Abstract
Formaldehyde is known as a highly toxic compound to humans and identified as a carcinogenic substance. In this study, Hantzsch reaction was utilized for the derivatization of trace amounts of formaldehyde in aqueous samples with acetylacetone in the presence of ammonia to form an extractable colored product named 3,5-diacetyl 1,4-dihydrolutidine (DDL) and its further extraction using two-phase hollow fiber liquid-phase microextraction. The main experimental variables affecting the extraction performance were investigated and optimized. Under the optimum conditions (sample volume 12 mL; reaction temperature 70 °C; ammonium acetate buffer solution 4 mL 0.1 mol L−1; acetylacetone 5 mL 0.15 mol L−1; solvent octanol, salt concentration 20% (w/v) NaCl; pH of donor phase 7.0; stirring speed 400 rpm and extraction time 30 min), the linear dynamic range, limit of detection (LOD as 3S b/m) and relative standard deviation (RSD %) of the proposed method were obtained as 5–250 μg L−1 (r 2 = 0.9979), 3.6 μg L−1 and 2.5%, respectively. Finally, the applicability of the proposed method was examined, and very good results were obtained. The results confirmed the applicability of the proposed method as a versatile, low-cost and sensitive preconcentration method for determination of low concentrations of formaldehyde in aqueous solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sample preparation is undoubtedly considered as an essential stage in the analytical process. The isolation and preconcentration of target analytes as well as the performance of a clean up step when dealing with complex matrices are known as the important stage of sample preparation [1]. Liquid-phase microextraction (LPME) was developed as a solvent-minimized sample pretreatment procedure, which is quick, minimizes the consumption of organic solvent and has a simple experimental setup [2]. Several static and dynamic models of LPME have been developed, including single-drop LPME (SD-LPME) [3], headspace LPME (HS-LPME) [4], continuous-flow LPME (CF-LPME) [5] and hollow fiber LPME (HF-LPME) [6].
In recent years, there was a growing interest in the use of porous hollow fiber-based LPME [7]. In 1999, Pedersen-Bjergaard and Rasmussen integrated the basic principle of supported liquid membrane (SLM) into a simple extraction unit for using commercial polypropylene HFs as membranes [8]. Among the different modes of LPME, HF-LPME gives higher sensitivity and better precision than the others due to the protection of organic phase by a fiber that eliminates the dissolution of the organic phase in comparison with SD-LPME. The general principle of membrane techniques is that the target analyte in the sample (donor phase) passes through the membrane. Membrane acts as a barrier and separates the donor and acceptor phases [9]. The membrane extraction techniques could be classified as porous and nonporous or one-, two- or three-phase extraction techniques [10]. In two-phase extraction systems, the solvent of membrane phase is the same as the donor or the acceptor phase, so only one-phase boundary exists. Three-phase HF-LPME involves extraction of ionizable or polar analytes from an aqueous sample (donor phase) into an aqueous phase inserted into the lumen of the hollow fiber (acceptor phase) through an intermediate organic phase which filled the pores of hollow fiber. Finally, the analytes will be ionized in the acceptor phase by acid–base reactions and thus become trapped in a non-extractable form [11]. Transfer of analytes from donor to acceptor phase can be promoted by adjustment of pH in two phases or can be done by active transport of analytes in which a carrier is added into the sample solution or membrane phase [12]. After trapping of target analytes in the acceptor phase, the extract is transferred to an analytical instrument, either manually or online [13]. In two-phase LPME, the selected acceptor solution, which represents the final extracting solvent, is usually an organic solvent such as n-octanol or toluene [14]. During procedure, the lumen of porous hollow fiber contains the organic extraction solvent and a thin layer of organic solvent is formed within its pores. The organic solvent acts as a selective barrier to trap the target analyte, and it depends on type of compounds. The analytes are extracted from the aqueous sample through the organic phase into the pores of the hollow fiber before entering the acceptor solution inside the lumen [15]. To perform two-phase HF-LPME, both micropores and the lumen of the hollow fiber must be filled with the organic phase [2, 3]. This technique is simple and inexpensive, with the further advantage that the fiber is disposable after use due to its low cost, thus overcoming carry over problems [15]. LPME has been applied successfully for the extraction and clean up of complicated samples such as drug/pharmaceuticals, environmental samples and foodstuff [14–21].
