Abstract
The removal of Cr(VI) ions from aqueous solution by human hair waste is investigated by using UV–Vis spectrophotometer technique. The morphological analysis of the human hair was also investigated by the scanning electron microscopy, Fourier transforms infrared spectroscopy and X-ray photoelectron spectroscopy. The influence of various physicochemical effective parameters such as pH, ionic strength, adsorbent amount, contact time, initial concentration of metal ion on removal of Cr(VI) ions by human hair process was also studied. The optimum conditions for this adsorption process were obtained at pH = 2 and contact time of 150 min while the highest Cr(VI) uptake is recorded for 0.5 g of the adsorbent per 100 ml of solution. Three isotherms models including Langmuir, Freundlich and Temkin were applied to describe the equilibrium data. It was found that the experimental data were well described by Freundlich isothermal model. The maximum adsorption capacity was found to be 11.64 mg g−1.The thermodynamic study data showed that the adsorption process of Cr(VI) on human hair is an endothermic, spontaneous and physisorption reaction. The kinetics of the adsorption process was studied using three kinetics models including Lagergren-first-order, pseudo-second-order and Elovich model. The obtained data are indicated that the adsorption processes of Cr(VI) over human hair could be described by the pseudo-second-order kinetic model.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water pollution is a serious environmental and public problem due to the toxic metals and organic compounds that are being disposed in groundwater. Therefore, we are faced with stringent regulations that have made it a major source of concern and a priority for most industrial sectors. Heavy metal ions, aromatic compounds (phenolic derivatives, and polycyclic aromatic compounds) and dyes are often found in the environment as a result of their wide industrial applications. They are common contaminants in wastewater, and many of them are known to be toxic or carcinogenic [1, 2]. For example, chromium(VI) has been shown to be toxic for bacteria, plants, animals and humans [3]. Chromium compounds are widely used by many modern industries such as leather tanning, electroplating, metal finishing and pigment production. Thus, a large amount of these compounds are being discharged into effluent industrial wastewaters [4,5,6,7]. For instance, 40% of the remaining chromium salts are generally discharged in the final effluents during the chrome tanning process [8, 9]. Undeniably, wastewater or drinking water containing high concentration of Cr(VI) causes severe environmental problems as well as prompt toxic and carcinogenic health implication for humans and animals [10]. Therefore, the removal of Cr(VI) from waters is an area of great interest for the scientific community. Chromium exists in the aqueous solution as both trivalent and hexavalent [Cr(VI)] ions states. Hexavalent chromium ion Cr(VI) is mainly in the form of chromate (CrO4 −) and dichromate (Cr2O7 2−) ions. Hexavalent chromium has considerably higher levels of toxicity than the other valence states [3].
Various technologies including ion exchange, filtration, chelation, osmosis, electrochemical operation, reduction and solvent extraction have been employed to remove Cr(VI) ions from drinking water and wastewater. However, these methods suffer from some disadvantages such as large volumes of reagents or energy expenditure, inadequate metal removal and production of toxic secondary waste. Therefore, the adsorption process, known as a highly efficient and low-cost method, has been recently applied for the treatment of wastewater containing heavy metals [11, 12]. Currently, the wastes natural bio-materials which are obtainable in large amounts in the environment are considered as low-cost adsorbents or bio-sorbent for removing heavy metals and other pollutants. Recently low-cost adsorbents such as pomace, olive oil industry waste [13], almond shells, cactus leaves, coal, wool, pine needles sawdust [14], active sludge [15], agricultural waste [16] and green coconut shell [17] have been applied to eliminate Cr(VI) from aqueous solutions. Interestingly, among the low-cost adsorbents, human hair can be utilized as a candidate since it is inexpensive, and easy to collect. In big cities, human hair often accumulates in large amounts in the solid waste currents and chokes the drainage systems, posing a multifaceted problem [18]. The best way to reduce such problems is by using human hair as a bio-sorbent for the removal of heavy metals such as Cr(VI) and also reduce housekeeping problem.
