Abstract
A new carbon paste electrode chemically modified with silver chloride is constructed. The silver chloride-modified carbon paste (SC-MCP) electrode was prepared by grinding the mixture of graphite powder and silicon oil (as a binder) with sufficient amount of silver chloride. Then, the electrode was placed in 0.1 mol L−1 NaOH and the electrode potential was cycled between 170 and 800 mV (vs. Ag/AgCl) at a scan rate of 50 mV s−1 for 20 cycles in a cyclic voltammetry regime until a stable voltammogram was obtained. The electrocatalytic activity of the SC-MCP electrode was examined for the oxidation of methanol. In CV studies, no oxidation response of methanol can be seen at the unmodified electrode, indicating the nonelectroactivity of methanol on this substrate. But at the SC-MCP electrode, a large anodic peak appears at 757.4 mV, indicating that the anodic oxidation of methanol could be catalyzed at SC-MCP electrode. This proves that the silver chloride bears the main role in electrocatalytic oxidation of methanol. Kinetic parameters such as the electron transfer coefficient (α) and the number of electrons involved in the rate-determining step (n α) for the oxidation of methanol was determined utilizing cyclic voltammetry (CV). The modified electrode shows a stable and linear response in the concentration range of 5 × 10−3–6 × 10−2 mol L−1 with a correlation coefficient of 0.9921.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The mechanism and kinetics of methanol oxidation have been studied under a wide range of conditions and on various electrodes including Pt [1], binary and ternary alloys [2, 3], modified electrodes [4, 5], nano composites [6, 7] and nickel [8–10]. It has been shown that one promising approach for minimizing over-voltage effects is through the use of an electrocatalytic process at chemically modified electrodes [11, 12].
Carbon paste electrode (CPE) is a special kind of heterogeneous carbon electrode consisting of mixture prepared from carbon powder (as graphite, glassy carbon and other carbonaceous materials) and a suitable water-immiscible or non-conducting binder [13]. The ease and speed of preparation and of obtaining a new reproducible surface, low residual current, porous surface, and low cost of carbon paste are some advantages of CPE over all other carbon electrodes. Therefore, CPE can provide a suitable electrode substrate for preparation of modified electrodes [14]. Modification of the paste matrix with various transition metal complexes [15–17] was reported in recent years. These electrodes have been widely used in electroanalysis due to their ability to catalyze the redox processes of some molecules of interest, since they facilitate the electron transfer [18].
A variety of mediators ranging from organic molecules to inorganic complexes such as prussian blue (PB) [19], polyoxometalates [20, 21] and ruthenium complexes [22] have been applied for the construction of CMCPE.
Ag is one of important metals and can be used not only as modification metal but also as substrate metal in surface modification [23]. We previously proved that the presence of halides causes an increased activity in the electrocatalytic behavior of copper [24–26]. It seems that the presence of halides can cause an increased activity in the electrocatalytic behavior of silver similar to copper.
The results showed that in comparison to bare carbon paste and silver electrodes, electrochemical behavior of methanol has been greatly improved at silver chloride-modified carbon paste (SC-MCP) electrode, indicating that the anodic oxidation of methanol could be catalyzed at silver chloride-modified carbon paste electrode.
Experimental
Reagents and instrumentation
Methanol and other reagents were of analytical grade supplied by Merck (Darmstadt, Germany) and Sigma Aldrich and were used without further purification. Distilled water was used for the preparation of all solutions. All electrochemical measurements were carried out in a three-electrode cell using an Autolab electrochemical system (Eco Chemie, Utrecht, the Netherlands) equipped with PGSTAT-12 and GPES software. A bare or modified carbon paste electrode (unmodified or modified) was used as a working electrode; Ag/AgCl/saturated KCl and a Pt wire were used as reference electrode and counter electrode, respectively. All experiments were carried out at room temperature.
Preparation of silver chloride-modified carbon paste electrode
The carbon paste electrode modified with silver chloride was prepared by hand mixing 71 % graphite powder, 9 % silver chloride and 20 % paraffin oil in an agate mortar to get homogeneous carbon paste. Then, paste was packed into the end of a polyethylene syringe (2 mm in diameter). A copper wire inserted into the carbon paste provided an electrical contact. Before each measurement, pushing an excess of paste out of the tube and then polishing the freshly exposed paste with weighing paper obtained a new surface. Also, unmodified carbon paste was prepared in the same way but without adding silver chloride to the mixture. Then, the electrode was placed in 0.1 mol L−1 NaOH and the electrode potential was cycled between 170 and 800 mV (vs. Ag/AgCl) at a scan rate of 50 mV s−1 for 20 cycles in a cyclic voltammetry regime until a stable voltammogram was obtained. The electrode was rinsed with distilled water, and applied for electrochemical studies.
