Abstract
In the present work, functionalized magnetic nano-adsorbent with amine groups (Fe3O4@SiO2@NH2) was prepared for the simultaneous removal of 2,4-Dichlorophenoxyacetic acid (2,4-D) and 2-methyl-4-chlorophenoxyacetic acid (MCPA) from aqueous solution. Characterization such as Fourier transform infrared spectroscopy, vibrating sample magnetometry, and scanning electron microscope confirmed that the magnetic nanoparticles structure of Fe3O4@SiO2 nano-adsorbent was successfully functionalized by amine groups. The impact of some influencing parameters such as contact time, pH, adsorbent dosage, 2,4-D and MCPA initials concentration and solution temperature were studied. The equilibrium data were analyzed by Langmuir and Freundlich adsorption isotherms and also two models kinetically of pseudo-first-order and pseudo-second-order. Findings of the present study showed that the synthesized amino-functionalized MNPs will be helpful in use as an effective recyclable adsorbent for the removal of phenoxy acid herbicides from aqueous solution due to its advantages such as facile and rapid separation of target molecules from solution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is an essential element for life and the lack of drinking water has always been an issue of main environmental concern all over the world. Water pollution because of synthetic organic and inorganic chemical compounds like pesticides is an important problem, which is related to their potential toxic, mutagenic and carcinogenic effects. Increasing use and disposal of pesticides group including herbicides, insecticides and fungicides by farmers lead to the presence of their residues in various environmental compartments [1,2,3]. Nowadays among the numerous pesticides, 2,4-Dichlorophenoxyacetic acid (2,4-D) and 2-methyl-4-chlorophenoxyacetic acid (MCPA), are both herbicides belongs to the chemicals group known as phenoxyacetic acid family, which applied to the post-emergency control of broad-leaved weeds and grasses in agricultural crops [4, 5]. 2,4-D and MCPA are commonly preferred because of their low-cost and good selectivity. Due to their widespread use, high solubility in water, low soil adsorption coefficient and slow biological degradation, phenoxyacetic acid herbicides have been detected as main pollutants in surface and ground waters all over the world [6,7,8]. Both compounds are classified as moderately toxic compounds by the World Health Organization (WHO), and classified, among other chlorophenoxy herbicides, as possible human carcinogens (Group 2B) by the International Agency for Research on Cancer (IARC) [6, 9, 10]. 2, 4-D can damages the liver and kidney and MCPA is harmful to the skin, eyes and the respiratory tracts, in addition, high concentrations ingestion can cause serious health effects such as abdominal pain, hypotension, hepatotoxicity, tachycardia, and kidney dysfunction [11,12,13,14]. Therefore, because of these harmful influence of 2,4-D and MCPA on human health and the environment, WHO have guideline-recommended a limit of 30 and 2 µg L−1 for 2,4-D and MCPA in drinking-water, respectively [15]. The chemical structures and the most important physicochemical properties of the both herbicides listed in Table 1.