Hair fiber consists of the cuticle, cortex and in some cases medulla which is mainly filled with about 65–95% keratin protein. Proteins are composed of long chains of different mixtures of the amino acids. Among various amino acids in human hair, cystine is one of the most important amino acid. Each of cystine unit consists of two cysteines with various chains adjacent to each other and connected by two sulfur atoms, forming a very strong bond known as a disulfide crosslink [19]. It is worth mentioning that hair is rich in peptide bonds and many –CO and –NH groups that make hydrogen bonds between neighboring chains [20]. Keratin protein contains C, O, N and S in proportion to the amino acid present. The hair surface is a mixture of all the possible organic functional groups present in the amino acids, such as sulfonate, carboxylate, amine, hydroxyl, hydrocarbon, disulfide [21]. The accumulation of negative charge on the hair surface results in the adsorption of positive ions in natural pH.
Efremenko and co-workers studied the uptake of macromolecules such as proteins on human hair [22]. Ekop et al. [23] investigated the adsorption of Zn(II) and Pb(II) ions from aqueous solutions onto hair, and thermodynamic variables such as ∆H 0, ∆S 0 and ∆G 0 were obtained from experimental data. Mahdavian [24] demonstrated that the removal of heavy metals by human hair, wool and goat hair could be effected by a magnetic field, pH and contact time.
This study investigated the adsorption process of Cr(VI)on human hair. The influence of different factors such as pH, contact time and temperature on adsorption capacity was also studied, and the thermodynamic variables (∆H 0, ∆S 0 and ∆G 0) were obtained from experimental data. Furthermore, the capability of human hair as an adsorbent for the removal of chromium(VI) ion in aqueous solution was investigated as well as the three isotherm models equations (Langmuir, Freundlich and Temkin). The maximum adsorption capacity of adsorbent, the intensity of the adsorbate on adsorbent surface and the potential possibility of the adsorbent were evaluated by the Langmuir, Freundlich and Temkin isotherms equations.
Materials and methods
Adsorbent preparation
Human hair samples were collected from different barber salons and were washed thoroughly with detergent at 60°C and then mixed together. The samples were rewashed with distilled water to neutralize the pH and then re-dried in an oven. Finally, the dried human hairs were cut into small pieces, grounded to a size of 1–2 mm and used in the adsorption experiments.
Materials and equipment
K2Cr2O7, 1,5-diphenylcarbazide, H2SO4, NaOH and HNO3 were purchased from Merck (Germany). The pH was adjusted using H2SO4 and NaOH with a digital pH-meter (Metrohm, Mode:l632). The absorbance measurement was taken by UV–Visible spectrophotometer (Cintra 101 GBC Scientific Equipment Ltd.). FT-IR spectra were recorded on a Fourier transforms infrared spectroscopy (FT-IR) (VERTEX70 Brucker) using a KBr disk. Scanning electronic microscope (SEM) (LEO1455VP) was used to study of the morphology of the surface of human hair.
Analysis of chromium(VI) ions
The concentration of the chromium(VI) ions was monitored by applying 1,5-diphenylcarbazide as the complexing reagent. Briefly, 0.025 g of 1,5- diphenylcarbazide powdered was dissolved in 10 ml of acetone. Then, 0.1 mL of this prepared reagent and one drop of concentrated nitric acid were added to 10 mL of the solution which was comprised of less than 2 mg L−1 of Cr(VI) ions. The absorbance of the purple colored complex solution for each sample was read at λ = 540 nm of a spectrophotometer [25], and consequently, the concentration was calculated based on Beer–Lambert rule.
Adsorption experiments
The stock standard solution (1000 ppm) of Cr(VI) was prepared by dissolving 2.828 g K2Cr2O7 in 1 L deionized water. The required concentration solution for the experiments was then prepared by diluting the stock standard solution. In order to investigate the effect of physicochemical factors such as pH, ionic strength, the amount of adsorbent, contact time and initial chromium ion concentration, the batch equilibrium method was used for the adsorption experiments. Therefore, the experiments were carried out in conical flask by adding 0.5 g of adsorbent (except for study the adsorbent dosage) to 100 mL of Cr(VI) solution with 50 mg L−1 concentration (except for study the initial Cr(VI) concentration). The initial pH of the solutions was adjusted by solution of NaOH and H2SO4 with concentration in range of 0.1–1 M. The mixture solution was then shaken for 180 min in the incubator with a constant agitation speed of 150 rpm at 25°C. All adsorption experiments were performed three times, and the mean and standard deviation values were used for data analysis. Then, the mixture was centrifuged and the initial and final metal ion concentration was determined by using an UV–Visible spectrophotometer. The removal of metal ions was then calculated by the following Eq. 1.
where C 0 (mg L−1) and C e (mg L−1) are the initial and equilibrium concentration of Cr(VI) ions, respectively. The amount of the adsorbed of chromium ions at equilibrium q e (mg g−1) was then obtained by Eq. 2 as below:
where V (L) is the volume of solution metal ion, and W (g) is the weight of the adsorbent.