Results and discussion
Electrochemical properties of the prepared SC-MCP electrode were investigated. For the activation of this electrode, the electrode was placed in 0.1 mol L−1 NaOH and the electrode potential was cycled between 170 and 800 mV (vs. Ag/AgCl) at a scan rate of 50 mV s−1 for 20 cycles in a cyclic voltammetry regime until a stable voltammogram was obtained (not shown). Results showed that with increase of the scan number, the currents for both anodic and cathodic peaks increase steadily for up to 20 runs. After 20 runs, the SC-MCP electrode shows reproducible cyclic voltammograms. A pair of redox peaks was observed which correspond to the couple Ag(II)/Ag(I) can be described by the reaction (1):

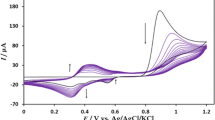
Next, the cyclic voltammograms of the modified electrode were recorded in 0.1 mol L−1 NaOH at various potential sweep rates (Fig. 1). With the increase of the scan rate, the redox current increased. The peak currents (I pa and I pc) are proportional to sweep rates in the range of 10–100 mV s−1 (Inset of Fig. 2), which showed the electrochemical activity of the surface redox couple. The value of Γ * was 6.31 × 10−5 mol cm−2 for n = 2, which were calculated from the slope of anodic peak current vs. scan rate using [27]:
where v is the sweep rate, A is the geometric surface area, and Γ is the surface coverage of the redox species.
To reveal the electrocatalytic activity of SC-MCP electrode toward the oxidation of methanol, the voltammetric experiments were carried out on both modified and unmodified CPEs in the presence of methanol. Figure 2 shows the cyclic voltammograms of SC-MCP electrode in the absence and presence of methanol in 0.1 mol L−1 NaOH solution. As shown, no oxidation response of methanol can be seen in the potential range from 170 to 850 mV on unmodified electrode, indicating the nonelectroactivity of methanol on this substrate. But at the SC-MCP electrode, a large anodic peak appears at 757.4 mV. In the negative scan, there is an oxidation peak at 500 mV, and it is caused by reoxidation of intermediate product of methanol. The great increase of the oxidized peak at 757.4 mV and the deoxidized peak at 500 mV indicates that the SC-MCP electrode displays preferable electrocatalytic activity toward methanol oxidation. Figure 2 also compares the cyclic voltammogram of SC-MCP electrode with that obtained by silver electrode. In comparison with the data at silver electrode, an increase of threefolds in peak current of methanol was observed at the SC-MCP electrode. Our measurements using K4Fe(CN)6/K3Fe(CN)6 system showed that the surface area of the bare CPE, silver and SC-MCP electrodes is almost the same. Thus, the increased activity of the electrode related to the presence of halide. The increased activity probably related to a more favorable adsorption of reactant or of intermediates leading to a higher surface concentration of electroactive molecules ready for being oxidized or it is due to the partial delocalization of the electronic density of methanol into the solid with possible consequent bond pre-dissociations which facilitates the oxidation or both.
Table 1 compares some electrochemical parameters of SC-MCP electrode for methanol oxidation with electrodes reported in literature. It was found that the electrochemical behavior of methanol was improved at SC-MCP electrode.
As suggested in literatures [24], the total oxidation process of methanol consists of a pattern of parallel reactions which can, in principle, be formulated as follows:

Both of these pathways require a catalyst, which should be able to (a) dissociate the C-H bond and (b) facilitate the reaction of the resulting residue with some O-containing species to form CO2 (or HCOOH). Generally accepted methanol oxidation process consists of the following steps that result in the formation of carboxyl intermediates and strongly adsorbed CO species [24–26, 28–34]:
The voltammetric signals were affected by the composition of the paste. It was observed that the sensitivity of the sensor first rapidly increases with increasing the silver chloride content in the paste up to about 9 %, and then started to level off and even slightly decreases with the higher loadings (Fig. 3). This is because the sites for adsorption increased with the increase of silver chloride percentage in the modified electrode, while the excess of silver chloride increases the resistance of the electrode. Hence, a silver chloride (9 %, w/w)-modified carbon paste electrode was used throughout this work.
