Abstract
The aim of the current study is to describe the synthesis, characterization, and measurement of optical and antifungal properties of Yb2TiO5@Ag nanocomposites (YTi@Ag) against three standard Candida species including Candida albicans (ATCC 1023), Candida krusei (ATCC 6258), and Candida tropicalis (ATCC 750). YTi@Ag nanocomposites were synthesized through the sol-gel assistant ultrasonic method in an aqueous solution. The antifungal abilities were performed base on macrodilution method as recommended by the Clinical and Laboratory Standards Institute (CLSI, document M27-A3). SEM, XRD, EDX, and FT-IR were applied to characterize structural and morphological characteristics of nanocomposites. In addition, several antifungal tests were performed to investigate the impact of YTi@Ag nanocomposites on the Candida species. Minimum inhibitory concentration (MIC50) measurements of the YTi@Ag NPs against C. albicans (ATCC 1023), C. krusei (ATCC 6258), and C. tropicalis (ATCC 750) after 24 and 48 h were 4 and 32 µg/ml, respectively. MIC50 results show that as-synthesized YTi@Ag nanocomposites are efficient as an alternative antibacterial/antifungal agent. The band gap of bare Yb2TiO5 and YTi@Ag nanocomposites is 3 and 2.85 eV, respectively. Ag-doped Yb2TiO5 has advantage over naked Yb2TiO5 which enhances separation of electron–hole pairs which improves antifungal activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the recent years, application of nanomaterials has experienced a rapid growth thanks to introduce novel physical and chemical properties, especially semiconductor nanoparticles [1,2,3]. Fungi, kingdom of mostly microscopic organisms, can easily grow on the surface of materials and seriously threaten human health. Besides, in the third millennium, fungal diseases are an important cause of morbidity and mortality, especially in patients who suffer from having defect in their immune function. Candida, one of the famous fungal opportunistic pathogens, is specified as the part of human normal flora proliferates in gastrointestinal, genitourinary tracts, and oral cavity [4, 5]. Candida species are able to produce different types of diseases including local infection (skin and mucous) or disseminated infections which penetrate through the organs with the aid of blood stream [6, 7]. There are five impressive Candida species which cause more than 90% of invasive infections including Candida albicans, Candida glabrata, Candida tropicalis, C. parapsilosis, and Candida krusei [8]. In spite of various created antifungal drugs which commonly were used in clinical treatments, only a few number of drugs are attainable for candidiasis infections [9]. However, Candida species, especially non-albicans species such as C. glabrata, become resistant to antifungal agents which cause an increase in treatment failure [10]. Therefore, it is necessary to study the novel drugs which have less influenced on the fungal resistance mechanism. Nanotechnology recently developed many biocidal nanomaterials which demonstrated good antifungal activities against various fungi [11,12,13,14,15]. Many nanoparticles have been studied as antimicrobial agents, such as those of chitosan [16], MgO [17], silica-coated Fe3O4 [18], silver and its derivatives [19,20,21], TiO2 [22], cobalt ferrite, and Fe3O4 [23, 24]. Ag compounds demonstrate having wide antimicrobial activities against bacteria, viruses, and fungi, with minimal development of microbial resistance. Antifungal and antibacterial properties of silver nanoparticles (AgNPs) originated from their ability for producing high levels of reactive oxygen species (ROS) and free radical species such as hydrogen peroxide, superoxide anion, hydroxyl radical, hypochlorous acid, and singlet oxygen [25,26,27]. In the absence of AgNPs, there is no substantial amount of ROS in cells and also it can be removed by antioxidant systems of the cell [28]. On the contrary, in the presence of AgNPs, they can inactivate dehydrogenases enzyme which causes an increase in ROS generation and consequently blocked respiration and growth of cells [29, 30]. On the other hand, AgNPs reduce producing level of antioxidant enzyme such as glutathione (GSH), superoxide dismutase, and catalase, which can accelerate the accumulation of ROS [31]. Eventually, accumulation of ROS causes apoptosis, lipid peroxidation, depletion of GSH, and DNA damage [32, 33]. The other thing is that AgNPs can release silver ions (Ag+) [34,35,36,37], which are dependent on their surface area [38]. There are several possible mechanisms for the antifungal and antibacterial properties of silver ions. First of all, it can interface with sulfhydryl groups in enzymes and proteins. In other words, Ag ions can bind to proteins in the cell membrane and form stable bonds which cause to deactivate proteins which are included in transmembrane ATP generation and mediate ion transport across cell membranes [39]. Second of all, they can take place between the purine and pyrimidine base pairs and disrupt the H-bonds between base pairs of the anti-parallel DNA strands, which prevent cell division and reproduction eventually [40, 41]. Furthermore, Ag+ as a heavy metal ion can cause cellular oxidative stress in microbes to increase. Herein, we applied simple two step sol-gel assistant ultrasonic method to synthesize YTi@Ag NPs in order to investigate its antifungal ability. The antifungal activities against Candida fungi group consisting of C. albicans (ATCC 1023), C. krusei (ATCC 6258), and C. tropicalis (ATCC 750) were investigated throughout the macrodilution method.
