Abstract
One-pot four-component reaction of aldehydes, dimedone, ethyl cyanoacetate or ethyl acetoacetate and ammonium acetate was studied in the presence of perchlorated Zr-MCM-41 (ClO4 −/Zr-MCM-41) nanoparticles for the synthesis of dihydropyridines. Zr-MCM-41 nanoparticles with various molar ratios of Si/Zr were synthesized by sol–gel method under mild conditions, and then ClO4 −/Zr-MCM-41 samples with different calcination temperatures were prepared and characterized by XRD, FT-IR and SEM techniques. The characterization results show the presence of perchlorate in the mesostructure of Zr-MCM-41 and the catalytic performance experiments show that ClO4 −/Zr-MCM-41 with Si/Zr molar ratio of 25 and calcinations temperature of 300 °C has the best catalytic activity. The catalyst can be recovered easily and reused many times with moderate decrease in activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multi-component reactions (MCRs) have been attracted considerable attention as a powerful synthetic tool for the creation of several bonds in a single operation. They have also many remarkable advantages such as user and environmental friendliness, step reduction, atom economy associated to their use, avoiding the complicated purification operations and allowing savings of both solvents and reagents. Recently, MCRs have been used as the best way for the synthesis of heterocycles, which are of considerable importance in the design and discovery of new biologically active molecules [1]. Among them, polyhydroquinolines as an important group of nitrogen containing heterocycles have attracted much attention because of their diverse therapeutic and pharmacological properties, such as calcium channel blockers, vasodilator, hepatoprotective, antiatherosclerotic, bronchodilator, antitumor, geroprotective and antidiabetic activity [2–4].
The general method for the synthesis of polyhydroquinolines involves the four-component reaction of aldehydes, ethyl cyanoacetate or ethyl acetoacetate, ammonium acetate and cyclic 1,3-dicarbonyl compounds such as dimedone, in the presence of acid or base catalysts. Many catalysts have been used for this transformation such as molecular iodine [5], ceric ammonium nitrate [6], Hafnium(IV) bis(perfluorooctanesulfonyl)imide [7], Yb(OTf)3 [8], Cu(OTf)2 [9], guanidine hydrochloride [10], trifluoroethanol [11], Pd-nanoparticles [12], Montmorillonite supported Ni0-nanoparticles [13], cerium(IV) ammonium nitrate [14], scandium triflate [15] and MCM-41 [16].
However, many of these methodologies suffer from one or more disadvantages such as the use of expensive catalyst, the use of large amount of catalyst, long reaction times and non-reusability of the catalyst. Therefore, there is a need to develop new methodologies using reusable catalytic systems for this type of reaction.
Recently, the application of mesoporous material as catalyst support has received more attention due to their remarkable advantages such as high surface area and the role of mesopores as nanoreactors [17]. MCM-41 with highly ordered mesostructure [18] has been used as catalyst and catalyst support in many organic reactions [19–22]. Although, MCM-41 has very high surface area, this compound lacks the Brønsted acid sites and usually exhibits only mild Lewis acidity [23]. Surface acidity enhancement of MCM-41 can be achieved by incorporation of foreign ions like Ti4+, Al3+, Zr4+, and Cu2+ into the Si framework [24] or surface modification by inorganic or organic materials [25].
In this paper, we aim to report preparation, characterization and catalytic application of perchlorated Zr-MCM-41 (ClO4 −/Zr-MCM-41) nanoparticles as a novel solid acid catalyst for the synthesis of polyhydroquinolines by one-pot, four-component reaction of aryl aldehydes, dimedone, ethyl cyanoacetate or ethyl acetoacetate and ammonium acetate (Scheme 1).
Experimental
Materials and methods
All chemicals were commercial products. MCM-41 was prepared by our previously reported method [26]. All reactions were monitored by TLC and all yields refer to isolated products. Melting points were obtained by Buchi B-540 apparatus and are uncorrected. 1H and 13C NMR spectra were recorded in CDCl3 on a Bruker AVANCE III (400 MHz for 1H and 100 MHz for 13C) spectrometer. Infrared spectra of the catalysts and reaction products were recorded on a Bruker FT-IR Equinax-55 spectrophotometer in KBr pellets. XRD patterns were recorded on a Bruker D8 ADVANCE X-ray diffractometer using nickel filtered Cu Kα radiation (λ = 1.5406 Å). The morphology was studied using a KYKY-EM3200 scanning electron microscopy.