Results and discussion
The characterization of human hair as an adsorbent was studied by using SEM, FT-IR and XPS techniques. The investigation of optimum experimental conditions for the adsorption process was systematically performed by studying the effect of solution pH, ionic strength, the amount of adsorbent (human hair), contact time and initial Cr(VI) ion concentration. The influence of the solution temperature was also investigated to clarify the nature of the adsorption process and thermodynamic parameters such as ∆H 0.
Characterization of the adsorbent
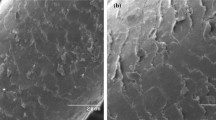
The surface contexture of human hair was characterized by using scanning electron microscopy (SEM), Fourier transforms infrared spectroscopy (FT-IR) techniques and X-ray photoelectron spectroscopy (XPS). The micrograph SEM images with magnifications 2000 times of human hair surface before and after the removal of Cr(VI) from the aqueous solution are illustrated in Fig. 1. As can be seen, the micrograph image of hair is smooth before the removal of Cr(VI) (see Fig. 1a). However, the SEM image of surface of hair indicated that the removal of Cr(VI) caused a craggy structure that could be attributed to the possibility of the attachment of Cr(VI) metals ions to the human hair (Fig. 1b).
The FT-IR spectrum of human hair was recorded in the region of 500–4000 cm−1 and is shown in Fig. 2. This FT-IR spectrum of human hair verified the possible contribution of the presence of the functional groups in human hair. The hydrocarbon, hydroxyl, carboxyl and amino groups were taken into account due to their presence in human hair. The hydroxyl stretching vibration appeared as a band in the region of 3730 cm−1, and N–H asymmetric and symmetric stretching vibration was observed in the region of 3601 cm−1. This could indicate the presence of O and N atoms in the texture of human hair that could be used as adsorbent agents for heavy metals. The peak at 1701 cm−1 could be assigned to C=O stretching vibration [8]. The peak at 1524 cm−1 may be due to N–H bending vibrations related to amide groups in the peptide bond. The C–N and C–H stretching vibration was demonstrated at 1058 and 2353 cm−1, respectively. The –OH, –NH and carboxyl groups played a major role in Cr(VI) adsorption on human hair [13].
Figure 3a shows a typical XPS spectra of the human hair surface [26]. The XPS survey spectra detected the presence of O, N, C and S atoms on the texture of human hair. The average composition of the hair surface was found to be 73.5% C atom, 17.2% O atom, 1.2% N atom and 2.0% S atom. The observed nitrogen existed in a single chemical state(~400 eV), due to the peptide bond between amino acids.
XPS spectra of survey (a) and S2p multiplex (b) spectra of hair surface [26]
It should be noted that in XPS multiplex spectra (Fig. 3a), the 2 s (~230 eV) and 2p (~164 eV) photoelectron transitions from S were present. Each of these S photoelectron transitions contained two distinguished peaks due to the disulfide (–S–S–) links of the cysteine amino acid and sulfonate (–SO _3 ) ion. The sulfur 2p(II) orbital’s peak was observed at the binding energy of 164 eV and could be related to the cystine in the hair(Fig. 3b).As mentioned in the literature upon aging of the hair, some of the disulfide crosslinks of cystine unit are oxidized, resulting in the formation of sulfonate ions at the surface which plays an important role in interactions during aqueous surface [21].