The cyclic voltammograms of SC-MCP electrode were recorded in different concentrations of NaOH solution containing 30 mmol L−1 methanol (not shown). It is shown that, the high catalytic peak current is achieved above a NaOH concentration of 0.1 mol L−1. So, 0.1 mol L−1 NaOH was chosen as an optimum supporting electrolyte.
To provide more evidence, the effect of the scan rate varying from 5 to 100 mV s−1 on the voltammetric response of SC-MCP electrode in a solution containing 30 mmol L−1 methanol was studied (Fig. 4a). The anodic currents increase and the peak potential shifts as the scan rate increases. When peak current values were plotted against v 1/2 (Fig. 4b), a linear relationship with R 2 = 0.9902 was obtained. This behavior suggests that the oxidation process is controlled by diffusion. Moreover, a plot of the scan rate normalized current (I/v 1/2) vs. the scan rate exhibits the typical shape of an electrochemical (EC′) catalytic process (Fig. 4c). The peak currents for anodic oxidation of methanol are not proportional to the scan rate. These results indicate that at sufficiently positive potential the reaction is controlled by surface-confined methanol species, which is the ideal case for quantitative applications. Also, it can be seen that the peak potential for the catalytic reduction of methanol shifts to more positive values by increasing the scan rate, suggesting a kinetic limitation in the reaction between the redox sites of silver chloride and methanol. A linear relationship was observed for E p vs. log ν (not shown) that can be expressed by the equation [27].
a Cyclic voltammograms of SC-MCP electrode in 0.1 mol L−1 NaOH containing 30 mmolL−1 of methanol at various potential scan rates (from inner to outer) 7–100 mV s−1. b Variation of the catalytic current with the square root of sweep rate. c Current function vs. v for 0.1 mol L−1 NaOH solution in the presence of 30 mmol L−1 methanol
On the basis of Eq. (17), the slope of E p vs. logν plot is b/2, where b indicates the Tafel slope. The slope of E p vs. log ν plot is ∂E p/∂ (logv) which was found to be 51.96, so b = 103.92. This slope indicates that a one-electron transfer process is the rate-limiting step assuming a transfer coefficient of α = 0.57 for methanol.
With the increase of methanol concentration, the anodic peak current gradually increased (Fig. 5). The characteristic shape of cyclic voltammogram in this potential region indicates that the signal is due to the oxidation of methanol. The catalytic peak current is proportional to the concentration of methanol in the range of 5 mmol L−1–60 mmol L−1. The linear regression equation is I (µA) = 30.357 C methanol (mmol L−1) + 894.24 (R 2 = 0.9947).
To evaluate the reaction kinetics, the oxidation of methanol on SC-MCP electrode was investigated by chronoamperometry. Chronoamperometry, as well as cyclic voltammetry has been employed for the investigation of the processes occurring via an E r C i mechanism [35]. Double steps chronoamperograms were recorded by setting the working electrode potentials to desired values and were used to measure the catalytic rate constant on the modified surface. Figure 6a shows a series of well-defined chronoamperograms for the SC-MCP electrode in the absence and presence of different concentrations of methanol at an applied potential of 700 mV vs. Ag/AgCl. The plot of net current with respect to the mines square roots of time presents a linear dependency (Fig. 6b). This indicates that the transient current must be controlled by a diffusion process. The transient current is due to catalytic oxidation of methanol, which increases as the methanol concentration is raised. No significant cathodic current was observed when the electrolysis potential was stepped to 0.00 mV (vs. Ag/AgCl), indicating the irreversible nature of the oxidation of methanol.
a Chronoamperograms of SC-MCP electrode in 0.1 mol L−1 NaOH containing different concentrations of methanol: 0.0, 5, 10, 15 and 20 mmol L−1, from a to e, respectively. Potential steps were 0.65 and 0.0 V, respectively. Insets variation of chronoamperometric currents at t = 15 s vs. concentration of methanol. b Plot of i vs. t −1/2 obtained from chronoamperometric measurements SC-MCP electrode in 0.1 mol L−1 NaOH containing different concentrations of methanol: 0.0, 5, 10, 15 and 20 mmol L−1, from a to e, respectively. c Dependency of I catal/I d on t 1/2 derived from CAs of a and e in panel A
The rate constants of the reactions of methanol and the ensuing intermediates with the redox sites of the SC-MCP electrode can be derived from the chronoamperograms according to Eq. (18) [27]:
where I catal is the catalytic current in the presence of methanol, I d the limiting current in the absence of methanol and λ = kCt (k, C and t are the catalytic rate constant, bulk concentration of methanol and the elapsed time, respectively) is the argument of the error function. For λ > 1.5, erf (λ 1/2) almost equals unity and Eq. (19) reduces to [27]:
From the slope of the I catal/I d vs. t 1/2 plot (Fig. 6c), the value of k for 20 mmol L−1 methanol was calculated to be 1.175 × 105cm3 mol−1 s−1.