Preparation of ClO4 −/Zr-MCM-41 nanoparticles
The synthesis of nanosized Zr-MCM-41 was carried out using tetraethyl orthosilicate (TEOS) as the Si source, zirconium tetrachloride as Zr source, cetyltrimethylammonium bromide (CTAB) as the template and ammonia as the pH control agent with the gel composition of Si:Zr:CTAB:NH4OH:H2O = 1:0.04:0.123:0.267:530 by modification of our previous method [27].
In a typical procedure, to a solution of CTAB (1.29 g, 3.54 mmol) in deionized water (270 mL), TEOS (6.4 mL) was added dropwise at 70 °C for about 1 h and then a solution of ZrCl4 (0.27 g, 1.15 mmol) in water (3 mL) was added dropwise. The mixture was allowed to cool to room temperature and then aqueous ammonia (10 wt %) was added until the pH of the solution was adjusted to 10.5. The gel was stirred for 12 h and then separated by centrifuge and washed with distilled water (20 mL) and EtOH (2 × 10 mL), respectively. The solid was dried in an oven at 120 °C for 4 h and then calcined at 550 °C for 4 h.
The ClO4 −/Zr-MCM-41 was prepared by impregnation of Zr-MCM-41 using HClO4 and the calcinations of obtained solid at various temperatures. In a typical experiment, Zr-MCM-41 (1 g) was added to a solution of 0.1 M HClO4 (15 mL) and allowed to stirred at ambient temperature for 4 h. The mixture was separated by centrifuge and washed with deionized water (2 × 3 mL). The obtained solid was dried in an oven at 120 °C for 4 h and then calcined at 250, 300, and 350 °C for 4 h. The obtained samples were denoted as PZM-120, PZM-250, PZM-300, PZM-350, respectively.
General procedure for the synthesis of polyhydroquinoline derivatives in the presence of ClO4 −/Zr-MCM-41
A mixture of dimedone (1 mmol), aldehyde (1 mmol), ethyl cyanoacetate or ethyl acetoacetate (1 mmol), ammonium acetate (1.3 mmol) and catalytic amount of ClO4 −/Zr-MCM-41 (10 mg) was stirred in ethanol (1 mL) at reflux condition. After completion of the reaction (monitored by TLC, eluent; n-hexane:EtOAc, 8:2), the catalyst was separated by centrifuge and washed with EtOH (2 × 3 mL). After evaporation of the solvent, the crude products were recrystallized from EtOH to give pure polyhydroquinolines.
Physical and spectroscopic data for new compounds
5f: MP: 182–183 °C, FT-IR (KBr) υ max : 3420, 3307, 3217, 1692, 1652, 1613, 1530, 1395, 1285, 1038, 757 cm−1. 1H NMR (400 MHz, DMSO-d 6 ): δ (ppm) = 0.93 (s, 3H), 1.02 (t, 3H, J = 7.2 Hz), 1.03 (s, 3H), 2.02 (d, 1H, J = 16.1 Hz), 2.25 (d, 1H, J = 16.1 Hz), 2.51 (m, 2H), 3.91 (q, 2H, J = 7.0 Hz), 4.83 (s, 1H), 7.23 (m, 4H), 7.65 (s, 2H, NH2). 13C NMR (100 MHz, DMSO-d 6 ): δ (ppm) = 14.1, 26.4, 28.6, 31.7, 32.2, 49.9, 55.67, 76.32, 113.9, 126.3, 127.3, 129.2, 131.8, 132.6, 142.7, 159.2, 162.2, 168.1, 195.6. Anal. Calcd for C20H23ClN2O3: C, 64.08; H, 6.18; N, 7.47. Found: C, 63.96; H, 5.98; N, 7.53.