Mechanism of Cr(VI) adsorption
The acceptable reason for the adsorption of Cr(VI) on human hair may include two aspects:
First, as the structure of the outermost surface of the human hair is shown in Fig. 4, the existence of the oxygen and nitrogen atom on the hair surface was proven via the XPS analysis. The adsorption abilities of Cr(VI) on human hair surface were demonstrated based on the structure of the Cr(VI) outermost d orbital electrons. A lone pair of electrons of nitrogen and oxygen atoms directly coordinated with the unoccupied 3d orbital of Cr(VI) corresponding to \( [\text{Ar}]3\text{d}^{0} \) [8]. Secondly, chromium species with negative charged ions such as HCrO4 − and CrO4 2− via electrostatic force were easily attracted to the functional groups on the hair surface such as carboxyl and amide which protonated at low a pH. In contrast, deporotonation of functional groups occurred at high pH and repulsion between negative charge could be a reason for the decrease in adsorption of Cr(VI) [27].
Schematic of the structural of outermost human hair surface [26]
Effect of pH
To investigate the effect of pH on the adsorption of Cr(VI) on human hair, removal experiments were carried out at a pH levels ranging from 1 to 6. Figure 5 demonstrates the removal of Cr(VI) initially increased with an increase in the pH of the solution and then lead to a decrease. The maximum value of the removal of Cr(VI) was achieved at a pH = 2. The adsorption of Cr(VI) on human hair was found to be significantly affected by the pH of solution. Such pH dependency may be due to the surface charge of hair which is strongly connected to the pH solution and the Cr(VI) as an adsorbate. The obtained results from the FT-IR analysis also indicated that human hair contains various functional groups such as hydroxyl, amino and carbonyl which could be influenced by the pH of the solution. The isoelectric point for human hair is around 3.7 [21]. Therefore, these polar functional groups were protonated at low pH and consequently, the surface of the human hair became positively charged.
There are three forms of Cr(VI) ions in the solution including: HCrO4 −, CrO4 2− and Cr2O7 2−. At low pH, the form of HCrO4 − is dominated; therefore, the active form of Cr(VI) which will adsorbed by human hair is HCrO4 − while the surface of the adsorbent is charged positively. By increasing the pH of the solution, the concentration of HCrO4 − shifts to the other forms, CrO4 2− and Cr2O7 2−. This may be due to the fact that Cr(VI) exists as an oxyanion (CrO4 2−, Cr2O7 2−, HCrO4 −) in aqueous solution in which HCrO4 − and Cr2O7 2− predominate at pH less than 6 [14]. The surface of the adsorbent is saturated with protons at low pH values. Thereby creating a positively charged surface increases the adsorption of the negatively charged Cr oxyanions onto the surface of the adsorbent. However, at high pH values the surface of the hair is saturated with abundant negative charge.
The pH for all samples was also measured after performing the adsorption process. However, as the main mechanism of adsorption is an electrostatic bind between human hair and Cr(VI) ions, no obvious change in pH was observed.
Effect of ionic strength
In order to study the influence of ionic strength on the removal of Cr(VI) ions by human hair, different solutions of 50 mg L−1 Cr(VI) at pH = 2, containing various concentration of NaCl (0.05–0.3 M) were used as feed. As indicated in Fig. 6, the result showed that NaCl ionic strength lower than 0.3 M did not significantly affect the removal of Cr(VI) in aqueous solution which is in consistent with previous reports [28]. Therefore, the ionic strength effect was ignored in future experiments.
Effect of adsorbent dosage
In order to identify the optimal amount of human hair require for the removal of Cr(VI), the effect of adsorbent dosage on the removal of Cr(VI) in an aqueous solution was investigated. Figure 7 shows the removal percentage versus the amount of human hair used. According to this graph, for the same initial Cr(VI) concentration(C 0 = 50 mg L−1),the removal percentage of chromium ions increases from 48.30 to 84.67% by increasing the human hair dosage from 0.2 to 1.6 g. Any further increases in the adsorbent dosage did not cause improvement in the percentage of removal. This may be due to the fact that more adsorption sites become available at higher amount of adsorbent which result in an increase in surface area and therefore higher removal of Cr(VI). The increase in removal percentage continues until all the adsorption sites on the hair are saturated with Cr(VI) ions. Thus, the optimum hair dosage was selected as 0.5 g for further experiments.