Experimental
Characterization
Materials and characterization: All chemical reagents in this experiment were of analytical grade and used without further purification, ytterbium salt, ethanol, silver nitrate, and tetra-n-butyl titanate (TNBT). X-ray diffraction (XRD) patterns were recorded by a Philips-X’Pert-Pro, X-ray diffractometer using Ni-filtered Cu Kα radiation at scan range of 10 < 2θ < 80. Scanning electron microscopy (SEM) images were obtained on LEO-1455VP equipped with an energy-dispersive X-ray spectroscopy. Fourier-transform infrared (FT-IR) spectra were recorded on Magna-IR, spectrometer 550 Nicolet with 0.125 cm−1 resolution in KBr pellets in the range of 400–4000 cm−1. The EDS analysis with 20 kV accelerated voltage was done.
Synthesis of Yb2TiO5 nanocomposites
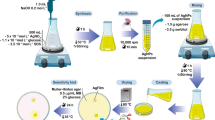
In a typical synthesis procedure, in two beakers, the stoichiometric amount (1:1) of tetra-n-butyl titanate (A) and ytterbium nitrate (B) was dissolved in ethanol, under stirring. Then, oxalic acid as a chelate agent was also dissolved in ethanol. Afterward, solutions A and B were mixed with the chelate agent solution under stirring at 90 °C for 30 min to form a gel. The final gel product was dried at 110 °C and then calcined at 800 °C for 3 h in a conventional furnace in air atmosphere.
Synthesis of YTi@Ag nanocomposites
In order to synthesize YTi@Ag nanocomposites, 0.5 g of as-synthesized Yb2TiO5 nanoparticles was dissolved in 20-ml ultra-pure water, while a 10 ml of silver nitrate solution (0.5 g) was added to it under stirring for 15 min. Afterward, above-mentioned solution was irradiated under ultrasonic probe, 180 W for 30 min. After irradiation, the system was allowed to cool to room temperature naturally, and the obtained precipitations were collected, washed three times with ethanol and distilled water, and dried under vacuum oven. It is worth mentioning that the electrostatic interaction (attraction) was happened between positive surface charge silver ions and negative surface charge Yb2TiO5 nanoparticles to make YTi@Ag nanocomposites.
Antifungal assay
The antifungal activity of YTi@Ag nanocomposites was performed according to the macrodilution method which is the recommended method by the Clinical and Laboratory Standards Institute (CLSI, document M27-A3). At first, YTi@Ag nanocomposites and Fluconazole were dissolved in distilled water and 100% dimethyl sulfoxide, respectively. Then, the above solutions were diluted, tenfold, by adding RPMI 1640 medium (with L-(3-N-morpholinepropanesulfonic acid). Afterward, 100 μl of aforementioned solutions was added into the wells micro-plate. It is noteworthy to mention that Fluconazole and YTi@Ag nanocomposites concentrations were 0.25–32 and 0.05–32 μg/ml, respectively. Then, three standard Candida species including C. albicans (ATCC 1023), C. krusei (ATCC 6258), and C. tropicalis (ATCC 750) were sub-cultured on the Sabouraud dextrose agar and incubated at 35 °C for 24 h. After incubation, they were suspended in 5 ml of sterile water and shook vigorously. In order to obtain 0.5 McFarland standard (1 × 106 to 5 × 106 CFU/ml) turbidity, the turbidity was adjusted spectrophotometrically at 625 nm. The suspension was diluted (1:100) and followed (1:20 dilution) with RPMI 1640 medium to provide final concentration around 0.5–2.5 × 103 colony-forming units (c.f.u)/ml. Then, 900 μg/ml of the above-prepared concentration was added to each well. In order to have positive control, one well was just filled with 1000 μg/ml Candida suspension. Finally, the micro-plates incubated in incubator in a humid environment, at 35 °C for 48 h. The MIC50 which considers as the lowest drug concentration and could create a ≥ 50% reduction on fungal growth in compared to the drug-free growth control was determined visually after 24 and 48 h. The other thing is that inverted microscope was used to better viewing and remove turbidity problems originated from nanoparticles. The inhibition percentage (MIC50) was calculated as follows (1):