5 g: MP: 155–158 °C, FT-IR (KBr) υ max: 3400, 3287, 2950, 1693, 1649, 1595, 1538, 1490, 1367, 1252, 1202, 1034, 865, 694 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 1.02 (s, 3H), 1.21 (s, 3H), 1.21 (t, 3H, J = 7.1 Hz), 2.21 (d, 1H, J = 12.8 Hz), 2.28 (d, 1H, J = 12.8 Hz), 2.45 (s, 2H), 3.8 (s, 3H, OMe), 4.1 (m, 2H), 4.73 (s, 1H), 6.23 (s, 2H, NH2), 6.68 (d.d, 2H, J = 7.7 Hz), 6.87 (s, 1H) 6.9 (d, 1H, J = 7.5 Hz) 7.15 (t, 1H, J = 7.7 Hz). 13C NMR (100 MHz, CDCl3): δ (ppm) = 14.2, 28.2, 28.9, 32.1, 34.3, 41.1, 51.1, 55.2, 60.1, 81.1, 110.9, 114.2, 116.9, 121.1, 129.1, 147.0, 159.1, 160.2, 162.1, 169.1, 196.0. Anal. Calcd for C21H26N2O4: C, 68.09; H, 7.07; N, 7.56. Found: C, 68.37; H, 6.99; N, 7.64.
5 h: MP: 179–180 °C, FT-IR (KBr) υ max: 3335, 3200, 2958, 1690, 1663, 1543, 1369, 1279, 1200, 1040, 707 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 0.99 (s, 3H), 1.12 (s, 3H), 1.16 (t, 3H, J = 7.0 Hz), 2.17 (d, 1H, J = 16.2 Hz), 2.25 (d, 1H, J = 16.2 Hz), 2.46 (s, 2H), 4.04 (m, 2H), 4.71 (s, 1H), 6.29 (s, 2H, NH2), 7.15 (m, 1H), 7.61 (m, 1H), 8.37 (m, 1H), 8.53 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 14.2, 27.5, 29.0, 39.9, 40.6, 50.6, 59.8, 79.7, 115.8, 122.8, 135.8, 141.3, 147.4, 150.0, 158.4, 161.7, 168.7, 196.2. Anal. Calcd for C19H23N3O3: C, 66.84; H, 6.79; N, 12.31. Found: C, 66.73; H, 6.61; N, 12.37.
5i: MP: 136–137 °C, FT-IR (KBr) υ max: 3435, 3305, 2951, 1695, 1521, 1350, 1202, 1196, 1038, 690 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 1.02 (s, 3H), 1.13 (s, 3H), 1.2 (t, 3H, J = 7.0 Hz), 2.18 (d, 1H, J = 12.7 Hz), 2.27 (d, 1H, J = 12.7 Hz), 2.46 (s, 2H), 4.08 (m, 2H), 4.7 (s, 1H), 6.28 (s, 2H, NH2), 7.1 (t, 1H, J = 7.6 Hz), 7.23 (d, 1H, J = 7.7 Hz), 7.26 (d, 1H, J = 7.8 Hz), 7.42 (s, 1H),. 13C NMR (100 MHz, CDCl3): δ (ppm) = 14.2, 27.1, 29.0, 32.1, 34.2, 41.1, 50.9, 60.1, 80.2, 116.0, 122.1., 127.2, 129.0, 130.1, 131.1., 147.9, 149.2, 157.9, 162.0, 169.1, 197.1. Anal. Calcd for C20H23BrN2O3: C, 57.29; H, 5.52; N, 6.68. Found: C, 57.11; H, 5.40; N, 6.54.
Results and discussions
The catalyst characterization
The Zr-MCM-41 and PZM samples were characterized by FT-IR, SEM and XRD techniques. The SEM images of Zr-MCM-41 and PZM-300 show spherical nanoparticles with the size of less than 100 nm (Fig. 1).