Effect of contact time
The effect of contact time on the removal of Cr(VI) on human hair was also investigated. For this purpose, the removal of Cr(VI) was studied at different initial Cr(VI) concentrations of 50, 70 and 100 ppm for 15–270 min which are plotted in Fig. 8. The graph displays that the increment of the adsorption capacity q t (mg g−1) of Cr(VI) ions by increasing the contact time. The maximum rate of removal was observed within 150 min after the start point. Thereafter, the removal rate reduced and the removal of Cr(VI) remains almost constant at a contact time of 270 min. This demonstrated that the nature of the adsorbent and accessibility of adsorption sites are affected by the time required to reach the equilibrium point.
Adsorption kinetics
In order to investigate potential rate controlling stages involved in the adsorption of Cr(VI) ions on human hair such as chemical reaction processes and mass transport, the kinetic models were applied to test the obtained experimental data. For this purpose, the well-known kinetic models of Lagergren-first-order, pseudo-second-order, Elovich and the intraparticle diffusion model were applied to the experimental removal data.
The Lagergren-first-order rate model was the first model used for describing the adsorption of liquid–solid systems based on the adsorbent capacity [29]. The nonlinear form of Lagergren-first-order kinetic model is stated by the following equation:
where q e and q t (mg g−1) are the adsorption capacity at equilibrium and at the time of t, respectively. k 1 is the rate constant of pseudo-first-order. The values of the k 1 and q e can be found from the slope and intercept of the plot of \( \ln \left( {q_{\text{e}} - q_{\text{t}} } \right) \) versus t for various concentrations of Cr(VI) ion.
The adsorption of removal of Cr(VI) by human hair could also be described by applying the pseudo-second-order kinetic model, in which the nonlinear form of this model is given in Eq. 4 [30]:
where k 2 (g mg−1 min−1) is the rate constant of pseudo-second-order. The k 2 and q e values are calculated from the slope and intercept of the plot of t/q t verses t, respectively.
The Elovich kinetics model equation is defined by Eq. 5.
where \( \alpha \;\left( {{\text{mg}}\;{\text{g}}^{ - 1} \;{ \hbox{min} }^{ - 1} } \right) \) is the initial adsorption rate, and \( \beta \;\left( {{\text{g}}\;{\text{mg}}^{ - 1} } \right) \) represents the surface coverage area and activation energy of chemisorption [31]. By rearranging Eq. 5 for the boundary condition, the nonlinear form of Elovich equation as below:
In which \( \alpha \) and \( \beta \) could calculated from the slope and intercept of the plot of q t verses ln t, respectively.
The kinetic models parameters were calculated and are listed in Table 1, and Fig. 9 shows the nonlinear form all various kinetics models. The comparison between nonlinear model form (Fig. 9a–c) of all the various models validate that kinetic of the removal of Cr(VI) process is described by the pseudo-second-order kinetic model better than the others .
In order to investigate the mechanism of the process of adsorption of Cr(VI) with human hair, the intraparticle diffusion model which was expanded by Webber and Morris [32] was applied to experimental data. This model can be demonstrated by Eq. 7.
where \( k_{\text{id}} \) is the intraparticle diffusion rate constant of the ith step, and \( C_{\text{i}} \) is proportional to thickness of boundary layer. The plot of \( q_{\text{t}} \) versus \( t^{0.5} \) is shown in Fig. 10 which illustrates that three steps with three various slopes, which are indicated multisteps, control the adsorption mechanism. The intraparticle diffusion constant of each step was calculated from the slope of the three linear lines which are given in Table 2. The results confirmed that at the beginning of the adsorption process, the Cr(VI) ions diffuse rapidly through the human hair pores and afterward, intraparticle diffusion slows down and finally, the equilibrium state is complete.
Effect of initial concentration of Cr(VI)
The isotherm adsorption study of the removal of Cr(VI) by human hair using different initial concentrations of Cr(VI) from 10 to 100 mg L−1 at optimum conditions was studied. Figure 11 shows that the adsorption capacity of the human hair increases by increasing the initial concentration of Cr(VI); however, the removal percentage is decreased. This can describe that the driving force for the mass transfer is great at high initial concentration.