A number of different methods have been reported to remove of pesticide from water include advanced oxidation [16, 17], biological treatment [18], activated carbon adsorption [5], and organic polymer resin [4]. Biological degradation methods have been used to pesticides removal and its environmental friendly method but incomplete degradation, time-consuming because of long acclimation period [19] and low temperature decreases the removal efficacy because it has the adverse effect on the growth of microorganisms [20]. Adsorption on activated carbon is a famous method for the removal of herbicides from aqueous solution but high energy demands and consequently, expensive regeneration costs are disadvantages of activated carbons [4]. Hence, it is imperative to develop new alternative sorbents with improved capacity for pesticides removal. Among of the offered adsorbents for removal of toxic pollutants in water treatment, application of magnetic nanoparticles (MNPs) has gained considerable attentions due to having large surface area, easy control, high efficacy and easy separation from solution by external magnetic field [21, 22]. Such facile separation is necessary to improve the operation efficiency. Moreover, reuse of magnetite nanoparticles would significantly reduce the treatment cost. Hydrophilic surface caused by silanol groups like (3-aminopropyl) triethoxysilane (APTES) improved aggregation adsorption on the external surface magnetite nanoparticles [23]. As shown in many literature APTES surface-functionalized magnetic nanoparticles is an important silanol groups in particular found applications in several biological applications including cell and enzyme separation [24], also it used as magnetically controlled drug carriers [25], as well as in water and waste water treatment field [26]. It should be noted a number of researchers have reported that, the amino-functionalized materials have excellent ability to adsorption a wide variety of heavy metal ions (i.e. Co+2, Cu+2, Ni+2, Pb+2 and Zn+2) [27] from aqueous solutions due to the powerful metal complexing capability of amino groups. Only a few describe the simultaneous adsorption of both 2,4-D and MCPA from aqueous solution [4, 10, 28, 29]. Hence, the present study was centered on the surface modification of MNPs using APTES for the adsorption and removal of 2,4-D and MCPA from aqueous solution. To the best of our knowledge, so far this has been the first report on the implementation of APTES for the removal of 2,4-D and MCPA through adsorption process. It should be also highlighted that due to existence of a great number of − NH2 groups located on the surface of the adsorbent, the efficiency of adsorption is significantly increased. Additionally, the magnetic properties of the mentioned adsorbent highly facilitates its recovery from any aqueous matrices.
Materials and method
Chemicals and materials
MCPA and 2,4-D with technical reagent grade were purchased from Sigma–Aldrich. Ferric chloride hexahydrate (FeCl3, 6 H2O), and ferrous chloride tetrahydrate (FeCl2, 4 H2O) were of reagent grade and used without further purification, tetraethylorthosilicate (TEOS), (3-aminopropyl) triethoxysilane (APTES) were purchased from Sigma–Aldrich, Ltd. Co.
Preparation of nano-adsorbent
Preparation of Fe3O4
Fe3O4 MNPs were prepared based on a modified co-precipitation method described previously because it’s a simple and convenient method to synthesize iron oxide (Fe3O4) under an inert atmosphere at room temperature [30, 31]. Precipitation Fe3O4 was done with a stoichiometric ratio of 2:1 (Fe3+/Fe2+). Firstly, 400 mL deionized water was purified with N2 for 20 min and then 11.4 g FeCl3·6H2O with 4.1 g FeCl2·4H2O were dissolved in deionized water under nitrogen atmosphere at 80 °C with vigorous stirring. 30 mL NH3.H2O (25% by w/w,) was added dropwise to the solution and reacted for 24 h by adjusting of pH ≈ 10. The mixture was held at 80 °C in a water bath for 1 h with continuous stirring and then cool down to room temperature. The black precipitate was aggregated by placing an external magnetic field, and the residual solution was removed. The black magnetic Fe3O4 MNPs were rinsed three times with deionized water and ethanol followed by drying at 50 °C under vacuum for 4 h.
Preparation of Fe3O4@SiO2
To prepare of Fe3O4@SiO2, a suspension of the obtained previous magnetic nanoparticles (Fe3O4 1.0 g) were re-dispersed in a mixture of 40 mL ethanol and 10 mL of deionized water using an ultrasonic water bath for 30 min. Afterward, 2 mL ammonia solution (25%), and 0.5 mL TEOS were added, and the mixture mechanically stirred at 50 °C for 6 h. The resulting Fe3O4@SiO2 was collected by an external magnetic separation, and washed with ethanol several times and followed by drying at 50 °C under vacuum for 12 h [9].
Preparation of Fe3O4 @ SiO2–NH2
In this step, 1.0 g of the Fe3O4@SiO2 nanoparticles was diluted with 25 mL dry toluene and methanol (volume ratio, 1:1) were added to a 250 mL three-necked flask and then ultrasonically dispersed for 15 min afterwards, treated with an addition of 2 mL (3-aminopropyl) trimethoxysilane (APTES) to a flask. The mixture was refluxed at 80 °C with constant stirring for 4 h under a N2 flow (40 ml min−1). The product was washed with ethanol several times and then drying at 60 °C under vacuum for 12 to obtain Fe3O4 @SiO2@NH2 [19, 32]. Figure 1 provide a scheme to illustrate the synthesis process of the introduced sorbent for removal of 2,4-D and MCPA.
