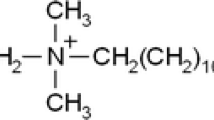Abstract
In this study, a rapid, sensitive and novel nanodrop spectrometric approach is used for the trace level determination of cationic surfactant methyltrioctylammonium chloride (MTOAC) based on the reaction of Mo(III)-SCN− complex with MTOAC in acidic condition. The molar absorptivity of red-colored Mo(III)-SCN− complex was enhanced in the presence of MTOAC that is attributed to a hyperchromic shift. The absorptivity value of the complex with respect to MTOAC is 9.8 × 104 L mol−1 cm−1 at λmax 415 nm. The quality parameters show the good analytical performance of the proposed method with detection limit (LOD) of 8.3 × 10−3 mg L−1 and limit of quantitation of 2.7 × 10−2 mg L−1. The calibration curve for MTOAC determination obeys Beer’s law with the concentration limit of 0.05–0.40 mg L−1 and determination coefficient (R2) 0.9992 along with slope and intercept 9.5 and 0.002, respectively. The proposed NDS method has been applied successfully in different environmental and biological samples for the determination of MTOAC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surfactants are also known as a surface active agent and have amphiphilic character. Generally, surfactant consists of hydrophilic and hydrophobic moieties. Hydrophilic group (head) is water-soluble and polar such as phosphate, sulfate, carboxylate or ammonium, whereas hydrophobic group (tail) includes hydrocarbon, fluorocarbon or aromatic ring. Surfactant has property to decrease the surface tension due to an amphiphilic character. It has an ability to form micelles and bilayer; thus, surfactants are broadly used in various industries like household, cosmetics, drug, pharmaceutics, leather, fiber, oil recovery, etc. [1,2,3,4].
Surfactants are classified into four classes based on the presence of hydrophilic groups. If hydrophilic moiety (head) group include carboxylate, phosphate or sulfate, it forms anionic surfactant, positively charged quaternary ammonium salts form cationic surfactants, uncharged hydrophiles form nonionic surfactants (fatty acids), and zwitterionic betaines form amphoteric surfactant. The head or hydrophilic polar part of the surfactant determine its chemical and physical qualities, and the hydrophobic moiety of surfactant also affects nature, i.e., the longer the carbon chain of the tail part, more it will become less water-soluble and nonpolar [5,6,7].
Cationic surfactants (CSs) are quaternary ammonium compounds (QACs), consisting of positively charged nitrogen atom with hydrophobic hydrocarbon chain substituent. Other substituents may be methyl or benzyl groups. Because of positive charge, cationic surfactant became able to absorbable on different surfaces. Therefore, it is commonly used to modify the surface properties. Cationic surfactants are most commonly used as softeners in apparel and fabric industry, laundry detergents, as antiseptics, in pigments as dispersants, in bio-industry as biocides in pharmaceuticals and antistatic agent and as corrosion inhibitors in metal industry [8, 9]. In spite of that, surfactants are widespread in various human activities also [10, 11]. Cationic surfactants (CSs) are widely used in commodity manufacture and in many industrial fields, but they also cause environmental pollution. Huge bodies of water get contaminated by disposal of surfactant containing wastewater effluent into it, resulting in damaging the aquatic ecosystem and ultimately affecting human health [12, 13]. Therefore, monitoring and control of the emission of surfactants in environment is quite essential.
Several works have been stated for the determination of cationic surfactant such as spectrofluorimetric [14], conductometric [15], liquid chromatography–mass spectrometry [16, 17], flow injection method [18, 19], MALDI [20] and multiwalled CNTs [21]. Various chemical reagents and dyes such as eosin-y [22], cresyl violet [23], brilliant blue [24], patent blue-V [25], benzothiaxolyldiazoaminoazobenzene [26] and nanoparticles [27] also have been used for the determination of CSs. However, these methods are not applicable for routine analysis due to the presence of interfering ions and tedious analysis and also the requirement of reagents is more with poor sensitivity.
