furo[2,3-d]pyrimidine-6,5′-pyrimidine]-2,2′,4,4′,6′(1′H,3H,3′H)-pentaone (6f–9f)
White solid; m.p. 234–236 °C (decomps.); FT IR (KBr) 3435, 3213, 2925, 2850, 1720, 1666, 1547, 1520, 1435, 1366 cm−1; 1H NMR (DMSO-d6, 300 MHz) δ 2.40, 3.29 (2 s, 6H, 2Me), overlapped with water of DMSO), 4.78 (s, 1H, CH-aliph.), 6.63 (d, 2H, J = 7.5 Hz, CH-ar.), 6.88 (d, 2H, J = 7.5 Hz, CH-ar.), 9.49 (s, 1H, OH), 11.11 (s, 1H, NH), 11.86 (s, 1H, NH); 13C NMR (DMSO-d6, 75 MHz) 166.4, 164.4, 164.1, 159.1, 158.0, 151.0, 150.0, 130.1, 124.7, 115.2, 90.6, 86.5, 56.6, 29.2, 27.4.
1′,3-Dimethyl-5-(3,4,5-trimethoxyphenyl)-1,5-dihydro-2H,2′H-spiro[furo[2,3-d]pyrimidine-6,5′-pyrimidine]-2,2′,4,4′,6′(1′H,3H,3′H)-pentaone (6k–9k)
White solid; m.p. 269–271 °C (decamps); FT IR (KBr) 3424, 2927, 2848, 1712, 1686, 1665, 1594, 1511, 1382, 1126, 1038 cm−1; 1H NMR (DMSO-d6, 300 MHz): δ 2.40, 2.49 (2 s, 3H), 3.07, 3,15 (s, 3H, CH-aliph), 3.61–3.76 (m, 9H, CH-aliph.), 3.15 (s, 3H, CH-aliph.), 4.8–4.9 (m, 1H, CH-aliph) a mixture of two diastereomers, 6.43–6.46 (s, 2H, CH-ar.), 11.12 (s, 1H, NH), 11.34 (s, 1H, NH); 13C NMR (DMSO-d6, 75 MHz) 164.3, 163.5, 163.14, 159.14, 152.9, 150.2, 138.1, 130.3, 107.0, 90.6, 60.4, 57.1, 56.4, 28.6, 27.3.
1′,3-Dimethyl-5-(pyridin-2-yl)-1,5-dihydro-2H,2′H-spiro[furo[2,3-d]pyrimidine-6,5′-pyrimidine]-2,2′,4,4′,6′(1′H,3H,3′H)-pentaone (6l–9l)
White solid; m.p. 272 °C (decomps.); FT IR (KBr) 3434, 3186, 2997, 1700, 1620, 1457, 1403, 1354 cm−1; 1H NMR (DMSO-d6, 300 MHz) δ 2.40 (s, 3H, Me), 3.30 (s, 3H, Me), 4.84 (s, 1H, CH-aliph), 7.20 (m, 1H, Pyr.), 7.30 (m, 1H, Pyr.), 7.73 (m, 1H, Pyr.), 8.43 (s, 1H, Pyr.), 11.21 (s, 1H, NH), 11.96 (s, 1H, NH); 13C NMR (DMSO-d6, 75 MHz) δ 166.3, 164.7, 164.0, 159.3, 154.8, 151.0, 149.9, 149.3, 149.3, 137.2, 123.8, 123.8, 89.4, 85.3, 58.7, 49.0, 31.1, 29.3, 27.4.
Supporting information
Full characterization and spectral data for compounds 6c–9c, 6e–9e, 6f–9f, 6k–9k and 6l–9l are available.