Analytical procedure
The concentration of 2,4-D and MCPA determined by means of chromatographic technique HPLC (CECIL 4100, USA) equipped with a UV–Visible detector (Adept CECIL 4100 UV–Visible detector). Separation of both herbicides was obtained with a Nucleodur Sphinx RP (25.0 cm × 4.6 mm) column (MZ-1 PerfectSil, Germany) at a flow-rate of 1 ml/min and detection was carried out at wavelengths of 230 nm. The mobile phase compound consist of a solvent A [0.05 M HK2PO4 as buffer solution at pH 3] and solvent B [methanol]. The linear gradient operated from 50:50 A: B to 20:80 A: B in 20 min. Under these conditions, the retention time of MCPA and 2,4-D were 15.4 and 17.24 min.
Adsorbent characterization
FT-IR measurement conducted by the KBr pellets method using a Perkin-Elmer (Germany) spectrometer under dry air at room temperature. The surface morphologies of the Fe3O4@SiO2@NH2 nano-adsorbent were analyzed by Scanning electron microscopy [SEM (KYKY, EM3200)]. The magnetic properties characterization of the Fe3O4@SiO2@NH2 nano-adsorbent was measured using a vibrating sample magnetometer (VSM, MDKFD, Iran).
Herbicide adsorption studies
Batch experiments of 2,4-D and MCPA were performed to determine the adsorption isotherms and adsorption kinetics. The stock solution of 1000 mg L−1 2, 4-D and MCPA were prepared by dissolving an appropriately weighed quantity of the both herbicides in double- distilled water. All the required testing solutions were prepared by diluting in deionized water. For equilibrium experiments, 0.6 g L−1 of the amino-functionalized Fe3O4@SiO2 nano-adsorbent (MNPs-NH2) was contacting with 200 mL of both herbicides solution in 250.0 mL Erlenmeyer flasks placed in a thermostatic shaker agitated at 200 rpm at 25 °C, and theirs residual concentrations were analyzed after 5, 15, 30, 50, 60, 70 and 80 min. The effect of pH on adsorption behaviors of 2,4-D and MCPA by the MNPs-NH2 was studied by varying the solution from 3.0 to 9.0. To the desired pH value, the initial pH of the solution was adjusted with 0.1 M H2SO4 or 0.1 M NaOH solutions. The other effect factors including 2,4-D and MCPA concentrations (10–150 mg L−1), nanoparticles dosages (0.1–1 g L−1) and temperature (25–45 °C) for removal two herbicides were investigated. At pre-determined intervals of time, by amino-functionalized Fe3O4@SiO2 nanomagnetic was separated from the aqueous solution under external magnetic field and solutions were analyzed for the final concentration of herbicides by using HPLC with wavelength at 230 nm. The 2,4-D and MCPA percentage adsorption efficiency (Removal%) was determined using Eq. (1):
where C 0 and C e are the initial amount of 2,4-D and MCPA concentrations (mg L−1), and the equilibrium amount of 2,4-D and MCPA concentration, respectively.
Equilibrium studies
Batch equilibrium studies of 2,4-D and MCPA on the amino-functionalized Fe3O4@SiO2 nanomagnetic were carried by contacting 0.6 g L−1 of the magnetic nanoparticle with 200 mL of varying concentrations of 2,4-D and MCPA from 10 to 150 mg L−1 for 5–80 min on a shaker at 200 rpm. After achieving adsorption equilibrium, the residual concentrations of herbicide determined using HPLC. The amount of 2,4-D and MCPA adsorbed on nanoadsorbent at time t (min), qt (mg mL−1), and equilibrium adsorption amount of the both herbicide; qe (mg g−1) were calculated using Eqs. 2 and 3:
where C 0, C e and C t are initial and final 2,4-D and MCPA herbicide concentrations (mg L−1) in the liquid-phase concentrations and time t, respectively, V is the volume of 2,4-D and MCPA solution (L) and W is the dry weight of the MNPs-NH2 nano-adsorbent (g).