Adsorption isotherms
The adsorption equilibrium is established when an adsorbent (human hair) attains contact with the adsorbate, Cr(VI) ions, for a sufficient amount of time, where its adsorbate concentration in the solution is in a dynamic balance with the interface concentration. The process can be summarized by the following reversible reaction:
The analysis of equilibrium data is important for developing an equation that can be used for the design and optimization of different operational conditions. The Langmuir, Freundlich and Temkin isotherms are commonly used for describing the adsorption equilibrium for water and wastewater treatment applications. The equilibrium relationships between the adsorbent and the adsorbate are described by the adsorption isotherms (Eq. 8). The Langmuir isotherm emphasizes monolayer adsorption without the interaction between the adsorbed molecules. The nonlinear form of the Langmuir isotherm is represented by Eq. 9.
where \( C_{\text{e}} \;\left( {{\text{mg}}\;{\text{L}}^{ - 1} } \right) \) is the equilibrium ion concentration in solution and \( q_{\text{m}} \;\left( {{\text{mg}}\;{\text{g}}^{ - 1} } \right) \) is the maximum adsorption capacity related to monolayer coverage on the surface as well as \( b\;\left( {{\text{L}}\;{\text{mg}}^{ - 1} } \right) \) denotes the Langmuir constant, corresponding to the adsorption energy [33].
The \( q_{\text{m}} \) and \( b \) values are calculated from the slope and intercept of the linear plots of \( {{C_{\text{e}} } \mathord{\left/ {\vphantom {{C_{\text{e}} } {q_{\text{e}} }}} \right. \kern-0pt} {q_{\text{e}} }} \) versus \( C_{\text{e}} \), respectively. The obtained data are listed in Table 3.
Moreover, the experimental data of the adsorption of Cr(VI) ions on human hair were fitted to the Freundlich adsorption isotherm. The Freundlich model is related to the multilayer adsorption mechanism on a heterogeneous surface in which the adsorption energy of a metal ion is binding to an adsorbent site under the influence of the occupancy of its neighboring sites. The nonlinear form of the Freundlich isotherm model is demonstrated by Eq. 10.
where \( n \) and \( K_{\text{f}} \) are Freundlich constants which are related to the adsorption intensity and adsorption capacity, respectively [34]. The values of \( n \) and \( K_{\text{f}} \) are obtained from the slope and intercept of the plot \( \ln q_{\text{e}} \) versus \( \ln C_{\text{e}} \), respectively.
The Temkin isotherm model contains a factor that considers the interaction between the adsorbent and adsorbate. The nonlinear form of Temkin isotherm is given by Eq. 11.
where \( B\ln A \) indicates the maximum binding energy \( ( {\text{kJ}}\;{\text{mol}}^{ - 1} ) \) related to the bond between the adsorbent and the adsorbate [35]. \( A \) and \( B \) are Temkin isotherm constants, in which \( B = \, RT/b \) and \( b\; ( {\text{kJ}}\;{\text{mol}}^{ - 1} ) \) correspond with the adsorption heat. The \( A \),\( B \) and \( b \) values were obtained from the intercept and slope of the plot \( q_{\text{e}} \) versus \( \ln C_{\text{e}} \) which are shown in Table 3.
According to the obtained results and nonlinear isotherm models in Fig. 12, the Freundlich isotherm model is the appropriate one for describing the Cr(VI) adsorption process on human hair. The adsorption capacity of Cr(VI) ions using human hair was compared with other adsorbent reported in Table 4. Regarding the adsorption of Cr(VI) ions, human hair performed better in comparison with the other sorbents and in fact it is the most available and inexpensive material used.
Adsorption thermodynamics
The influence of temperature on the adsorption of Cr(VI) ions on human hair was investigated by conducting isotherm experiments at 293, 303, 313 and 323 K for an initial Cr(VI) concentration 50 mg L−1. It was found that by increasing the temperature, the removal percentage of Cr(VI) ions was enhanced. The results showed that the adsorption of Cr(VI) ions over human hair is endothermic in nature. The determination of the thermodynamic parameters such as standard Gibbs free energy change \( (\Delta G^{0} ) \), standard enthalpy change \( (\Delta H^{0} ) \) and standard entropy change \( (\Delta S^{0} ) \) were important to discover the spontaneity of the adsorption process. These parameters were calculated by using the following equations:
where \( C_{\text{a}} \) and \( C_{\text{e}} \) are the Cr(VI) concentration on the adsorbent and in the solution at an equilibrium point, respectively. The thermodynamic equilibrium constant K C is related to the Gibbs free energy change using Vant Hoff’s equation as follows:
where \( \Delta G^{0} \; ( {\text{J}}\;{\text{mol}}^{ - 1} ) \) is the standard Gibbs free energy change, R is the universal gas constant (8.314 J mol−1 K−1), and T is the temperature (K) of the adsorption process. According to Eq. 14, Gibbs free energy change is dependent on the enthalpy and entropy changes of the adsorption at constant a temperature.
