Abstract
In this work, choline chloride/zinc chloride was employed as a deep eutectic solvent (DES) in the green synthesis of coumarin derivatives using Knoevenagel condensation. Coumarins are important organic structures with useful application in various fields. The procedure consisted of the reaction between salicylaldehyde derivatives and active methylene compounds (dimethyl malonate, ethyl cyano acetate and ethyl 3-oxo-3-phenylpropanoate) to produce various coumarin derivatives. The general procedure is easy because of simple preparation and isolation of desired products. Moreover, the method is environmental friendly because of avoiding use of toxic solvents or hazardous catalysts. In the presented method, only 10 % of choline chloride/zinc chloride was employed as a non-toxic and biologically degradable media. All reactions have been completed under mild conditions with high yields (61–96 %). The employed DES plays both roles of solvent and catalyst in this reaction. Moreover, it has been reused four times in this procedure without important decreasing in the yield of reaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coumarins are important class of organic structures with useful applications in pharmaceutical, optical devices, dyes, antimicrobials, chemotherapeutics and solar cells [1]. Because of these applications, they have been attracted much interests and various strategies for their synthesis have been designed [2]. Coumarins could be prepared using different methods including Pechmann [3], Knoevenagel [4, 5], Perkin [6, 7], Reformatsky [8] and Wittig [9] reaction. During these years, Knoevenagel condensation has been one of the simplest and the most important methods for the synthesis of coumarin derivatives. This method consisted of the reaction between salicylaldehyde and carbonyl compounds with active methylene in presence of catalyst [10]. The reaction usually proceeds using base, acid or heterogeneous catalysts [11]. However, because of the importance of coumarin and its derivatives, developing new methods in this category is still the subject of interest. In this line, the use of homogeneous [12] solid acid [13] and solid base catalysts [14] has been reported as new catalysts for this reaction. Most of these methods have some limitations such as using hazardous materials (or solvents) or expensive catalysts, low yields or harsh reaction conditions. In addition, because of the importance of environmental protection in this century, it will be necessary to produce coumarins using the green and environmental friendly procedure. Chemists have directed their researches, employing more safe materials and non-toxic methods to avoid environmental problems. In this line, ionic liquids and their new generations, deep eutectic solvents (DESs), have been recently used in the synthesis of various organic structures. DESs are more useful than simple ionic liquids because of playing two roles (solvent and catalyst), having less viscosities (than simple ionic liquids) and melting at less temperatures [15–17]. Moreover, they have shown high potencies in the synthesis of various organic structures [18–23]. DESs are free of some disadvantages related to simple ionic liquids, such as high cost and environmental problems [24–27]. They are mostly biodegradable, recyclable and cheap alternative for the most of solvents. Moreover, they could be easily prepared by mixing two or more components to form the eutectic with a melting point below each individual component. Therefore, to continue our previous studies in multicomponent synthesis and using ionic liquids [28, 29], we have attempted to employ a cheap DES in the synthesis of coumarin derivatives. In this work, the use of choline chloride/zinc chloride DES as a green and efficient media in the synthesis of coumarin derivatives has been reported. We have prepared some coumarin derivatives to show the versatility of this method and in comparing with the previous reports [30–32], we have avoided using any toxic catalyst or solvent to ensure that this method will be considered as a green method. In addition, the effect of various parameters and the recyclability of employed DES have been evaluated. The methodologies and results obtained in this study will be discussed in the next sections.
Experimental
Chemicals and apparatus
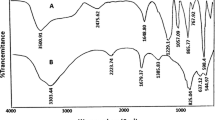
All compounds have been prepared from Aldrich and Merck companies and used without further purifications. Melting points were determined using Gallen Kamp melting point apparatus. Thin-layer chromatography was used to monitor the reaction and check purities. IR spectra (KBr) were recorded by JASCO FT-IR. 1H-NMR and 13C-NMR spectra were recorded by Brucker Ultrashield 400 MHz. NMR chemical shifts were expressed in ppm versus the chemical shift of tetramethylsilane (TMS) as an internal reference.