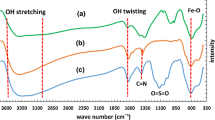
The FT-IR spectra of PZM-300, PZM-120, HClO4, Zr-MCM-41, MCM-41, and ZrO2 are shown in Fig. 2. MCM-41 shows characteristic peaks at 410, 810, 960, and 1083 cm−1 (Fig. 2a). The spectrum of ZrO2 shows corresponding peaks at 498, 584, 754, 1060, 1127, 1200, 1350, and 1450 cm−1. The Zr-MCM-41 calcined at 550 °C (ZM-550) sample exhibits characteristic peaks of MCM-41 at 455 and 804 cm−1 and also the peak at 1010 and 1350 cm−1 corresponded to ZrO2. In the spectrum of PZM-120 (Fig. 2e), weak peak at 630 cm−1 corresponded to HClO4. The main peak of HClO4 overlaps with the main peak of Zr-MCM-41 in the region of 1100 cm−1.
Perchlorate peaks in the range of 1089 and 1145 cm−1 overlap with the strong peak of MCM-41 around 960 and 1083 cm−1 resulting in increase of the intensity and broadening of this peak. In the spectrum of PZM-300 (Fig. 2f), the characteristic peaks of perchlorate clearly cannot be observed. This may be due to changes in symmetry groups because of the formation of covalent bonds with silica during calcination. This may be also due to the removal of nonbonded perchlorate ions on the surface of Zr-MCM-41 during the calcination process.
The low angle XRD patterns of MCM-41, ZM-550 and PZM-300 are shown in Fig. 3. The characteristic peaks of MCM-41 (Fig. 3a) have appeared at 2θ = 2.42 °, 4.14 °, 4.74 °, and 6.18 °. As shown in Fig. 3b after surface modification with Zr, the intensity of the main pak of MCM-41 was decreased and its width was increased. These changes are due to decrease in long-range order of hexagonal mesostructure of MCM-41 by incorporation of foreign ion.
The catalytic acidity of MCM-41 and PZM-300 were compared by potentiometric titration using 0.02 N solution of n-butylamine in acetonitrile. According to this method, the initial electrode potential (E i) indicates the maximum acid strength of the surface sites [28]. Potentiometric titration curves show that PZM-300 (E i = 520) has the greatest potential than MCM-41 (E i = 77), PZM-250 (E i = 511) and PZM-350 (E i = 156). The results show that PZM-300 is the strongest acid catalyst and has more acidic sites than others (Fig. 4).
To investigate the distribution of Brønsted and Lewis acid sites on the catalyst surface, the pyridine adsorption techniques were used. Figure 5 shows the FT-IR spectra of the PZM-300 and pyridine adsorbed PZM-300 at ambient temperature and heated at 100 °C up to 400 °C. The spectrum of pyridine adsorbed PZM-300 at ambient temperature (Fig. 5b) shows the contribution of pyridine adducts in the region of 1400–1650 cm−1. This spectrum shows the peaks at 1446 and 1602 corresponded to Lewis acid sites. Weak peak at 1490 cm−1 shows the combination band of pyridine bonded to Lewis and Brønsted acid sites. The peak at 1544 cm−1 is attributed to Brønsted acid sites. The peak at 1635 cm−1 in the spectrum of the catalyst before pyridine adsorption and also after pyridine adsorption is due to the presence of water on the preparation of the pellet sample. This peak may overlap with the weak peak of Brønsted acid site in this region [26, 29]. Peaks of Lewis acid sites (1446 and 1490 cm−1) are stronger than Brønsted acid sites which represents higher number of Lewis acid sites. Lewis acid sites are more powerful because in the high temperature at 400 °C the peak of pyridine adsorbed Lewis acid site (1464 cm−1) is also visible.
The loading amounts of perchlorate on the samples were investigated by determination of the Cl content using potentiometric titration. The wt % of ClO4 − by this method was obtained 25.7, 12.8 and 5.7 % for PZM-120, PZM-300 and PZM-350, respectively. Reduction of ClO4 − from 25.7 % in PZM-120 to 12.8 % in PZM-300 may be due to decomposition of free HClO4 on the surface. Reduction of the perchlorate content in the PCAM-350 sample confirms rapid decomposition of perchlorate at the temperature of higher than 300 °C according to literature report [30].