Formaldehyde (HCHO) is the simplest aldehyde that is an important precursor to many chemical compounds and commonly known as a preservative in medical laboratories that is called formalin. Formaldehyde is a colorless and strong-smelling gas under normal conditions and is soluble in water. It is also found in other products such as chemicals, particle board, household products, glues, permanent press fabrics, paper product coatings, fiberboard and wood product [22]. Formaldehyde is one of the exceptionally important air pollutants in residential and industrial environment [23]. It has created intense concern because it caused eye irritation and increasing risks of allergy in children [24]. Thus, it is necessary to develop a simple, specific and sensitive sample preparation method for the detection of trace quantities of this compound in environmental water and wastewaters. Up to now, some extraction methods were introduced to extract and separate trace amounts of formaldehyde from aqueous samples [25, 26].
In this work, detection and quantification of trace amounts of formaldehyde in aqueous samples was studied using two-phase HF-LPME as a preconcentration step prior to UV–Vis spectrophotometry. The well-known Hantzsch reaction which involves the cyclization between acetylacetone and formaldehyde in the presence of ammonium acetate was used for the derivatization of trace amounts of formaldehyde in water samples.
The derivatization procedure and extraction conditions were optimized using univariate method, and the applicability of the proposed method was studied in real aqueous samples.
Experimental
Reagents and chemicals
All the materials used in this research were prepared from Merck Company (Darmstadt, Germany). A stock standard solution of formaldehyde (1000 mg L−1) was prepared by appropriate dilution of 124 µL of 37% (v/v) formaldehyde solution (GR for analysis, d = 1.09 g mL−1) in 50 mL of double-distilled water. The working standard solutions were prepared by diluting the stock solution with double-distilled water. A 0.1 mol L−1 ammonium acetate solution (M W = 77.0825 g mol−1, as a pH adjusting buffer) was prepared by dissolving 0.386 g of salt in 50 mL double-distilled water. A 0.15 mol L−1 acetylacetone (as a reducing reagent) was prepared in double-distilled water. Sodium hydroxide (0.01 mol L−1) and hydrochloric acid (0.01 mol L−1) were used to adjust the pH of solution in the range of 5.0–7.0.
Apparatus
A double-beam UV–Vis spectrophotometer (MADAPA. model 6300, China) was used for trace determination of formaldehyde. Since the volume of extraction solvent was so small, a quartz microcell (path length of 1 cm and internal volume of 350 μL (was employed for spectrophotometric measurements. All absorption measurements were taken in a maximum absorption wavelength of 375 nm. A pH meter (model GP353, EDTA, England) equipped with a combined glass electrode was used for adjusting the pH of solutions. All the extractions were carried out using an Accurel Q3/2 polypropylene hollow fiber membrane from Membrana GmbH (Wuppertal, Germany) with a 600-μm internal diameter, 200-μm wall thickness and 0.2-μm pore size. A magnetic stirrer (Labnico, Netherlands) and a stirrer bar (4 mm × 14 mm) were used to enhance mass transfer of analyte from donor to acceptor phase during the two-phase HF-LPME. An ultrasonic bath system (DSA100-SK2-4OL China) was used to speed up formation reaction of DDL product. All glasswares were washed with double-distilled water and were put in the oven prior to use.
HF-LPME procedure
For each extraction, 12 mL of the aqueous solution containing formaldehyde (as donor phase) was poured into a 25-mL sample vial having a 4 mm × 14 mm magnetic stirring bar. Four milliliters of ammonium acetate buffer solution (0.1 mol L−1, for adjusting solution pH) and 5 mL of acetylacetone (0.15 mol L−1, as reductant agent) were added to the sample solution, and the pH of solution was adjusted in the range of 6.5–7.5. Before each extraction, the sample solution was put in the ultrasonic bath (30 min at 70 °C) to promote reaction between formaldehyde and acetylacetone to form extractable colored product of 3, 5-diacetyl 1,4-dihydrolutidine. Before extraction, 4.2 g NaCl was added to solution to make a 20% (w/v) NaCl solution and 18 mL of this mixture was used for two-phase HF-LPME.