Results and discussion
Structural study
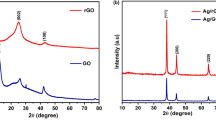
Figure 1a, b demonstrates the comparison of XRD patterns of Yb2TiO5 and YTi@Ag nanocomposites, respectively. Figure 1a shows cubic phase of YTi (a = b = c = 10.0300 Å) with a space group of Fd-3m and JCPDS No. 33-1457. Furthermore, XRD pattern of YTi@Ag nanocomposites illustrates cubic phase of Yb2TiO5 (JCPDS No. 33-1457) along with cubic phase of Ag (JCPDS No. 87-0717 and space group Fm-3m). No other crystalline phases are detected in the YTi@Ag nanocomposites. The crystallite diameter (Dc) of YTi@Ag nanocomposites (15.7 nm) was calculated through the Scherrer Eq. (2):
where β is the breadth of the observed diffraction line at its half intensity maximum, K is the so-called shape factor (0.9), and λ is the wavelength of X-ray source used in XRD. In order to further prove the chemical structure and composition of YTi@Ag nanocomposites, energy-dispersive spectroscopy (EDS) was performed, as shown in Fig. 2. Based on Fig. 2, nanocomposites consist of only Yb, Ti, O, and Ag atoms. FT-IR analysis was performed to confirm the presence of certain functional groups, as shown in Fig. 3. According to Fig. 3, the absorption bands at 3443 and 1633 cm−1 can be attributed to the stretching and bending vibrations of H2O molecules that were absorbed on the surface of the nanoparticles [42]. The weak absorption peak at 594 cm−1 is attributed to the Ti–O bonds [3]. Moreover, an absorption peak at 491 cm−1 is assigned to Yb–O band. Scanning electron microscopy (SEM) was applied to demonstrate the morphology of YTi@Ag nanocomposites. Figure 4a, b shows the SEM images of Yb2TiO5 and YTi@Ag nanocomposites. Based on Fig. 4a, product mainly consists of homogenous nanoparticles, while adding Ag causes to somewhat increase the particles size and agglomeration of YTi@Ag (Fig. 4b). On the one hand, the surface charge of Yb2TiO5 is intrinsically negative due to the presence of five oxygen groups with negative charge. Therefore, Yb2TiO5 nanoparticles do not show aggregation or have very small amount of aggregation, due to the electrostatic repulsion between negative charges on their nanoparticles surface, as we can see in Fig. 4a. On the other hand, doping silver nitrate creates aggregation because silver ions have positive charge and after mixing with Yb2TiO5 nanoparticles, which have negative surface charge, aggregates nanoparticles due to the electrostatic interaction between positive charge of silver ions and negative surface charge of Yb2TiO5 (Fig. 4b). TEM image of Yb2TiO5 (Fig. 4c) and YTi@Ag nanocomposites (Fig. 4d) is in agreement with their SEM images and confirms that adding Ag causes an increase in particle size along with agglomeration. Besides, TEM image of Yb2TiO5 consists of particles with average size 25–35 nm, while the size of particles in nanocomposites is 45–60 nm. The optical properties of the as-synthesized Yb2TiO5 and YTi@Ag nanocomposites have been analyzed by UV–Visible spectroscopy, as shown in Fig. 5a, b, respectively. Based on the Tauc’s relationship (α = α0(hѵ − Eg)n/hѵ), Eg value decreased from 3 to 2.85 eV after doping Ag with Yb2TiO5 which is mainly due to the efficient substitution of Ag with ytterbium (Yb) atoms. The substitution process lowers the conduction band of the host material (Yb2TiO5) to more negative values. Moreover, surface oxygen vacancies and defect state may form because of doping that can capture the photoinduced electron during photocatalytic process, which can enhance photocatalytic inactivation of fungal. Therefore, silver-doped Yb2TiO5 is suitable candidate for enhancing antibacterial properties.