Adsorption isotherms of 2,4-D and MCPA
Two adsorption isotherm models were tested for their ability to describe the experimental adsorption data of the 2,4-D and MCPA on Fe3O4@SiO2@NH2, namely the Langmuir and Freundlich were used to compare the adsorption performances for the purpose of optimizing the design of an adsorption system. The Longmuir isotherm is originally based on assumption of that the adsorption occurs on homogenous monolayer with constant energy, whereas Freundlich isotherm is an empirical assuming that the adsorption process takes place on heterogeneous or multilayer surfaces. The linear forms of Langmuir and Freundlich model are expressed in Eqs. (4) and (5) respectively:
where q m is the maximum amount of the 2,4-D and MCPA per unit mass of adsorbent (mg g−1), k l is Langmuir adsorption constant (L mg−1) and C e is the equilibrium concentration of the 2,4-D and MCPA in the solution (mg L−1). k f (mg g−1)(L mg−1)1/n and n are the constants parameters related to the adsorption capacity of the sorbent and intensity of Freundlich, respectively.
Adsorption kinetics and of 2,4-D and MCPA
Kinetic adsorption process of both herbicides at the initial concentration of 100 mg L−1 onto MNPs-NH2 analysis by two kinetic models, pseudo-first-order and pseudo-second-order models reaction represented by the following Eqs. (6) and (7):
pseudo-first-order model [33]:
And pseudo- second-order model is [34]:
where q t and q e (mg g−1) are the amount of adsorbed 2,4-D and MCPA at equilibrium adsorbent at time t respectively. k 1 (1/min) and k 2 (g (mg min)−1) are rate constants related to the first and second order sorption, respectively.
Results and discussion
Characterization of nano-adsorbents
FT-IR analysis
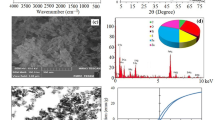
To proof the functionalization of the magnetic nanoparticles with amine groups, FT-IR were performed in the range of 500–4000 cm−1. As can be seen in the Figure 1S [ESM (Electronic supplementary material)], As shown in Fig. 4, Fe3O4@SiO2@NH2 nanoparticles possess two distinct IR-absorption peaks at 570 cm−1 assigned to the vibrations of Fe–O. Furthermore, for, Fe3O4@SiO2@NH2 the peaks at ~ 1120 and 1050 cm−1 represent the vibration of aromatic Si–O–H and Si–O-Si bonding, respectively. The peaks at ~ 1420 and 3400 cm−1 are also assigned to the N–H stretching. As can be concluded, the FT-IR spectroscopy analysis verified the formation of the amino group on silica-coated Fe3O4 NPs.
SEM and VSM analysis
SEM images of Fe3O4 and Fe3O4@SiO2@NH2 nanoparticles are shown in Fig. 2a, b respectively. Figure 2b clearly illustrates the distribution of amino-functionalized silica over the surface of the magnetite nanoparticles. VSM magnetization curve of Fe3O4 and Fe3O4@SiO2@NH2 nanoparticles at room temperature is depicted in Fig. 3. The saturated magnetization values of Fe3O4 and Fe3O4@SiO2@NH2 nanoparticles were determined to be 58.9 and 31.2 emu/g, respectively. These results also indicate that the Fe3O4@SiO2@NH2 nanoparticles show an excellent magnetic response to a magnetic field. Therefore, it could be separated easily and rapidly due to this high magnetic sensitivity. This value is smaller than the magnetization value of amino-functionalized Fe3O4@SiO2@NH2 (45.93 emu/g) reported by Safari et al. [35].