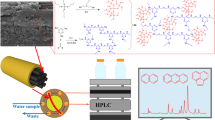
For each two-phase HF-LPME, the polypropylene hollow fibers were cut into pieces with 8.8 cm length (25-μL internal capacity for extraction solvent). For decreasing the memory effect, each piece was used only once. Before use, possible contaminants were removed by sonicating each hollow fiber in ethanol (10 min) and next drying in the oven (20 min at 80 °C). Twenty-five microliters of octanol as extraction solvent (acceptor phase) was drawn into a 25-μL Hamilton microsyringe (model 702 NR, Bonaduz, Switzerland). One end of hollow fiber was connected to the needle of microsyringe containing extraction solvent. Before use, hollow fiber was inserted in octanol as extraction solvent (10 s) to fill the pores and then in double-distilled water (10 s) to eliminate extra octanol. The extraction phase filled the channel and pores of hollow fiber. Finally, the end of hollow fiber was closed with a piece of aluminum foil. The hollow fiber was then introduced into the aqueous sample (18 mL) at U-shaped configuration, and the top of the vial was covered via parafilm (Fig. 1). During the extraction period (30 min), the colored product (DDL) was extracted by octanol filled in the lumen of hollow fiber.
At the end of extraction period, the microsyringe was removed from vial and octanol (23 µL) was drawn into the microsyringe. After that, the extraction phase was poured into a 1-mL vial, was diluted with 50 μL of pure octanol and transferred into the quartz microcell for spectrophotometric measurements.
The blank solution was prepared during the extraction from an aqueous phase without formaldehyde. The spectrum was recorded in the wavelength range of 300–750 nm, and finally, 375 nm was used as maximum wavelength for further measurements.
Results and discussion
In this study, the extraction and spectrophotometric determination of low concentration of formaldehyde was planned based on the Hantzsch reaction firstly explored by Nash in 1953 [27]. The reaction is characterized by a cyclization of acetylacetone and formaldehyde in the presence of excess ammonium acetate salt in approximetry neutral solutions at 70 °C to form a yellow product called 3,5-diacetyl 1, 4-dihydrolutidine (DDL) (2,4-dimethyl-1,4-dihydropyridine-3,5-dimethylcarboxylate) (Fig. 2).
Finally, DDL as the resulting colored product was extracted into the extraction organic solvent filled in the lumen of hollow fiber. UV–Vis spectrum was recorded after completion of extraction, and absorbance was measured at 375 nm. Furthermore, effect of experimental parameters affecting the extraction efficiency was investigated using univariate optimization method. In all optimization experiments, samples containing 100 µg L−1 of formaldehyde were used.
Selection of extraction solvent type
In two-phase HF-LPME, the selection of organic solvent is of great importance in order to obtain an efficient extraction. For selection of organic solvent, several factors should be considered. The solvent should be immiscible with water and be easily immobilized in the pores of hollow fibers. The organic solvent should have high solubility for the analyte. Also, it should have low volatility to prevent solvent loss during extraction. Therefore, 1-octanol, 1-hexanol and 1-hexyl-4-methyl imidazolium hexafluoro phosphate ([C6MIM]PF6, an ionic liquid solvent) were examined, and octanol showed better extraction efficiency and was selected as proper and optimal solvent to fill the pores and internal lumen of the hollow fiber [28]. The examined ionic liquid ([C6MIM] PF6) was improper for extraction due to its very high viscosity that creates problems in handling with microsyringe.
Effect of acetylacetone concentration on the HF-LPME efficiency
Acetylacetone is a yellow reagent with good solubility in water. In the presence of ammonia, it reacts with formaldehyde to make a colored product called diacetyl dihydrolutidine [26]. To investigate the effect of acetylacetone concentration, its values were changed from 0.05 to 0.5 mol L−1 while keeping other variables constant. Due to the presence of different concentrations of acetylacetone, various blanks were made for each concentration of acetylacetone and the absorbance difference (ΔA) were measured at 375 nm. According to the results (Fig. 3), with increase in acetylacetone concentration up to 0.15 mol L−1, more extraction was occurred that shows the formation of more product. At higher concentrations of reagent, ΔA decreases probably due to existence of higher concentration of free acetylacetone and its influence on absorbance. Therefore, all subsequent experiments were performed at 0.15 mol L−1 of acetylacetone.