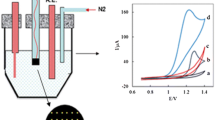
The pseudo-steady-state polarization curves of the electro-oxidation of methanol on SC-MCP electrode at a number of methanol concentrations are presented in Fig. 7. The rotation rate of the electrode is maintained at 3000 rpm to avoid the interference of the mass transfer in the kinetic measurements. The oxidation process was found to begin at nearly 515 mV (vs. Ag/AgCl) and to reach a plateau at 605 mV (vs. Ag/AgCl), while the oxygen evolution starts at still higher potentials. In the course of reaction, the coverage of Ag(II) increases and reaches a saturation (steady state) level and the oxidation current follows accordingly.
To obtain information on the rate-determining step, Tafel plots were drawn under a controlled rotating disc speed at different methanol concentrations (Inset of Fig. 7), derived from data of the rising part of the current–voltage curves at a low scan rate of 7 mV s−1. The anodic electron transfer coefficient of α = 0.69 and the exchange current density (j 0) of 8.85 × 10−7 Acm−2 were obtained.
Analytical characteristics of SC-MCP electrode for the amperometric determination of methanol were estimated. Figure 8 shows the current–time responses of the modified electrode to methanol which was successively added to the electrochemical cell containing 0.1 mol L−1 NaOH under hydrodynamic conditions, while the electrode potential was kept at 0.6 V. As shown in the figure, a well-defined response was observed during the stepwise increasing of methanol concentration in the range 60–220 µmol L−1. The linear regression equation of calibration curve is expressed as I (µA) = 1.0567 C methanol µmol L−1 +89.52 with a correlation coefficient of 0.9978 (n = 9). A calibration plot constructed from data of Fig. 8 gives a limit of detection (LOD) and sensitivity 8.21 and 1.0567µA/µmol L−1, respectively [36].
By repetitive CV of the SC-MCP electrode for approximately 20 times in NaOH solution at a scan rate of 50 mV s−1, the peak current value decreases less than 9 %, indicating good stability. The modified electrode retained its initiate activity for more than 40 days when kept in air at ambient conditions. A decrease of 12 % was observed in the current response of the electrode at the end of 40th day. In addition, repetitive recording of cyclic voltamograms in methanol solution tested the reproducibility of the electrocatalytic effect of the modified CPE. It was found that the relative standard deviation (R.S.D.) of the peak currents of 30 mmol L−1 methanol for seven replicate determinations was 2.7 %.
Conclusion
In the present study, the electrochemical behaviors of methanol at SC-MCP electrode have been investigated. The modified electrode exhibits excellent and persistent electrocatalytic behavior toward methanol oxidation compared with the bare carbon paste electrode. For SC-MCP electrode, kinetic parameters such as the electron transfer coefficient (α), catalytic reaction rate constant (k) and the number of electrons involved in the rate-determining step (n α) for oxidation of methanol at the SC-MCP surface were calculated as 0.66, 1.175 × 105 and 1, respectively.