Equation 15 is obtained by combination of Eq. 13 and 14 [13].
The values of \( \Delta H^{0} \) and \( \Delta S^{0} \) were obtained from the slope and intercept of plot of \( \ln K_{\text{C}} \) versus \( 1/T \), respectively (Fig. 13). \( \Delta G^{0} \) values are determined using Eq. 14. The negative value of \( \Delta G^{0} \) shows the feasibility and the spontaneous nature of the adsorption process. The positive value of \( \Delta H^{0} \) indicates that the adsorption reaction is endothermic and the positive value of \( \Delta S^{0} \) showed the increase in the randomness at the solid–solute interface and the attraction of the adsorbent for the Cr(VI) ions. The values of thermodynamic parameters are presented in Table 5. The results indicate that the \( \Delta G^{0} \) values were negative and increase with increasing the solution temperature. These results suggest that the process of adsorption of Cr(VI) on human hair is spontaneous and the removal percentage is favored at high temperature. The values of heat of adsorption, \( \Delta H^{0} \) is positive for Cr(VI) ions, indicated that the adsorption process of Cr(VI)ions on human hair is endothermic. A positive value of \( \Delta S^{0} \) suggest that Cr(VI) ions increased the randomness of the process. This exclusive behavior could be caused by an increase in the active sites on hair or cracks on the surface due to the increasing temperature. The obtained thermodynamic parameters, listed in Table 5, concluded that the adsorption process is physisorption, spontaneous and endothermic.
Conclusions
Overall, it could be concluded that human hair is introduced as a bio-sorbent for the removal of Cr(VI) ions from aqueous solution. Human hair tissue contains C, O, N and S in proportion to the amino acid, in which a lone pair of electrons of nitrogen and oxygen atoms on the surface of hair can be directly coordinated to the unoccupied 3d orbital of a Cr(VI) ion in a solution. As illustrated, this method acts as a suitable procedure for the reuse of waste materials and the removal of heavy metals such as Cr(VI) in wastewater. In this study pH, ionic strength, contact time, initial concentration and adsorbent amount are optimized for maximum removal. Theses optimal values are pH = 2, contact time = 150 min, initial concentration = 50 ppm and the amount of human hair = 0.5 g. The calculated thermodynamic parameters are indicated that the adsorption process of Cr(VI) ions on human hair is an endothermic and spontaneous reaction and also it is in the range of physisorption reaction. The adsorption isotherm results demonstrate that the removal is best described by Freundlich isotherm with the maximum adsorption capacity \( 1 1. 6 4 {\text{ mg}} . {\text{g}}^{ - 1} \). Moreover, it is observed that the kinetic of Cr(VI) ions adsorption on human hair is followed by the pseudo-second-order kinetics model. Consequently, it can be concluded that the application of human hair as an adsorbent is economical in comparison with commercially available adsorbent, such as activated carbon. Therefore, the use of human hair for removing heavy metals from wastewater not only solves the housekeeping problem, but also could be used as an effective adsorbent for wastewater treatment.