Effect of acetylacetone concentrations on the HF-LPME of formaldehyde. Conditions: sample, 12 mL with pH = 6.5; 4 mL 0.1 mol L−1 ammonium acetate buffer, 5 mL acetylacetone reacted in ultrasound water bath (30 min at 70 °C); extraction solvent: 25 µL octanol, NaCl concentration: 20% (w/v); stirring speed: 300 rpm and extraction time: 30 min
Effect of salt effect on the HF-LPME efficiency
To study the salt effect on the HF-LPME efficiency, a series of aqueous formaldehyde samples containing various concentrations of NaCl in the range of 0–25% (w/v) were prepared and extracted by the proposed HF-LPME procedure. The results (Fig. 4) showed the increase in extraction efficiency using NaCl up to 20% (w/v) due to “salting-out” and after that the extraction efficiency was decreased. This can be explained by the engagement of more water molecules in the hydration spheres around the ionic salt that reduces the amount of water molecules available to dissolve the analyte. This will derive additional extractable product into the octanol on the pores of hollow fibers. As shown in Fig. 4, the extraction efficiency decreased with increasing NaCl concentration higher than 20% that can be related to the enhancement of viscosity and density of the donor phase that reduces mass transfer into the organic phase. Based on these observations, an overall salt concentration of 20% (w/v) was used for further studies.
Effect of NaCl concentration on the HF-LPME of formaldehyde. Conditions: sample, 12 mL with pH = 6.5; 4 mL 0.1 mol L−1 ammonium acetate buffer, 5 mL 0.15 mol L−1 acetylacetone reacted in ultrasound water bath (30 min at 70 °C); extraction solvent: 25 µL octanol; stirring speed: 300 rpm and extraction time: 30 min
Effect of pH of donor phase on the HF-LPME efficiency
To study the effect of pH on the extraction efficiency, pH of solution was studied in the range of 4.0–7.5 while keeping other variables constant. This range of pH values has a significant influence on reaction of formaldehyde with acetylacetone to form DDL. According to the results (Fig. 5), in the pH range of 6.5–7.5, absorption was high and constant, showing the best extraction efficiency. So, pH = 7.0 was selected as optimum value for further studies.
Effect of pH of donor phase on the HF-LPME of formaldehyde. Conditions: sample, 12 mL; 4 mL 0.1 mol L−1 ammonium acetate buffer, 5 mL 0.15 mol L−1 acetylacetone reacted in ultrasound water bath (30 min at 70 °C); extraction solvent: 25 µL octanol; NaCl concentration: 20% (w/v); stirring speed: 300 rpm and extraction time: 30 min
Effect of stirring rate on the HF-LPME efficiency
Like other microextraction techniques, the extraction in HF-LPME can be enhanced by agitation of the sample solution. In two-phase HF-LPME, the organic solvent is sealed and protected by the hydrophobic hollow fiber membrane [29]. Agitation of the donor solution increases mass transfer and reduces the required extraction time by raising the diffusion rate of analyte from donor to acceptor phase [30]. In order to investigate the influence of agitation speed, different stirring rates ranging from 200 to 500 rpm were investigated. Figure 6 shows that extraction increase by changing stirring rates up to 400 rpm. Therefore, 400 rpm was chosen as optimum value for further works.
Effect of stirring rate on the HF-LPME of formaldehyde. Conditions: sample, 12 mL with pH = 7.0; 4 mL 0.1 mol L−1 ammonium acetate buffer, 5 mL 0.15 mol L−1 acetylacetone reacted in ultrasound water bath (30 min at 70 °C); extraction solvent: 25 µL octanol; NaCl concentration: 20% (w/v) and extraction time: 30
Effect of Hantzsch reaction time on the HF-LPME efficiency
The reaction time of Hantzsch reaction was investigated using ultrasound bath using 15, 30, 45 and 60 min at 70 °C to form DDL product.
The experimental results showed that optical absorbance was constant in a time interval of 30 min. So, this time was chosen to complete Hantzsch reaction.
Effect of extraction time on the HF-LPME efficiency
During the two-phase HF-LPME, extractable product (DDL) should transfer from donor to acceptor phase and the mass transfer of analyte through interfaces requires time. To investigate the effect of time, different extraction times in the range of 2–60 min were examined. According to the results, the extraction was increased rapidly up to 30 min, and after that, it was nearly constant up to 60 min. Based on these observations, 30 min was selected for the next experiments.