The catalyst activity
The catalytic performance of perchlorated Zr-MCM-41 (PZM samples) was investigated in the four-component one-pot reaction of aryl aldehydes, dimedone, ethyl cyanoacetate or ethyl acetoacetate and ammonium acetate to the synthesis of polyhydroquinolines. Initially, the optimization experiments were performed in the reaction of benzaldehyde (1 mmol), dimedone (1 mmol), ethyl cyanoacetate or ethyl acetoacetate (1 mmol) and ammonium acetate (1.3 mmol) in EtOH at reflux condition as the model reaction and results are summarized in Table 1.
To optimize the amount of PZM-300 in the model reaction, the reaction was performed in the presence of various amounts of PZM-300 (Table 1, entries 2, 9, 10) and results show that in terms of the reaction time and the yield of the product, the use of 10 mg PZM-300 has the best catalytic activity.
To investigate the effect of the molar ratio of Si/Zr on the catalytic activity of ClO4 −/Zr-MCM-41, the model reaction was performed in the presence of 10 mg of the catalyst with various Si/Zr molar ratios (Table 1, entries 1–3). The results show that molar ratio of Si/Zr = 25 have the best catalytic activity.
To show efficiency of the support on the catalytic activity of perchlorated Zr-MCM-41, the model reaction in the presence of 10 mg Zr-MCM-41 was performed under the same reaction conditions and lower yield of the product was obtained after 45 min (Table 1, entry 7). In a blank reaction (without the catalyst), 75 % yield of the products was achieved after 75 min.
To investigate the effect of solvent in the reaction, the model reaction in the presence of 10 mg PZM-300 was carried out in the various solvents (Table 1, entries 2, 11–14). The best solvent is EtOH with the best yield and time for the reaction.
In spite of these results, the reaction of benzaldehyde (1 mmol), dimedone (1 mmol), ethyl cyanoacetate or ethyl acetoacetate (1 mmol) and ammonium acetate (1.3 mmol) in the presence of 10 mg PZM-300 in EtOH at reflux condition was selected as the best condition for the synthesis of polyhydroquinolines.
Following the obtained results, the reaction of various aldehydes with both electron-donating and electron-withdrawing substituents, dimedone (1 mmol), ethyl cyanoacetate or ethyl acetoacetate and ammonium acetate were carried out in the presence of 10 mg PZM-300 in EtOH at reflux condition and the corresponding polyhydroquinoline derivatives were obtained in good yields (Table 2, entry 5a–n).
A plausible mechanism for the synthesis of polyhydroquinolines catalyzed by PZM-300 is explained in Scheme 2.
To investigate the reusability of the catalyst, the catalyst recovered and reused in the model reaction three times. The results show moderate decrease in the catalytic activity (Table 3). After third run, the catalyst was washed by EtOH, dried at 120 °C for 2 h and recalcined at 300 °C for 2 h. This catalyst was used in the model reaction (run 4). The result shows increase in catalytic activity in comparison to run 3 (Table 3, run 4). This result indicates that deactivation may be due to partial blockage of surface or leaching of ClO4 − from the surface of the catalyst.
Conclusion
In conclusion, we prepared perchlorated Zr-MCM-41 (PZM) as a new solid acid catalyst and it has been characterized using various techniques such as SEM, XRD and FT-IR. The performance of the catalyst was investigated in the synthesis of polyhydroquinolines. The study of heat treatment on the catalytic activity of PZM samples showed that the catalyst with calcination temperature of 300 °C (PZM-300) and 12.8 wt % loading amount of perchlorate had the best catalytic activity. High yields of the products, ease of work up, simple purification and green condition makes this method attractive for the synthesis of polyhydroquinolines.