General procedure for preparation of DES
Choline chloride/zinc chloride DES was prepared according to the procedure reported in the literature [17]. The preparation method involved mixing of choline chloride (1 mol) with zinc chloride (2 mol) at 100 °C till a clear solution was obtained. The mixture (prepared DES) was used without any purification (Scheme 1).
General procedure for synthesis of coumarin derivatives
In a 50 mL round-bottom flask equipped with condenser arranged on magnetic stirrer, 1 mmol of salicylaldehyde (or its derivatives) and 1 mmol of diethylmalonate (or similar active methylene compounds) were added to 10 mol % DES and the reaction mixture was stirred at 80 °C in oil bath. The reaction was monitored by TLC to show the completion of the reaction. After terminating reaction, 2.5 mL of water was added and precipitated product was filtered and dried. The product was purified by crystallization using ethanol. The filtrate, containing catalyst, was recycled directly in next runs without further purification. The structures of all the products were determined by comparison of their melting points, FT-IR, 1H-NMR and 13C-NMR spectra data with those of authentic samples. Selected spectroscopic data for sample structures are as follows. More characterization data could be found in the supporting information.
Methyl 2-oxo-2H-chromene-3-carboxylate (1a)
Yield 96 %: pale yellow solid. m.p. 109–110 °C; IR (KBr, cm−1): 1748, 1698, 1616, 1564, 1454, 1439, 1312, 1272, 1248. 1HNMR (400 MHz, CDCl3): 8.60 (s, 1H), 7.64–7.68 (m, 2H), 7.35–7.41 (m, 2H), 3.99 (s, 3H). 13CNMR (100 MHz, CDCl3): 175.5, 149.2, 134.8, 134.5, 130.6, 129.6, 129.1, 124.9, 117.1, 116.9, 53.0. Elemental Anal. C11H8O4 C, 64.71; H, 3.95; O, 31.34, Found: C, 64.42; H, 3.640; O, 31.331.
Result and discussion
A mixture of choline chloride/zinc chloride has been employed as DES for the synthesis of coumarin derivatives (Scheme 2). The DES has dual roles of catalyst as well as solvent in this synthesis. To examine the versatility of the employed DES in this synthesis, simple and substituted salicylaldehydes (4-hydroxy- and 5-bromo-) with three different active methylene compounds (dimethyl malonate, ethyl cyano acetate and ethyl 3-oxo-3-phenylpropanoate) have been used to prepare different coumarin derivatives. To obtain the best conditions (amount of catalyst, reaction time and temperature), some preliminary reactions were done based on the reaction of salicylaldehyde and diethyl malonate (the model reaction) and the results are listed in Table 1. Based on the previous reports on the same reactions, 10 mol % of DES in overnight conditions (20 h) was used to obtain the best reaction temperatures. In the optimization of reaction temperature, according to the obtained results (entries 1–3 of Table 1), the maximum yield was obtained at 80 °C.
It should be noticed that the reaction did not proceed successfully without using DES. To obtain the best amount of DES, these conditions were used with different amounts of DES (5, 10, 15 and 20 mol %). These experiments showed that increasing the amount of DES from 5 to 10 mol % extensively increases the reaction yield. However, by increasing this amount to larger values, any significant increasing could not be observed in the reaction yield (entries 4–7 of the Table 1). Therefore, the reaction proceeds completely with 10 mol % the DES. To optimize the reaction time (using optimum values of the other conditions), the time was decreased (from 20 h, entries 9–12 Table 1) and the obvious decreasing in the reaction yield was observed. Therefore, 20 h has been considered as the best time for this reaction. The results obtained from these optimization processes were employed for the synthesis of the other coumarin derivatives through Knoevenagel condensation in the presence of choline chloride/zinc chloride DES (Scheme 2). When our results have been compared with previous reports related to the synthesis of coumarins, it will be defined that we avoid using any toxic catalyst or solvent to ensure that this method could be considered as a green method. In addition, we have used a simple procedure (in preparation and separation of product) and mild condition.