References
D.J. Brown, in Comprehensive Heterocyclic Chemistry, vol. 3, ed. by A.R. Katritzky, C.W. Rees (Pergamon, Oxford, 1984)
H. Wamhoff, J. Dzenis, K. Hirota, Adv. Heterocycl. Chem. 55, 129 (1992)
S.I. Naya, H. Miyama, K. Yasu, T. Takayasu, M. Nitta, Tetrahedron 59, 1811 (2003)
V. Cody, N. Galitsky, J.R. Luft, W. Pangborn, A. Gangjee, R. Devraj, S.F. Queener, R.L. Blakley, Acta Crystallogr. Sect. D. 53, 638 (1997)
R.G. Melik-Ogandzhanyan, V.E. Khachatryan, A.S. Gapoyan, Russ. Chem. Rev. 54, 262 (1985)
J.D. Figuera-Villar, C.L. Carneiro, E.R. Cruz, Heterocycles 34, 891 (1992)
E. Campaigne, R.L. Ellis, M. Bradford, J. Ho, J. Med. Chem. 12, 339 (1969)
F. Blume, F. Arndt, R. Ress, Ger. Patent 3712782 (1988)
K. Krasnov, V. Kartsev, Russ. J. Org. Chem. 41, 901 (2005)
S. Kotha, A.C. Deb, R.V. Kumar, Bioorg. Med. Chem. Lett. 15, 1039 (2005)
J.F. Gerkens, Eur. J. Pharmacol. 134, 293 (1987)
J. Duan, Z. Lu, US Patent. 6936620B2 (2005)
B.S. Jursic, E.D. Stevens, Tetrahedron Lett. 44, 2203 (2003)
A.V. Moskvin, N.R. Reznikova, B.A. Ivin, Russ. J. Org. Chem. 38, 463 (2002)
B.S. Jursic, F. Douelle, E.D. Stevens, Tetrahedron 59, 3427 (2003)
S. Spange, M. Bauer, B. Walfort, H. Lang, J. Org. Chem. 71, 7850 (2006)
B.S. Jursic, D.M. Neumann, K.L. Martin, E.D. Stevens, Org. Lett. 4, 811 (2002)
B.S. Jursic, D.M. Neumann, Z. Moore, E.D. Stevens, J. Org. Chem. 67, 2372 (2002)
A. Renard, J. Lhomme, M. Kotera, J. Org. Chem. 67, 1302 (2002)
I.V. Paramonov, N.A. Belyaev, T.V. Glukhareva, A.S. Volkov, E.V. Deeva, Y.Y. Morzherin, Chem. Heterocycl. Compd. 42, 127 (2006)
S.I. Naya, K. Yoda, M. Nitta, Tetrahedron 61, 8616 (2005)
C.J. Burns, C.A. Martin, K.B. Sharpless, J. Org. Chem. 54, 2826 (1989)
T. Katsuki, A.W.M. Lee, P. Ma, V.S. Martin, S. Masamune, K.B. Sharpless, D. Tuddenham, F.J. Walker, J. Org. Chem. 47, 1373 (1982)
K. Berijani, A. Morsali, J.T. Hupp, Catal. Sci. Technol. 9, 3388 (2019)
A.A. Choliq, R. Nakae, M. Watanabe, T. Misaki, M. Fujita, Y. Okamoto, T. Sugimura, Bull. Chem. Soc. Japan. 92, 1175 (2019)
N. Haruna, D.E. Acosta, S. Nakagawa, K. Yamaguchi, A. Tai, T. Okuyama, T. Sugimura, Heterocycles 62, 375 (2004)
I. Arai, A. Mori, H. Yamamoto, J. Am. Chem. Soc. 107, 8254 (1985)
A. Mori, I. Arai, H. Yamamoto, H. Nakai, Y. Arai, Tetrahedron 42, 6447 (1986)
E.A. Mash, K.A. Nelson, Tetrahedron 43, 679 (1987)
E.A. Mash, K.A. Nelson, Tetrahedron Lett. 27, 1441 (1986)
K.R. Prasad, B. Swain, Tetrahedron Asymmetry 19, 1134 (2008)
M. Markert, I. Buchem, H. Krüger, R. Mahrwald, Tetrahedron Asymmetry 15, 803 (2004)
K.R. Prasad, P. Anbarasan, Tetrahedron Asymmetry 17, 2465 (2006)
A.C. Pinto, C.B.L. Freitas, A.G. Dias, V.L.P. Pereira, B. Tinant, J.-P. Declercq, P.R.R. Costa, Tetrahedron Asymmetry 13, 1025 (2002)