In the proposed work, determination of cationic surfactant MTOAC was performed with Mo-SCN− complex. MTOAC, also called as Aliquat 336, is a quaternary ammonium salt cationic surfactant which is commonly used as a phase transfer catalyst, metal extraction agent and in several medical as well as industrial applications. The waste products containing such surfactants are discharged into water bodies, leading to aquatic toxicity which can cause hazardous effect on aquatic system as well as human body. It can interrupt the normal physiological function of organs by damaging the enzyme activity. It also accumulates in the human body, which is difficult to degrade. MTOAC causes mouth ulcer and damage to throat if ingested. Other symptoms like diarrhea, fever, nausea, vomiting, dermal necrosis, lung malfunctioning, uncontrolled blood pressure, hypotension, serious corneal damage to eyes are also reported in many cases [28, 29].
In order to prevent such types of noxious problem, determination of MTOAC cationic surfactant is needful. The proposed NDS method which requires less than 1 μL of sample is suitable for onsite and accurate determination of MTOAC. The work is based on absorptivity enhancement due to an associated ion pair complex in the presence of cationic surfactant MTOAC. During experimental work, all parameters were optimized for the analysis. The novel and simple NDS method was applied successfully for the determination of MTOAC in real samples such as industrial effluents and biological samples.
Experimental
Apparatus
All spectral measurements were taken using nanodrop spectrophotometer (NDS) (Thermo Scientific Inc. TM 1000, Wilmington, Delaware, USA). In NDS, 0.5 µL of sample is sufficient for analysis with high sensitivity and accuracy.
Reagent and chemicals
In the proposed experimental work, all the reagents used were of analytical grade. 100 mg L−1 Mo(VI) solution has been prepared by appropriate dilution of a stock standard solution of 1000 mg L−1 Mo(VI) (E. Merck, Germany). A 0.25 M ascorbic acid (4.4% w/v) was used for the reduction of metal. 3.5 M NH4SCN (26.6% w/v) was used for the formation of the Mo-SCN− complex. 5.0 M H2SO4 was used to maintain the pH throughout the experiment.
Cationic surfactants, methyltrioctylammonium chloride (MTOAC, 98.0%) and tetraoctylammonium bromide (TOAB, 99.0%) were from Sigma-Aldrich Co. St. Louis, USA. Dodecyltrimethylammonium bromide (DTAB, 99.0%), sodium lauryl sulfate (SLS, 98.0%), sodium dodecylbenzenesulfonate (SDBS, 98.0%) and nonionic surfactant such as Tween-80, Brij-35 were obtained from Merck, Mumbai, India.
Collection and preparation of samples
Proposed method was employed for the analysis of MTOAC in industrial wastewater and biological samples. The industrial wastewater effluent samples were collected from industrial area of different region of Chhattisgarh, i.e., Raipur, Raigarh and Korba. The biological samples such as human blood and urine were obtained from pathology clinic, Raipur. All samples were kept in polyethylene bottles (100 mL). The samples were preliminary treated by HNO3 solution and finally filtered with Whatman paper-42. The standard addition method which was free from matrix interferences was applied for the analysis of methyltrioctylammonium chloride (MTOAC) in samples.
Procedure
A 1.0 mL of micro-tube contains 500 µL of 50 mg L−1 Mo(VI) solution and 150 µL of 0.5 M NH4SCN solution, then 50 µL ascorbic acid (0.013 M) was added to 100 µL of 0.5 M H2SO4 medium mixed with MTOAC surfactant (0.05–0.40 mg L−1), and the absorptivity measurement was taken on nanodrop spectrophotometer (NDS) against reagent blank at λmax 415 nm. The same analytical procedure was also applied for the analysis of MTOAC surfactant in biological (blood and urine) and industrial wastewater samples by standard addition method.
Results and discussion
Optimization of different analytical parameters
Effect of acid
Various acids such as HNO3, HCl and H2SO4 were examined as a medium for the analysis of MTOAC during extraction of metal complex. However, in the presence of HCl and HNO3 complex showed low molar absorptivity and unstable complexing at ambient temperature (25 ± 2 °C). Thus, H2SO4 has been chosen for further analysis. Effect of acid was observed to range from of 0.5 M to 5.0 M H2SO4, and the optimal value was found to 3.0 M for H2SO4 as shown in Fig. 1.