Effect of initial 2,4-D and MCPA concentration and contact time on pesticides adsorption
The herbicide initial concentration plays a key role in domination mass transfer resistance of the adsorbate between aqueous solution and solid phase [36]. Control experiments were conducted to determine the effects of varying initial concentration of 2,4-D and MCPA from 10 to 150 mg L−1 with 0.6 g L−1 of nanoscale MNPs-NH2 in the optimized contact time (60 min) and results shows in Fig. 4. To ensure that full equilibrium was achieved, the experimental data were measured at 60 min. As seen in Fig. 4, a higher initial concentration of 2,4-D and MCPA leads to decrease in the adsorption efficiency of both the herbicides on Fe3O4@SiO2@NH2. The lower adsorption at higher concentration resulted from an increased ratio of initial number of moles of 2,4-D and MCPA to the available surface area; hence fractional adsorption becomes dependent on initial concentration. For a given adsorbent dose the total number of available activated sites is fixed thereby adsorbing nearly the same amount of both herbicide, therefore, resulting in a decrease in the percentage removal of the 2,4-D and MCPA corresponding to an increase in initial adsorbate concentration. Similar results were also reported for the adsorption of phosphate adsorption on iron hydroxide-eggshell from aqueous solution [37].
The pesticides removal percentage onto magnetic nanoparticles modified with amine groups at various contact times (5–80 min) at 100 mg L−1 was investigated to evaluate the required equilibrium time. Short equilibrium time indicates high tendency of 2,4-D and MCPA for rapid migration and diffusion to the Fe3O4@SiO2@NH2 surface. It was found that the adsorption rate is rapid at the initial stages and the system reaches equilibrium at about 60 min. The rapid adsorption at the initial contact time can be attributed to the availability of the reactive site of adsorbent.
Effect of adsorbent dosage on pesticides adsorption
The effects of different Fe3O4@SiO2@NH2 dosage on the removal of 2,4-D and MCPA were investigated. The adsorption percentage of MCPA and 2,4-D increases from 31.5–95 to 30.2–92% respectively after 60 min adsorption (optimum contact time), as initial Fe3O4@SiO2 nano-adsorbent (MNPs-NH2) concentrations increased from 0.1 to 1 g L−1. The phenomenon may be attributed to that the increased surface area and the more active sites with increasing Fe3O4@SiO2@NH2 dosage, which reaction occurs and consequently increases the rate of reaction [38]. However, adsorption rate of the both herbicide almost increased with the increasing of the dosage of MNPs-NH2 nanoparticles but with continuous increase of the dosage of Fe3O4@SiO2 nano-adsorbent (MNPs-NH2) over than 0.6 g L−1, the increasing of the adsorption rate of 2,4-D and MCPA trend constant gradually.
Effect of solution pH on 2,4-D and MCPA adsorption
The pH of the solution can effect on the 2,4-D and MCPA adsorption over the Fe3O4@SiO2@NH2 adsorbent. As shows in Fig. 5, herbicides adsorption were strongly pH dependent, because it can determines the degree of ionization, the surface charge of the adsorbent and the molecular characteristics of herbicides. The effect of pH value on the adsorption properties was studied by mixing 200 mL of both herbicides (100 mg L−1) with 0.6 g L−1 amino-functionalized MNPs by varying pH ranging from 3.0 to 9 for 60 min.
It can be seen in the Fig. 5 adsorption increase with increasing of pH from 3 to 7. After pH of 7, the adsorption was quantitative until pH of 8 and further increase of pH after pH of 8 causes a decrease in the adsorption efficiency. At alkaline solution (pH ≥ 8), the herbicide exist in the ionized form (negatively charged) which has a negative impact on the adsorption rate, on the other hand in the pH range of more than 7, the surface charge of Fe3O4@SiO2–NH2 was nearly zero or negative, hence the acidic herbicides were rejected from negatively charged of nano adsorbents by charge repulsion or diffusion, which does not benefit for adsorption. According to the obtained data for adsorption efficiency of target molecules, pH of 7.0 was chosen as the optimum pH for subsequent experiments [39]. Contaminant with negatively charged such as 2,4-D and MCPA, were more readily absorbed on positively charged the Fe3O4@SiO2@NH2 by electrostatic interaction. The influence of solution initial pH was ascribed to the electrostatic interaction between the MNPs-NH2 surfaces with the 2,4-D and MCPA molecules. [35]. In addition to the electrostatic interaction between positively-charged 3-aminopropyl triethoxysilane via: ≡Si-R-NH3 +-----−OOC-R [23], other force that influence on adsorption are hydrogen bonding and van der Waals interaction between the Fe3O4@SiO2@NH2 surfaces with the 2,4-D and MCPA, also combination of these force could enhanced adsorption capacity of Fe3O4@SiO2@NH2 [40].