References
H. Nonaka, Y. Matsumura, J. Electroanal. Chem. 520, 101 (2002)
C.L. Green, A. Kucernak, J. Phys. Chem. B 106, 1036 (2002)
A.S. Arico, Z. Poltarzewski, H. Kim, A. Morana, N. Giordano, V. Antonucci, J. Power Sources 55, 159 (1995)
M. Jafarian, M.G. Mahjani, H. Heli, F. Gobal, H. Khajesharifi, M.H. Hamedi, Electrochim. Acta 48, 3423 (2003)
S.M. Golabi, A. Nozad, Electroanalysis 15, 278 (2003)
F.E. Jones, S.B. Milen, B. Gurau, E.S. Smotkin, S.R. Stock, C.M. Lukehart, J. Nanosci. Nanotechnol. 2, 81 (2002)
J. Luo, M.M. Maye, Y. Lou, L. Han, M. Hepel, C.J. Zhong, Catal. Today 77, 127 (2002)
A.A. El-Shafei, J. Electroanal. Chem. 471, 89 (1999)
M.S. Kim, T.S. Hwang, K.B. Kim, J. Electrochem. Soc. 144, 1537 (1997)
M.A. Abdel Rahim, R.M. Abdel Hameed, M.W. Khalil, J. Power Sources 134, 160 (2004)
M.A. Abdel Rahim, H.B. Hassan, R.M. Abdel Hamid, J. Power Sources 154, 59 (2006)
S.M. Golabi, A. Nozad, Electroanalysis 16, 199 (2004)
K. Kalcher, Electroanalysis 2, 419 (1990)
Hamid R. Zare, Navid Nasirizadeh, Int. J. Electrochem. Sci. 4, 1691 (2009)
K.I. Ozoemena, R.S. Staden, T. Nyokong, Electroanalysis 21, 1651 (2009)
S. Shahrokhian, M. Amiri, Solid State. Electrochem. 11, 1133 (2007)
E.V. Ivanova, V.S. Sergeeva, J. Oni, Ch. Kurzawa, A.D. Ryabov, W. Schuhmann, Bioelectrochem. 60, 65 (2003)
M. Amiri, Z. Pakdel, A. Bezaatpour, S. Shahrokhian, Bioelectrochemistry 81, 81 (2011)
D. Moscone, D.DQOttavi, D. Compagnone, G. Palleschi. Anal. Chem. 73, 2529 (2001)
X.L. Wang, E.B. Wang, Y. Lan, C. Hu, Electroanalysis 14, 1116 (2002)
B.R. Limoges, R.J. Stanis, J.A. Turner, A.M. Herring, Electrochim. Acta 50, 1169 (2005)
X.L. Wang, Z.B. Han, H. Zhang, E.B. Wang, C.W. Hu, Electroanalysis 15, 1460 (2003)
C. Jin, Z. Zhang, Z. Chen, Q. Chen, Electrochim. Acta 87, 860 (2013)
G. Karim-Nezhad, P.S. Dorraji, Electrochim. Acta 55, 3414 (2010)
G. Karim-Nezhad, B. Zare Dizajdizi, P.S. Dorraji, Catal. Communi. 12, 906 (2011)
G. Karim-Nezhad, A. Pashazadeh, S. Pashazadeh, J. Korean Chem. Soc. 57, 322 (2013)
M. Hasanzadeh, G. Karim-Nezhad, N. Shadjou, B. Khalilzadeh, L.A. Saghatforoush, S. Earshad, I. Kazeman, Chin. J. Chem. 27, 638 (2009)
G. Karim-Nezhad, S. Pashazadeh, A. Pashazadeh, Anal. Bioanal. Electrochem. 5, 719 (2013)
L. Niu, Q. Li, F. Wei, S. Wu, P. Liu, X. Cao, J. Electroanal. Chem. 578, 331 (2005)
M.H. Pournaghi-Azar, B. Habibi, J. Electroanal. Chem. 601, 53 (2007)
F. Vigier, F. Gloaguen, J.M. Léger, C. Lamy, Electrochim. Acta 46, 4331 (2001)
P. Ross, N. Markovic, CATTECH 4, 110 (2000)
H. Razmi, E. Habibi, J. Solid State Electrochem. 13, 1897 (2009)
H. de Santana, M.L.A. Temperini, J. Electroanal. Chem. 316, 93 (1991)
A.J. Bard, L.R. Faulkner, Electrochemical methods: fundamentals and applications, 2nd edn. (Wiley, New York, 2001)
J.C. Miller, J.N. Miller, Statistics for Analytical chemistry, 2nd edn. (Prentice Hall, New York, 1994)
G. Karim-Nezhad, S. Pashazadeh, A. Pashazadeh, Chin. J. Catal. 33, 1809 (2012)
H. Razmi, Es. Habibi, H. Heidari. Electrochim. Acta 53, 8178 (2008)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karim-Nezhad, G., Pashazadeh, S. Development of a new modified carbon paste electrode by silver chloride: application to electrocatalytic oxidation of methanol. J IRAN CHEM SOC 13, 307–314 (2016). https://doi.org/10.1007/s13738-015-0738-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-015-0738-1