Effect of different reducing agents
In the proposed work, various reducing agents such as ascorbic acid [30], SnCl2 [31] and hydrazinium hydrogen sulfate [32] were examined. The present study shows Mo(VI) is finally reduced to Mo(III). With SnCl2 and hydrazinium hydrogen sulfate, reproducible results are obtained only within a narrow range concentration of the reagents, whereas ascorbic acid showed maximum color with a definite excess of the reagent, but any further increase in the concentration of ascorbic acid did not alter the molar absorptivity. So ascorbic acid was chosen for further detailed experiment. 0.15 M ascorbic acid was chosen for detailed experiment.
Effect of ammonium thiocyanate concentration
The complexation effect of ammonium thiocyanate with Mo(III) was also observed [33] in the range of 0.25–2.0 M concentration for the determination of MTOAC. Maximum absorbance was achieved at 1.4 M SCN−; thus, 1.4 M was used for further experiment. Effect of SCN− concentration on absorptivity measurement is shown in Fig. 2.
Effect of Mo concentration
In this experiment, determination of MTOAC was done by complex formation of Mo with SCN−. Mo(VI) solution was treated with thiocyanate solution, an interaction was proceeding at an appreciable speed, when it was reduced to Mo(III) trivalent red complex [34], and it was studied for further investigation. Mo in +3 state behaves as class-B acceptor or soft metal and SCN− is soft ligand or base, so they form complex through soft–soft interaction [35]. In this experiment, concentration of Mo(III) was optimized from 0.10 to 1.0 mg L−1 for effective complex formation and color development and the optimum absorbance was found to be 0.7 mg L−1 of Mo concentration as shown in Fig. 3.
Absorption spectra
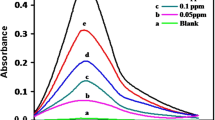
In terms of molar absorptivity measurement, various kinds of surfactant such as (anionic, cationic and nonionic) were examined as shown in Table 1. Molar absorptivity of the complex increased remarkably upon the addition of cationic surfactant, because of the formation of ion-associated complex. For cationic surfactants, the range of molar absorptivity was from 3.2 × 104 to 9.8 × 104 L mol−1 cm−1, in which MTOAC revealed maximum absorbance at λmax 415 nm as shown in the absorption spectra of cationic surfactants (Fig. 4). The absorption spectra of [Mo(SCN)] MTOAC complex are displayed in Fig. 5.
Study of diverse ions for the analysis of MTOAC
In the present investigation, the effect of diverse ions due to the presence of anion, cation, oxidizing agents, reducing agents or surfactant ions which might be associated with the sample or analyte was examined separately in 1.0 mg L−1 concentration of MTOAC. The most common co-existing interfering ions were determined. The tolerance limits of various interfering species are summarized in Table 2.
Composition of the surfactant-assisted Mo-SCN− complex
In the present work, there is extensive electrostatic binding between negatively charged SCN− complex of Mo(III) with MTOA+ (methyltrioctylammonium cation). Appearance of dark red color at λmax 415 nm is attributed to hyperchromic shift. The composition or mole ratio of Mo(III) to SCN− and MTOAC in complex was calculated by curve-fitting method [36]. The graph was plotted between log distribution ratio of Mo(III) or log D and molar concentration of [SCN]− or [MTOAC] or log M shown in Fig. 6a, b. Distribution ratio (D) = (Aeq/Amax − Aeq) where Aeq and Amax denote absorbance value when the SCN− and MTOAC concentration was in equilibrium and in constant excess, respectively. Calculated slope for the curve log D versus log M = [SCN−] was 3.7 and for the curve log D versus log M = [MTOAC] was 0.7 which were nearer to integer 4.0 and 1.0. Therefore, the molar stoichiometric ratio for Mo/SCN/MTOAC is supposed to be 1:4:1. So the expected reaction involved in this experiment can be expressed as:
-
1.
Formation of thiocyanate complex
$${\text{Mo}}^{3 + } + \, 4{\text{ SCN}}^{ - } \to \, \left[ {{\text{Mo}}\left( {\text{SCN}} \right)_{4} } \right]^{-}$$(1) -
2.