Method validation
Analytical figures of merit
To evaluate the quantitative parameters of the proposed two-phase HF-LPME method for formaldehyde determination, the figures of merit of this method was investigated under the optimized experimental conditions (aqueous sample containing formaldehyde 12 mL; ammonium acetate buffer solution 4 mL 0.1 mol L−1; acetylacetone 5 mL 0.15 mol L−1 reacted in ultrasound water bath (30 min at 70 °C); extraction condition includes: extraction solvent octanol, salt concentration 20% (w/v); pH of donor phase (ammonium acetate buffer) 7.0; stirring speed 400 rpm and extraction time 30 min). Using figures of merit of each analytical method, it is possible to compare the efficiency of various analytical methods with each other and also evaluate the ability of an analytical method for particular applications. For evaluation of linear dynamic range (LDR), 13 spiked standard samples of formaldehyde in the range of 0.1–500 µg L−1 were prepared. Each standard sample was extracted by the proposed method under the optimized conditions, and the extractant was measured spectrophotometrically. The LDR was found to be linear in the range of 5–250 µg L−1 with r 2 = 0.9979.
The limit of detection (LOD) of the proposed method was calculated from CLOD = 3S b/m, where m is the slope of the calibration curve for two-phase HF-LPME of formaldehyde from standard solutions at optimum conditions and S b is the standard deviation of five replicate extraction of blank. The blank solutions were prepared as the same as the samples without formaldehyde and were extracted at optimum conditions by the proposed method and finally measured by spectrophotometer at 375 nm. The calculated LOD of the method was obtained as 3.6 μg L−1. Preconcentration factor (PF) of the method was obtained as 188.0 (at the formaldehyde concentration of 25 µg L−1 and optimum experimental conditions). Enhancement factor (EF) was calculated as 200 by dividing the slope of calibration curve after preconcentration (in acceptor phase) to that obtained without preconcentration (in the donor phase at concentration range of 0.5–50 μg L−1) and the relative standard deviation (RSD %) was obtained as lower than 5.5% using five replicate extractions from 50 μg L−1 formaldehyde solutions.
In order to investigate the applicability of the method for real samples, extraction from two real tap and well water samples was considered. At first, each sample was extracted by the proposed method in optimal conditions. To evaluate the accuracy of the method, 50 μg L−1 of formaldehyde was added to each sample and then the spiked samples were extracted (five replicate extractions) with the proposed two-phase HF-LPME. The summarized results are presented in Table 1. The good agreement between the found and added values (relative recoveries (%) between 98 and 106%) shows the applicability of the proposed extraction method for the preconcentration and determination of formaldehyde in aqueous solutions.
Comparison of the proposed method with other methods
A comparison between the figures of merit of the proposed HF-LPME method with a variety of techniques reported in the literature for quantitative determination of formaldehyde in aqueous samples is summarized in Table 2. It clearly shows that our proposed method has good sensitivity and precision, wide linear dynamic range and low LOD in comparison with some of the other techniques.
Low consumption of organic solvent and sample solution, simplicity and low cost of the extraction device, minimum carry over and cross-contamination and production of a clean extracting phase are the benefits of the proposed method. It is clear that by utilizing special quartz cell having lower internal volume or using nanodrop spectrometers, the need for dilution of extractant phase removed and higher preconcentration factors can be achieved. Also, this can be obtained during extraction from more volumes of samples.
Conclusion
The present study developed two-phase HF-LPME method coupled with Hantzsch reaction using acetylacetone reagent for preconcentration and determination of trace amounts of formaldehyde prior to UV–Vis spectrophotometry. The method has a high preconcentration factor, simplicity and convenience in operation. A little amount of organic solvent is applied which is environmentally friendly. Due to the simplicity and low cost of the extraction device, the hollow fibers can be discarded after each extraction to avoid carry over and cross-contamination. This serves to maintain high reproducibility and repeatability of the method. Excellent clean up property of the hollow fibers facilitate their application for the extraction from polluted samples. Also, the content of formaldehyde after the derivatization reaction and HF-LPME is measured by a spectrophotometer that is known as a common instrument in chemical and environmental laboratories.