Antifungal assessment
A possible mechanism that could explain interaction between nanocomposite and antifungal is that YTi@Ag nanocomposites could utilize electrostatic interactions, hydrophobic effects, or van der Waals forces to be adsorbed on the cell membrane and release silver ions (Ag+) and ROS. The as-released Ag+ and ROS might have damaged the cell membrane throughout the mechanisms including protein coagulation, cell wall pits, inactivation of the respiratory chain, membrane permeability induction, and biosorption process [43]. YTi@Ag nanocomposites can benefit from the advantages of producing ROS by Yb2TiO5 along with realized silver ions (Ag+) and ROS from YTi@Ag nanocomposites to demonstrate higher antifungal activity compared with pure silver nanoparticles. The antifungal activities of three different Candida species in the presence of YTi@Ag nanocomposites and Fluconazole (Fig. 6a, b, respectively) and silver nanoparticles for 24 and 48 h are listed in Table 1. Serial dilutions for YTi@Ag (1–8) wells were 32, 16, 4, 2, 1, 05, 0.25, and drug free, respectively. The one thing is that, based on Table 1, MIC50 was significantly increased with increase in incubation time in the presence of YTi@Ag nanocomposites, while it was slightly increased in the presence of Fluconazole, except C. krusei. The other thing is that the MIC50 of YTi@Ag nanocomposites is higher than that of silver nanoparticles.
Conclusions
In summary, Yb2TiO5@Ag nanocomposites were synthesized through the sol-gel assistant ultrasonic method. Besides, various concentrations (0.25–32 and 0.05–32 μg/ml) of YTi@Ag nanocomposites were applied to investigate its antifungal activity against three standard Candida species including C. albicans (ATCC 1023), C. krusei (ATCC 6258), and C. tropicalis (ATCC 750) in comparison with Fluconazole. Antifungal results demonstrate that maximum MIC50 for three standard Candida species was 4 and 32 µg/ml after 24 and 48 h, respectively. In addition, MIC50 value of Yb2TiO5@Ag nanocomposites is higher than pure silver nanoparticles due to the synergetic effect of Ag+ and ROS in nanocomposite.
References
S.M. Hosseinpour-Mashkani, A. Sadeghinia, Z. Zarghami, K. Motevalli, J. Mater. Sci. Mater. Electron. 27, 365 (2016)
M. Mansournia, S. Rafizadeh, S.M. Hosseinpour-Mashkani, J. Mater. Sci. Mater. Electron. 26, 5839 (2015)
S.M. Hosseinpour-Mashkani, M. Maddahfar, A. Sadeghinia, M. Ramezani, J. Mater. Sci. Mater. Electron. 26, 3359 (2015)
J.D.C.O. Sardi, D.R. Silva, M.J.S. Mendes-Giannini, P.L. Rosalen, Microb. Pathog. 125, 116 (2018)
L.A.N. Egue, J.P.K.M. Bouatenin, F.K. Nguessan, M. Koussemon-Camara, Microb. Pathog 124, 5 (2018)
R. López-Martínez, Clin. Dermatol. 28(2), 178 (2010)
D. Kontoyiannis, E. Mantadakis, G. Samonis, J. Hosp. Infect. 53(4), 243 (2003)
D.M. MacCallum, Int. J. Microbiol. 2012, 363764 (2011)
B.P. Mathew, M. Nath, Chem. Med. Chem. 4(3), 310 (2009)
R. Razzaghi, M. Momen-Heravi, M. Erami, M. Nazeri, Curr. Med. Mycol. 2(3), 20 (2016)
G. Li, H. Zhao, J. Hong, K. Quan, Q. Yuan, X. Wang, Colloids Surf. B 160, 220 (2017)
S. Gowri, R. Rajiv Gandhi, M. Sundrarajan, J. Mater. Sci. Technol 30, 782 (2014)
A.S.H. Hameed, C. Karthikeyan, V.S. Kumar, S. Kumaresan, S. Sasikumar, Mater. Sci. Eng. C 52, 171 (2015)
P. Balashanmugam, M.D. Balakumaran, R. Murugan, K. Dhanapal, P.T. Kalaichelvan, Microbiol. Res. 192, 52 (2016)
D. Sharma, J. Rajput, B.S. Kaith, M. Kaur, S. Sharma, Thin Solid Films 519, 1224 (2010)