References
A. Shaabani, A. Maleki, A.H. Rezayan, A. Sarvary, Mol. Diversity 15, 41 (2010)
V. Klusa, Drug Future 20, 135 (1995)
R.G. Bretzel, C.C. Bollen, E. Maeser, K.F. Federlin, Am. J. Kidney Dis. 21, S53 (1993)
R. Boer, V. Gekeler, Drug Future 20, 499 (1995)
S. Ko, M.N.V. Sastry, C. Lin, C.-F. Yao, Tetrahedron Lett. 46, 5771 (2005)
S. Ko, C.-F. Yao, Tetrahedron 62, 7293 (2006)
M. Hong, C. Cai, W.-B. Yi, J. Fluorine Chem. 131, 111 (2010)
L.-M. Wang, J. Sheng, L. Zhang, J.-W. Han, Z.-Y. Fan, H. Tian, C.-T. Qian, Tetrahedron 61, 1539 (2005)
K.K. Pasunooti, C. Nixon Jensen, H. Chai, M.L. Leow, D.-W. Zhang, X.-W. X-W, J. Comb. Chem. 12, 577 (2010)
S.M. Baghbanian, S. Khaksar, S.M. Vahdat, M. Farhang, M. Tajbakhsh, Chin. Chem. Lett. 21, 563 (2010)
A. Heydari, S. Khaksar, M. Tajbakhsh, H.R. Bijanzadeh, J. Fluorine Chem. 130, 609 (2009)
M. Saha, A.K. Pal, Tetrahedron Lett. 52, 4872 (2011)
L. Saikia, D. Dutta, D.K. Dutta, Catal. Commun. 19, 1 (2012)
C.S. Reddy, M. Raghu, Chin. Chem. Lett. 19, 775 (2008)
J.L. Donelson, R.A. Gibbs, S.K. De, J. Mol. Catal. A: Chem. 256, 309 (2006)
L. Nagarapu, M.D. Kumari, N.V. Kumari, S. Kantevari, Catal. Commun. 8, 1871 (2007)
H. Kosslick, G. Lischke, B. Parlitz, W. Storek, R. Fricke, Appl. Catal. A: Gen. 184, 49 (1999)
C. Kresge, M. Leonowicz, W. Roth, J. Vartuli, J. Beck, Nature 359, 710 (1992)
M. Abdollahi-Alibeik, M. Pouriayevali, Catal. Commun. 22, 13 (2012)
M. Abdollahi-Alibeik, A. Rezaeipoor-Anari, Cat. Sci. Tech. 4, 1151 (2014)
M. Abdollahi-Alibeik, M. Pouriayevali, Reac. Kinet. Mech. Cat. 104, 235 (2011)
M. Abdollahi-Alibeik, E. Heidari-Torkabad, C. R. Chim. 15, 517 (2012)
D. Nedumaran, A. Pandurangan, Microporous Mesoporous Mater. 169, 25 (2013)
Y.-W. Chen, H.-Y. Lin, J. Porous Mater. 9, 175 (2002)
P. Sivaguru, K. Parameswaran, M. Kiruthiga, P. Vadivel, A. Lalitha, J. Iranian Chem. Soc. 12, 95 (2015)
M. Abdollahi-Alibeik, A. Rezaeipoor-Anari, Cat. Sci. Tech. 4, 1151 (2014)
M. Abdollahi-Alibeik, G. Ahmadi, Res. Chem. Intermed. 41, 8173 (2015)
M. Abdollahi-Alibeik, F. Nezampour, Reac. Kinet. Mech. Cat. 108, 213 (2012)
M. Abdollahi-Alibeik, A. Moaddeli, New J. Chem. 39, 2116 (2015)
M.P. Henderson, V.I. Miasek, T.W. Swaddle, Can. J. Chem. 49, 317 (1971)
S. Kumar, P. Sharma, K.K. Kapoor, M.S. Hundal, Tetrahedron 64, 536 (2008)
M. Saha, T.S. Luireingam, T. Merry, A.K. Pal, J. Heterocycl. Chem. 50, 941 (2013)
E. Mosaddegh, A. Hassankhani, Arabian J. Chem. 5, 315 (2012)
S. S. Mansoor, K. Aswin, K. Logaiya, S. Sudhan, Arabian J. Chem. (2012)
B. Bandgar, P. More, V. Kamble, J. Totre, Arkivoc 2008, 1 (2008)
Acknowledgments
We are thankful to the Yazd University Research Council for partial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdollahi-Alibeik, M., Hoseinikhah, S.S. ClO4 −/Zr-MCM-41 nanoparticles prepared at mild conditions: a novel solid acid catalyst for the synthesis of polyhydroquinolines. J IRAN CHEM SOC 13, 1339–1347 (2016). https://doi.org/10.1007/s13738-016-0848-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-016-0848-4