Effect of temperature on 2,4-D and MCPA adsorption
The equilibrium uptake of 2,4-D and MCPA by Fe3O4@SiO2@NH2 was also affected by temperature and increased with increasing temperature between 25 and 45 °C (Fig. 6). At the optimized pH (pH of 7.0) for 100 mg L−1 initial concentration of 2,4-D and MCPA, the adsorption efficiency of target molecules was increased with increasing temperature from 25 to 45 °C. Figure 6 shows the amount of 2,4-D and MCPA adsorbed versus contact time. It is famous that solubility of the organic compound could effective on adsorption; i.e. less solubility is due to the higher adsorption probability [41].
Adsorption kinetics
Kinetic studies were conducted and the experimental results are summarized in Table 2. As mentioned two different models kinetic including pseudo-first-order and pseudo-second-order were applied to simulate the investigate kinetic data of 2,4-D and MCPA on Fe3O4@SiO2@NH2 adsorbent. The results are presented in Table 2. The pseudo- second -order model gave best fitting with high correlation coefficient (R 2 ˃ 0.99) values and the experiment qe (exp) (158 mg g−1 for 2,4-D and 157 mg g−1 for MCPA) and calculated qe (cal) (155.1 mg g−1 for 2,4-D and 153.5 mg g−1 for MCPA) were close together which shows good also very well agreement. Therefore, from the achieved result we conclude that the pseudo-second-order model is more suitable for adsorption process of 2,4-D and MCPA on Fe3O4@SiO2@NH2. _ENREF_64.
Adsorption isotherms
The adsorption isotherms of both herbicides on MNPs-NH2 nanoparticles were studied and all the model parameters and the correlation coefficient (R 2) obtained from the two isotherm models are also given in Table 3. The maximum adsorption amounts were found to be approximately 166.6 and 153.8 for 2,4-D and MCPA respectively. To further elucidate of the phenoxy acid herbicides adsorption mechanism, batch tests were conducted to study Freundlich and Langmuir isotherms models adsorption. It’s clear from Table 3 that the R 2 value 0.65 for MCPA and 0.72 for 2,4-D of the Freundlich model is lower than Langmuir isotherm that used in this study. So according to these results it can be concluded that the Freundlich isotherm model is not suitable to describe the equilibrium adsorption of 2,4-D and MCPA on MNPs-NH2 nanoparticles. Compared with the Freundlich model, Langmuir model give better fit with higher R 2 values > 0.99 for 2,4-D and MCPA. Therefore; the adsorption process of both herbicides follows better from Langmuir model. This result suggests that the 2,4-D and MCPA adsorption on MNPs-NH2.nanoparticles was monolayer. Similar results have been reported by Nejati et al. [39, 42] in 2,4-D and MCPA removal from aqueous solution and Wang et al. to tannic acid adsorption on an Fe3O4@SiO2–NH nano-adsorbent [39, 41, 42]. The n parameter values in Freundlich formula show the type of adsorption to be favorable (range of 2–10), moderately difficult (in the range of 1–2) or poor adsorption (n < 1). The main properties of the Langmuir isotherm can be describe in view of dimensionless separation factor R L:(R L = 1/(1 + k L C 0)). The factor of R L demonstrates the kind of the isotherm to be irreversible if R L = 0, favorable (0 < R L > 1), linear adsorption (R L = 1) and if R L > 1 the process is unfavorable. The value of R L for the present system comes out to be 0.02 and 0.009 for 2,4-D and MCPA, respectively, communicating the favorable adsorption of the target molecules onto Fe3O4@SiO2@NH2.