G. Fiorani, O. Saoncella, P. Kaner, S.A. Altinkaya, A. Figoli, M. Bonchio, M. Carraro, J. Clust. Sci. 25, 83 (2014)
H. Beyzaei, R. Aryan, H. Molashahi, M.M. Zahedi, A. Samzadeh-Kermani, B. Ghasemi, M. Moghaddam-Manesh, J. Iran. Chem. Soc. 14, 1023 (2017)
D. Azarifar, H. Ebrahimiasl, R. Karamian, M. Ahmadi-Khoei, J. Iran. Chem. Soc. 16, 341 (2019)
M.A. Azeez, A. Lateef, T.B. Asafa, T.A. Yekeen, A. Akinboro, I.C. Oladipo, E.B. Gueguim-Kana, L.S. Beukes, J. Clust. Sci. 28, 149 (2017)
P. Karthiga, S. Rajeshkumar, G. Annadurai, J. Clust. Sci. 29, 1233 (2018)
E.E. Elemike, D.C. Onwudiwe, O.E. Fayemi, A.C. Ekennia, E.E. Ebenso, L.R. Tiedt, J. Clust. Sci. 28, 309 (2017)
M. Ghanbari-Mehrabani, R. Karimian, R. Rakhshaei, F. Pakdel, H. Eslami, V. Fakhrzadeh, M. Rahimi, R. Salehi, H. Samadi-Kafil, Int. J. Biol. Macromol. 116, 966 (2018)
A.A. Agale, S.M. Janjal, S.T. Gaikwad, A.S. Rajbhoj, J. Clust. Sci. 28, 477 (2017)
P.M. Anjana, M.R. Bindhu, M. Umadevi, R.B. Rakhi, J. Mater. Sci. Mater. Electron. 29, 6040 (2018)
R. Zhao, M. Lv, Y. Li, M. Sun, W. Kong, L. Wang, S. Song, C. Fan, L. Jia, S. Qiu, Y. Sun, H. Song, R. Hao, ACS Appl. Mater. Interfaces 9(18), 15328 (2017)
E.Z. Gomaa, J. Gen. Appl. Microbiol. 63(1), 36 (2017)
P. Siritongsuk, N. Hongsing, S. Thammawithan, S. Daduang, S. Klaynongsruang, A. Tuanyok, R. Patramanon, PLoS ONE 11(12), e0168098 (2016)
B. Ramalingam, T. Parandhaman, S.K. Das, ACS Appl. Mater. Interfaces 8(7), 4963 (2016)
M.A. Quinteros, V. Cano Aristizábal, P.R. Dalmasso, M.G. Paraje, P.L. Páez, Toxicol In Vitro 36, 216 (2016)
H.L. Su, C.C. Chou, D.J. Hung, S.H. Lin, I.C. Pao, J.H. Lin, F.L. Huang, R.X. Dong, J.J. Lin, Biomaterials 30(30), 5979 (2009)
Y.G. Yuan, Q.L. Peng, S. Gurunathan, Int. J. Mol. Sci. 18(3), 569 (2017)
P. Korshed, L. Li, Z. Liu, T. Wang, PLoS ONE 11(8), e0160078 (2016)
W. Lee, K.J. Kim, D.G. Lee, Biometals 27(6), 1191 (2014)
X. Zhong, Y. Song, P. Yang, Y. Wang, S. Jiang, X. Zhang, Changyi Li. PLoS ONE 11(1), e0146957 (2016)
J.C. Jin, X.J. Wu, J. Xu, B.B. Wang, F.L. Jiang, Y. Liu, Biomater. Sci. 5(2), 247 (2017)
P.C. Lombardo, A.L. Poli, L.F. Castro, J.R. Perussi, C.C. Schmitt, ACS Appl. Mater. Interfaces 8(33), 21640 (2016)
T. Kim, G.B. Braun, Z.G. She, S. Hussain, E. Rouslahti, M.J. Sailor, ACS Appl. Mater. Interfaces 8(44), 30449 (2016)
K. Zawadzka, K. Kądzioła, A. Felczak, N. Wrońska, I. Piwoński, A. Kisielewska, K. Lisowska, New J. Chem. 38(7), 3275 (2014)
U. Klueh, V. Wagner, S. Kelly, A. Johnson, J.D. Bryers, J. Biomed. Mater. Res. 53(6), 621 (2000)
D.W. Hatchett, H.S. White, J. Phys. Chem. 100(23), 9854 (1996)
D.R. Monteiro, L.F. Gorup, A.S. Takamiya, E.R. de Camargo, A.C. Filho, D.B. Barbosa, J. Prosthodont. 21(1), 7 (2012)
A. Javidan, S. Rafizadeh, S.M. Hosseinpour-Mashkani, Mater. Sci. Semicond. Process. 27, 468 (2014)
Z. Kanwal, M. Akram Raza, S. Riaz, S. Manzoor, A. Tayyeb, I. Sajid, S. Naseem, R. Soc. Open Sci. 6(5), 182135 (2019)
Acknowledgements
This study financial supported by research project (98105) of Kashan University of Medical sciences and Health Services.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nazeri, M., Hosseinpour Mashkani, S.M. & Chelongarian, R. Synthesis of YTi@Ag nanocomposite and investigation of its structural and antifungal properties. J IRAN CHEM SOC 17, 103–110 (2020). https://doi.org/10.1007/s13738-019-01756-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01756-6