Z. Araźny, Z. Czarnocki, K. Wojtasiewicz, J.K. Maurin, Tetrahedron Asymmetry 11, 2793 (2000)
L. Flores-Santos, E. Martin, M. Diéguez, A.M. Masdeu-Bultó, C. Claver, Tetrahedron Asymmetry 12, 3029 (2001)
P.B.W. McCallum, M.R. Grimmett, A.G. Blackman, R.T. Weavers, Aust. J. Chem. 52, 159 (1999)
D.D. Tanner, G. Lycan, N.J. Bunce, Can. J. Chem. 48, 1492 (1970)
A. Alberola, C. Andres, A.G. Ortega, R. Pedrosa, M. Vicente, Synth. Commun. 16, 1161 (1986)
J. von Braun, K. Heider, E. Müler, Chem. Ber. 51, 273 (1918)
S. Chambert, F. Thomasson, J.-L. Dĕcout, J. Org. Chem. 67, 1898 (2002)
N. Noroozi Pesyan, M. Rezaee, Monatshefte fűr chemie 145, 1165 (2014)
E. Kashani, N. Noroozi Pesyan, H. Rashidnejad, A. Poursattar Marjani, H. Yaghoobnejad Asl, J. Iran. Chem. Soc. 14, 2143 (2017)
N. Noroozi Pesyan, A. Gharib, M. Behroozi, A. Shokr, Arabian J. Chem. 10, S1558 (2017)
N. Noroozi Pesyan, H. Rashidnejad, J. Iran. Chem. Soc. 14, 1365 (2017)
E. Kashani, N. Noroozi Pesyan, H. Rashidnejad, Synthesis 48, 2079 (2016)
M. Jalilzadeh, N. Noroozi Pesyan, F. Rezaee, S. Rastgar, Y. Hosseini, E. Şahin, Mol. Divers. 15, 721 (2011)
H. Zhian, N. Noroozi Pesyan, M. Alinejad, H. Rashidnejad, B. Notash, J. Heterocycl. Chem. 55, 2563 (2018)
M.-E. Capella-Peiró, S. Carda-Broch, L. Monferrer-Pons, J. Esteve-Romero, Anal. Chim. Acta 517, 81 (2004)
O. Pelletier, J.A. Campbell, J. Pharm. Sci. 51, 594 (1962)
O. Pelletier, J.A. Campbell, J. Pharm. Sci. 50, 926 (1961)
N. Noroozi Pesyan, S. Rastgar, Y. Hosseini, Acta Cryst. E 65, O1444 (2009)
Y. Hosseini, S. Rastgar, Z. Heren, O. Büyükgüngör, N. Noroozi Pesyan, J. Chin. Chem. Soc. 58, 309 (2011)
M. Jalilzadeh, N. Noroozi Pesyan, J. Korean Chem. Soc. 55, 940 (2011)
M. Jalilzadeh, N. Noroozi Pesyan, Bull. Korean Chem. Soc. 32, 3382 (2011)
D.J. Brown, S.F. Mason, Chemistry of Heterocyclic Compounds, the Pyrimidines, vol. 16 (Wiley, New York, 1962)
A. Vogel, Textbook of Practical Organic Chemistry, 4th edn. (Longman, New York, 1978)
W.W. Hartman, E.E. Dreger, Org. Synth. Coll. 2, 150 (1943)
Acknowledgements
We gratefully acknowledge financial support by the Research Council of Urmia University (Grant Research No. #9-10523).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shahvirdi, S., Rashidnejad, H. & Noroozi Pesyan, N. The organocatalytic role of L-(+)-tartaric acid in the synthesis of 5-aryl-1,1′- and 5-aryl-3,1′-dimethyl-1H,1′H-spiro[furo[2,3-d]pyrimidine-6,5′-pyrimidine]2,2′,4,4′,6′(3H,3′H,5H)-pentaones. J IRAN CHEM SOC 18, 457–465 (2021). https://doi.org/10.1007/s13738-020-02041-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-020-02041-7