Comparison with other adsorbents
To point out of the advantages of amino-functionalized MNPs-SiO2 as a nano-adsorbent for removal of 2,4-D and MCPA we compared obtained results in this work with previously published articles (Table 4). It is clear from this table that amino-functionalized magnetic nano-adsorbent is an efficient and environmentally friendly nano-adsorbent which could be useful in the removal of pollutant in water and wastewater treatment. As demonstrated in Table 4, the present the Fe3O4@SiO2@NH2 by had a higher maximum removal efficiency comparing to other treatment process in previous studies.
Conclusion
Amino-functionalized magnetic nanoparticle was prepared for the adsorption of 2,4-D and MCPA from aqueous solution. The characterization of the sorbent by FTIR, VSM and SEM confirmed that the successfully synthesis of desired functionalized magnetic nanoparticles. The adsorption capacity and removal time of the target pesticides by the sorbent at optimized condition were appropriate. Adsorption kinetics of 2,4-D and MCPA can be well described by pseudo-second-order kinetics. Furthermore, the adsorption isotherm was very well agreement by Langmuir model. Results of this study will be helpful in use of the amine-functionalized magnetic nanoparticles as a high potential adsorbent for 2,4-D and MCPA removal in aqueous solution.
References
M. Behbahani, F. Najafi, S. Bagheri, M.K. Bojdi, P.G. Hassanlou, A. Bagheri, Environ. Monit. Assess. 186, 2609 (2014)
F. Omidi, M. Behbahani, H.S. Abandansari, A. Sedighi, S.J. Shahtaheri, J. Environ. Health Sci. Eng. 12, 1 (2014)
F. Omidi, M. Behbahani, S. Samadi, A. Sedighi, S.J. Shahtaheri, Iranian. J. Pub. Health 43, 645 (2014)
I. Vergili, H. Barlas, Desalination 249, 1107 (2009)
I. Cansado, P. Mourão, J. Gomes, V. Almodôvar, Ciência Tecnol. Mater. 29, 224 (2017)
S.-P. Tong, R. Shi, H. Zhang, C.-A. Ma, J. Hazard. Mater. 185, 162 (2011)
M. Dehghani, S. Naseri, M. Karamimanesh, J. Environ. Health. Sci. Eng. 12, 87 (2014)
S. Mendiola-Alvarez, J. Guzmán-Mar, G. Turnes-Palomino, F. Maya-Alejandro, A. Hernández-Ramírez, L. Hinojosa-Reyes, Environ. Sci. Pollut. Res. 24, 12673 (2017)
D. Loomis, K. Guyton, Y. Grosse, F. El Ghissasi, V. Bouvard, L. Benbrahim-Tallaa, N. Guha, H. Mattock, K. Straif, L. IARC. Lancet Oncol. 16, 891 (2015)
A. Derylo-Marczewska, M. Blachnio, A. Marczewski, A. Swiatkowski, B. Tarasiuk, J. Therm. Anal. Calorim. 101, 785 (2010)
N. Gao, K. Cai, X. Guo, Y. Zhang, S. Yang, D. Hu, Int. J. Environ. Anal. Chem. 94, 594 (2014)
D.M. Roberts, R. Seneviratne, F. Mohammed, R. Patel, L. Senarathna, A. Hittarage, N.A. Buckley, A.H. Dawson, M. Eddleston, Ann. Emerg. Med. 46, 275 (2005)
M. Shankar, S. Anandan, N. Venkatachalam, B. Arabindoo, V. Murugesan, Chemosphere 63, 1014 (2006)
Y. Tang, G. Zhang, C. Liu, S. Luo, X. Xu, L. Chen, B. Wang, J. Hazard. Mater. 252, 115 (2013)
Guidelines for drinking-water quality, recommendations, 3rd edn. (World Health Organization, Genova, 2004), p. 191