Formation of associated ion complex with MTOAC
$$\left[ {{\text{Mo}}\left( {\text{SCN}} \right)_{4} } \right]^{-} + {\text{ MTOA}}^{ + } \to \, \left[ {{\text{Mo}}\left( {\text{SCN}} \right)_{4} } \right]{\text{MTOA}}$$(2)
Analytical and statistical variables measured by NDS
The calibration graph was plotted, and it is dynamically linear from 0.05 to 0.40 mg L−1 following Beer’s law. Determination coefficient value (R2), slope and intercept were calculated as 0.9992, 9.5 and 0.002, respectively. The increased molar absorptivity value of [Mo(SCN] MTOAC complex is 9.8 × 104 L mol−1 cm−1 at λmax 415 nm. The limit of detection [37] was obtained as 8.3 × 10−3 mg L−1, and limit of quantitation (LOQ) value of the method was 2.7 × 10−2 mg L−1. The calibration curve for the determination of MTOAC is shown in Fig. 7.
Comparison of present work with others
The statistical variable of proposed work has been compared with established reported methods with respect to precision, accuracy as well as sensitivity of data. The comparison parameter (LOD) for the determination of cationic surfactant is shown in Table 3. On the basis of observation, the existing method is quite easier and cost-effective and found to be accurate compared to other reported approaches for the determination of CSs. However, the nanodrop spectrophotometric system is quick and reproducible (i.e., low sample throughput) with lesser consumption of analytes.
Application
The present work focused on simple and rapid nanodrop spectrophotometric approach for the MTOAC determination in environmental and biological fluids samples via Mo-SCN− complex. To examine the validity of investigatory work, known concentration of MTOAC was spiked with industrial water samples and biological samples (urine and blood) by triplicate analysis (n = 3) and recovery of spiked samples has been calculated and found between 88.00 and 98.5% with RSD ± 0.89–1.45% as demonstrated in Table 4.
Conclusion
The newly described nanodrop method is being much sensitive, selective and rapid for the determination of cationic surfactant MTOAC at sample source in wastewater effluents and biological (blood, urine) sample containing 0.0083 mg L−1. The method overcomes most of the limitations such as interfering diverse ions, prolonged analysis and more consumption of reagents as well as analyte, reported for cationic surfactant determination. The method has useful advantages such as simplicity, short time analysis with high accuracy, lower reaches of sample solution (≤ 1.0 μL), portable and easy handling. The proposed work will further be extending to pharmaceutics and toxicology sciences.
References
R. Kurrey, M. Mahilang, M.K. Deb, K. Shrivas, Trends Environ. Anal. Chem. (2019). https://doi.org/10.1016/j.teac.2019.e00061
L.L. Schramm, E.N. Stasiuk, D.G. Marangoni, Annu. Rep. Prog. Chem. Sect. C Phys. Chem. (2003). https://doi.org/10.1039/B208499F
A.S. Bisht, Commercial Surfactants for Remediation: Mobilization of Trace Metals from Estuarine Sediment and Bioavailability (Springer, Berlin, 2018). https://doi.org/10.1007/978-981-13-0221-3
O. Galović, M. Samardžić, M. Hajduković, M. Sak-Bosnar, Sens. Actuators B 236, 257 (2016)
M.J. Rosen, J.T. Kunjappu, Surfactants and Interfacial Phenomena (Wiley, Chichester, 2012)