References
R. Khosravi, M. Fazlzaehdavil, B. Barikbin, A.A. Taghizadeh, Appl. Surf. Sci. 292, 670 (2014)
S. Aghajani, A. Afkhami, M. Mohseni, T. Madrakian, J. Iran. Chem. Soc. 12(11), 2007 (2015)
Y. Zhao, S.Yangh, D. Ding, J.Chen, Y. Yang, Z. Lei, C. Feng, Z. Zhang, J. Colloid. Interf. Sci, 395, 198 (2013)
W. Cao, Z. Dang, X.Y. Yi, C. Yang, G.N. Lu, Y.F. Liu, S.Y. Huang, L.C. Zheng, Environ. Tecchnol. 34(1), 7 (2013)
J. Xie, X. Gu, F. Tong, Y. Zhao, Y. Tan, J. Colloid. Interface Sci 455, 55 (2015)
S. Hojati, A.R. Esfahani, A. Azimi, M. Farzadian, J. Taiwan Inst. Chem. Eng 49, 172 (2015)
Z. Huang, X.l. Wang, D. Yang, Water. Sci. Eng. 8(3), 226 (2015)
R.J. Santhi, V. Vetriselvi, Water Resour. Ind. 10, 39 (2015)
S.H. Araghi, M. Entezari, M. Chamsaz, Microporous Mesoporous Mater. 218, 101 (2015)
M.Cieslak-Golonka, Polyhedron Report NO. 61, 15, 3667 (1995)
Y. Xie, H. Li, X. Wang, I.S. Ng, Y. Lu, K. Jing, J. Taiwan Inst. Chem. Eng 45, 1773 (2014)
M. Rajasimman, P. Karthic, J. Taiwan Inst. Chem. Eng 41, 105 (2010)
E. Malkoc, Y. Nuhojlu, M. Dundar, J. Hazard. Mater, B 138, 142 (2006)
M. Dakiky, M. Khamis, A. Manassra, M. Mer, eb. Adv. Environ. Res. 6, 533 (2002)
J. Wu, H. Zhang, P.J. He, Q. Yao, L.M. Shao, J. Hazard. Mater. 176, 697 (2010)
S.P. Dubey, K. Gopal, J. Hazard. Mater. 145, 465 (2007)
S. Kumar, B.C. Meikap, Desalination Water. Treat 52, 3122–3132 (2014)
B. Bhushan, Progress. Mater. Sci. 53, 585 (2008)
A. Gupta, J. Waste. Manag. 2014, 1 (2014)
A. Ghosh, S.R. Collie, Def. Sci. J 64(3), 209 (2014)
B.C. Beard, J. Hare, J. Surfactants Deterg. 2, 145 (2002)
I. Efremenko, R. Zach, Y. Zeiri, J. Phys. Chem, C 111, 11903 (2007)
A.S. Ekop, N.O. Eddy, J. Chem. 7(4), 1296 (2010)
L. Mahdavian, Afr. J. Microbiol. Res 6(1), 183 (2012)
ASTM, Standard Test Methods for Chromium in Water. Annual Book of ASTM Standards, D1687-02 (2007)
M. Okamoto, K. Ishikawa, N. Tanji, S. Aoyagi, Surf. Interface Anal. 44, 736 2011
N. Tahri Joutey, H. Sayel, W. Bahafid, N. El-Ghachtouli, Rev. Environ. Contam. Toxicol. 233, 45 (2015)
E. Aranda-Garcia, L. Morales- Barrera, G. Pineda-Camacho, E. Cristiani-Urbina, Environ. Monit. Assess. 186, 6207 (2014)
S. Lagergren, Kungliga Sven. Vetensk. Psakademiens Handlingar 24(4), 1 (1898)
Y.S. Ho, G.M. Kay, Inst. Chem. Eng. B 76, 332 (1998)
M.J.D. Low, Chem. Rev. 60(3), 267 (1960)
W.J. Weber, J.C. Morris, J. Sanit, J. Sanit. Eng. Div. 89, 31 (1963)
L. Langmuir, J. Am. Chem. Soc. 40, 1361 (1918)
H.M.F. Freundlich, J. Phys. Chem. 57, 385 (1906)
M.I. Temkin, V. Pyzhev, Acta Physiochim USSR 12, 327 (1940)
P. S. Blanes, M. E. Bordoni, J. C. Gonzalez, S. I. Garcia, A. M. Atria, L. F. Sala, S. E. Bellu, J. Environ. Chem. Eng. 4, 516 (2016)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Abbasi, F., Farrokhnia, A., Bamdad, M. et al. The kinetic and thermodynamic study of the removal of Cr(VI) ion from aqueous solution by human hair waste. J IRAN CHEM SOC 14, 1741–1752 (2017). https://doi.org/10.1007/s13738-017-1115-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-017-1115-z