M.J. Farré, M.I. Maldonado, W. Gernjak, I. Oller, S. Malato, X. Domènech, J. Peral, Chemosphere 72, 622 (2008)
D. Pęziak-Kowalska, F. Fourcade, M. Niemczak, A. Amrane, Ł. Chrzanowski, G. Lota, Environ. Technol. 38, 1093 (2017)
A.J. González, M.S. Fortunato, A. Gallego, S.E. Korol, Water Air Soil Pollut. 228, 300 (2017)
S. Mangat, P. Elefsiniotis, Water Res. 33, 861 (1999)
M. Cycoń, A. Żmijowska, Z. Piotrowska-Seget, Open. Life Sci. 6, 188 (2011)
M. Bhaumik, A. Maity, V. Srinivasu, M.S. Onyango, J. Hazard. Mater. 190, 381 (2011)
L. Li, M. Fan, R.C. Brown, J. Van Leeuwen, J. Wang, W. Wang, Y. Song, P. Zhang, Crit. Rev. Environ. Sci. Technol. 36, 405 (2006)
L.N. Robinson, Water resources research progress (Nova Science Publisher Inc., New York, 2008)
M.J. Sweetman, C.J. Shearer, J.G. Shapter, N.H. Voelcker, Langmuir 27, 9497 (2011)
L.H. Reddy, J.L. Arias, J. Nicolas, P. Couvreur, Chem. Rev. 112, 5818 (2012)
Y.-G. Zhao, H.-Y. Shen, S.-D. Pan, M.-Q. Hu, Q.-H. Xia, J. Mater. Sci. 45, 5291 (2010)
M. Kumar, D. Rathore, A.K. Singh, Talanta 51, 1187 (2000)
W. Kaminski, K. Kusmierek, A. Swiatkowski, Adsorption 20, 899 (2014)
K. Kuśmierek, M. Sankowska, A. Świątkowski, Desalt. Water Treat. 52, 178 (2014)
A.H. Lu, E.E.L. Salabas, F. Schüth, Angew. Chem. Int. Edit. 46, 1222 (2007)
M. Mikhaylova, D.K. Kim, C.C. Berry, A. Zagorodni, M. Toprak, A.S. Curtis, M. Muhammed, Chem. Mater. 16, 2344 (2004)
Y. Liu, Y. Li, X.-M. Li, T. He, Langmuir 29, 15275 (2013)
V. Vimonses, S. Lei, B. Jin, C.W. Chow, C. Saint, Chem. Eng. J. 148, 354 (2009)
Y.-S. Ho, G. McKay, Process Biochem. 34, 451 (1999)
J. Wang, S. Zheng, Y. Shao, J. Liu, Z. Xu, D. Zhu, J. Colloid Interface Sci. 349, 293 (2010)
M. Anbia, A. Ghaffari, Appl. Surf. Sci. 255, 9487 (2009)
N.Y. Mezenner, A. Bensmaili, Chem. Eng. J. 147, 87 (2009)
L. Ding, X. Lu, H. Deng, X. Zhang, Ind. Eng. Chem. Res. 51, 11226 (2012)
K. Nejati, S. Davari, Z. Rezvani, M. Dadashzadeh, J. Chin. Chem. Soc. 62, 371 (2015)
M. Ghambarian, M. Behbahani, A. Esrafili, H.R. Sobhi, J. Sep. Sci. 40, 3479 (2017)
J. Wang, C. Zheng, S. Ding, H. Ma, Y. Ji, Desalination 273, 285 (2011)
K. Nejati, S. Davary, M. Saati, Appl. Surf. Sci. 280, 67 (2013)
S. Sanchis, A. M. Polo, M. Tobajas, J. J. Rodriguez, A. F. Mohedano, Chemosphere 93, 115 (2013)
G.J. Zhou, G.-G. Ying, S. Liu, L.J. Zhou, Z.F. Chen, F.Q. Peng, Environ. Sci. Process. Impacts 16, 2018 (2014)
T.Y. Kim, S.S. Park, S.J. Kim, S.Y. Cho, Adsorption, 14, 611 (2008)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohammadi, F., Esrafili, A., Kermani, M. et al. Application of modified magnetic nanoparticles with amine groups as an efficient solid sorbent for simultaneous removal of 2,4-Dichlorophenoxyacetic acid and 2-methyl-4-chlorophenoxyacetic acid from aqueous solution: optimization and modeling. J IRAN CHEM SOC 15, 421–429 (2018). https://doi.org/10.1007/s13738-017-1243-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-017-1243-5