K. Agrawal, G. Agnihotri, K. Shrivas, G.L. Mundhara, K.S. Patel, P. Hoffmann, Microchim. Acta (2004). https://doi.org/10.1007/s00604-004-0228-0
J. Falbe (ed.), Surfactants in Consumer Products: Theory, Technology and Application (Springer, Berlin, 1987). https://doi.org/10.1007/978-3-642-71545-7
E. Olkowska, Ż. Polkowska, J. Namieśnik, Talanta 88, 1 (2012)
P. Bassarab, D. Williams, J.R. Dean, E. Ludkin, J.J. Perry, J. Chromatogr. A 1218, 673 (2011)
M.E. Fait, L. Bakas, G.L. Garrote, S.R. Morcelle, M.C. Saparrat, Appl. Microbiol. Biotechnol. 103, 97 (2019)
F. Laffleur, S. Pschick, J. Barthelmes, S. Hauptstein, A. Bernkop-Schnurch, Curr. Drug Deliv. 15, 351 (2018)
Y. Wang, Y. Zhang, X. Li, M. Sun, Z. Wei, Y. Wang, X. Feng, Sci. Rep. 5, 10107 (2015)
F.G. Bartnik, F. Wingen, Food Cosmet. Toxicol. 17, 633 (1979)
F. Suling, C. Xingguo, F. Jing, H. Zhide, Int. J. Environ. Anal. Chem. 85, 63 (2005)
S.E. Moore, M. Mohareb, S.A. Moore, R.M. Palepu, J. Colloid Interface Sci. 304, 491 (2006)
H.F. Schröder, J. Chromatogr. A 1020, 131 (2003)
W. Hsin-Yi, S. Chia-Lung, L. Ton, C. Tai-Yuan, L. Lan-Chi, L. Kuan-Yu, C. Hui-Chuan, C. I-Chi, L. Shih-Yuan, L. Pao-Chi, Talanta 194, 778 (2019)
T. Masadome, Anal. Lett. 31, 1071 (1998)
A. Safavi, M.A. Karimi, Anal. Chim. Acta 468, 53 (2002)
K. Shrivas, H.F. Wu, J. Mass Spectrom. 42, 1637 (2007)
K. Shrivas, H.F. Wu, Anal. Chim. Acta 628, 198 (2008)
S.P. Liu, G. Zhou, Z. Liu, Fresenius’. J. Anal. Chem. 363, 651 (1999)
E.M. Costi, M.D. Sicilia, S. Rubio, D. Pérez-Bendito, Anal. Chim. Acta 577, 257 (2006)
H.W. Gao, L.X. Zheng, H.D. Mei, Instrum Sci. Technol. 32, 271 (2004)
M. Idouhar, A. Tazerouti, J. Surf. Deterg. 11, 263 (2008)
S. Li, S. Zhao, Anal. Chim. Acta 501, 99 (2004)
A. Sharma, K. Tapadia, Orient. J. Chem. 32, 2641 (2016)
C.L. Yuan, Z.Z. Xu, M.X. Fan, H.Y. Liu, Y.H. Xie, T. Zhu, J. Chem. Pharm. Res. 6, 2233 (2014)
D. Attwood, A.T. Florence, Aspects of Surfactant Toxicity, vol. 614 (Springer, Dordrecht, 1983)
B. Tamhina, M.J. Herak, V. Jagodic, Anal. Chim. Acta 76, 417 (1975)
A.A. Bergh, G.P. Haight Jr., Inorg. Chem. 1, 688 (1962)
K. Nytko, H. Sikorska-Tomicka, Microchem. J. 25, 548 (1980)
J. Lewis, R.S. Nyholm, P.W. Smith, J. Chem. Soc. (Resumed) (1961). https://doi.org/10.1039/JR9610004590
C.F. Hiskey, V.W. Meloche, J. Am. Chem. Soc. 62, 1565 (1940)
P.C.H. Mitchell, Coord. Chem. Rev. 1, 315 (1966)
L.G. Sillen, Acta Chem Scan. 10, 186 (1956)
G.D. Christian, Analytical Chemistry, 6th edn. (Willey, Hoboken, 2007)
Acknowledgements
We gratefully acknowledge National Institute of Technology, Raipur (CG), for providing essential laboratory facility and also University Grants Commission (UGC), New Delhi, India, for financial support as fellowship (JRF student id-141394).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Rights and permissions
About this article
Cite this article
Gupta, S.K., Tapadia, K. & Sharma, A. A sensitive nanodrop method for the micro-level determination of cationic surfactant methyltrioctylammonium chloride (MTOAC) in biological fluids and environmental samples. J IRAN CHEM SOC 18, 343–349 (2021). https://doi.org/10.1007/s13738-020-02029-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-020-02029-3