References
I. Perevia, I. Lavilla, Trends Anal. Chem. 29, 617 (2010)
C. Bendicho, I. Lavilla, F. Pena-Pereira, V. Romero, J. Anal. At. Spectrom. 27, 1831 (2012)
M.A. Jeannot, F.F. Cantwell, Anal. Chem. 69, 235 (1997)
M.A. Farajzadeh, L. Khoshmaram, Anal. Bioanal. Chem. Res. 1, 1 (2014)
A. Sarafraz-Yazdi, A. Amiri, Trends Anal. Chem. 29, 1 (2010)
S. Bjergaard, K.E. Rasmussen, J. Chromatogr. A 118, 132 (2008)
J. Lee, H.K. Lee, K.E. Rasmussen, S.P. Bjergaard, Anal. Chim. Acta 624, 253 (2008)
S.P. Bjergaard, K.E. Rasmussen, Anal. Chem. 71, 2650 (1999)
J.Å. Jönsson, Chromatographia 57, 317 (2003)
M. Ghambarian, Y. Yamini, A. Esrafili, Mcirochim. Acta 177, 271 (2012)
J.F. Peng, J.F. Liu, X.L. Hu, J. Chromatogr. A 1139, 165 (2007)
Sh. Shariati, Y. Yamini, A. Esrafili, J. Chromatogr. B 877, 393 (2009)
K.E. Rasmussen, F. Barahona, A. Gjelstad, P.S. Bjergaard, J. Chromatogr. A 1217, 1989 (2010)
A. Gjelstad, P.S. Bjergaard, Sci. Chromatogr. 5, 181 (2013)
S.P. Bjergaard, K.E. Rasmussen, J. Chromatogr. A 1109, 183 (2006)
S.P. Bjergaard, H. Tung, K.E. Rasmussen, J. Chromatogr. A 998, 61 (2003)
E. Tahmasebi, Y. Yamini, A. Saleh, J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 877, 1923 (2009)
A. Esrafili, Y. Yamini, Sh. Shariati, M. Moradi, M. Ghanbarian, J. Liq. Chromatogr. Relat. Technol. 35, 343 (2012)
M. Charalabaki, E. Psillakis, D. Mantzavinos, Chemosphere 60, 690 (2005)
J. Salafranca, D. Pezo, C. Nerın, J. Chromatogr. A 1174, 85 (2007)
A. Esrafili, Y. Yamini, Sh. Shariati, Anal. Chim. Acta 604, 127 (2007)
K.M. Al-Azzam, A. Makahleah, B. Saad, S.M. Mansor, J. Chromatogr. A 23, 3654 (2010)
Q. Li, M. Oshima, Talanta 72, 1675 (2007)
M.H. Garrett, M.A. Hooper, B.M. Hooper, P.R. Rayment, M.J. Abramson, Allergy 54, 330 (1999)
P.A. Martos, J. Pawliszyn, Anal. Chem. 70, 2311 (1998)
M. Arvand, E. Bozorgzadeh, Sh. Shariati, M. Zanjanchi, Environ. Monit. Assess. 184, 7597 (2012)
T. Nash, Biochem. J. 55, 416 (1953)
M. Arvand, E. Bozorgzadeh, Sh. Shariati, J. Food Comp. Anal. 31, 275 (2012)
A. Kashtiaray, H. Faranhani, H.M. Sobhi, S. Farhadi, AJAC 2, 429 (2011)
Z. Es-haghi, AJAC 2, 1 (2011)
Q. Li, M. Oshima, S. Motomizu, Talanta 72, 1675 (2007)
M. Sáenz, J. Alvarado, F. Pena-Pereira, S. Senra-Ferreiro, I. Lavilla, C. Bendicho, Anal. Chim. Acta 687, 50 (2011)
D. Zhang, J. Zhang, M. Li, W. Li, G. Aimaiti, G. Tuersun, J. Ye, Q. Chu, Food Chem. 129, 206 (2011)
Z. Li, H. Ma, H. Lu, G. Tao, Talanta 74, 788 (2008)
G. Zurek, U. Karst, J. Chromatogr. A 864, 191 (1999)
S. Dong, P.K. Dasgupta, Environ. Sci. Technol. 21, 581 (1987)
A.A. Hill, R.J. Lipert, J.S. Fritz, M.D. Porter, Talanta 77(4), 1405 (2009)
N.G. Yasri, H. Seddik, M.A. Mosallb, Arab. J. Chem. 8(4), 487 (2015)
K. Motyka, A. Onjia, P. Mikuska, Z. Vecera, Talanta 71, 900 (2007)
M. Safari, Y. Yamini, E. Tahmasebi, F. Latifeh, J. Sep. Sci. 38, 3421–3427 (2015)
Acknowledgements
Financial support by Rasht Branch, Islamic Azad University, Grant No. 4.5830, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ganjikhah, M., Shariati, S. & Bozorgzadeh, E. Preconcentration and spectrophotometric determination of trace amount of formaldehyde using hollow fiber liquid-phase microextraction based on derivatization by Hantzsch reaction. J IRAN CHEM SOC 14, 763–769 (2017). https://doi.org/10.1007/s13738-016-1026-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-016-1026-4