Furthermore, other DESs (choline chloride/SnCl2 and various molar ratios of choline chloride/ZnCl2) were employed on the model reaction to examine their efficiencies in this reaction and the results are summarized in Table 2. It should be mentioned that using another ionic liquids and basic DES in the synthesis of coumarin derivatives has been previously reported [11, 33–37] and we have only focused on acidic DESs in this synthesis. When ChCl.ZnCl2 was employed in the model reaction, the reaction yield was decreased (to only 25 % after 20 h) and using ChCl.3ZnCl2, any significant increasing in the reaction yield has not been observed. In addition, ChCl.2SnCl2 was also employed as another DES in the model reaction and the obtained yield for the reaction was only 35 % after 20 h. Therefore, ChCl.2ZnCl2 has been considered as the best DES for this reaction.
As it shown in the Scheme 2, the reaction yield for different derivatives was between 61 and 96 %. Using simple salicylaldehyde, the reactions have more yields than substituted salicylaldehyde and dimethyl malonate has the maximum yield among all active methylene compounds. Therefore, the highest yield was observed in the reaction of salicylaldehyde with dimethyl malonate and the minimum yield was observed in the reaction of 5-bromo salicylaldehyde with ethyl 3-oxo-3-phenylpropanoate. It seems that the electron donor groups reduce the reactivity of salicylaldehyde derivatives.
The role of DES in the mechanistic details of these syntheses is depicted in Scheme 3 for the model reaction. This mechanism has been presented based on the acidic nature of DES that participates in the reaction as choline cation and ZnCl3 − anion. Zinc chloride acts as Lewis acid and the chloride ion may be involved in the reaction as Lewis base to adsorb a proton of diethylmalonate. More details about the role of DESs in the organic synthesis were defined in the reported work of Abbot [38].
To complete this work, the potency of ChCl.2ZnCl2 for further use (reusability) in the model reaction was examined and the results are shown in Table 3. For each step, the DES was separated from the reaction mixture by phase extraction and the extracted DES has been used without future purification in the next run. These experiments showed that further reactions (up to four consecutive runs) using recycled eutectic solvent have been performed without significant loss of yield. Therefore, this DES could be employed in this synthesis at least four runs without remarkable loss of activity.
Conclusion
This work presents the high-yield synthesis of coumarin derivatives by Knoevenagel condensation using only 10 % of choline chloride/zinc chloride as a DES. The reaction involves salicylaldehyde derivatives and active methylene compounds such as dimethyl malonate, ethyl cyano acetate and ethyl 3-oxo-3-phenylpropanoate. The employed DES plays role of solvent in addition to its catalytic effect and it could be recycled four times without significant decreasing in the reaction yield. This efficient method is environmental friendly because of avoiding use of toxic solvents and using choline chloride as a cheap, biologically degradable and non-toxic molecule. Moreover, the general method is simple and it uses mild condition without producing remarkable byproducts.
References
R. Okennedy, R.D. Thornes, Coumarins: biology, applications and mode of action, 1st edn. (Wiley, Chichester, 1997), pp. 103–136
M. Maheswara, V. Siddaiah, G.L.V. Damu, Y.K. Rao, C.V. Rao, J. Mol. Catal. 255, 49 (2006)
S.M. Sethna, R. Phadke, Org. React. 7, 1 (1953)
G. Jones, Org. React. 15, 204 (1967)
F. Bigi, L. Chesini, R. Maggi, G. Sartori, J. Org. Chem. 64, 10334 (1999)
B.J. Donnelly, D.M.X. Donnelly, A.M.O. Sullivan, Tetrahedron 24, 2617 (1968)
J.R. Johnson, Org. React. 1, 210 (1942)
R.L. Shirner, Org. React. 1, 1 (1942)
I. Yavari, R. Hekmat-shoar, A. Zonuzi, Tetrahedron Lett. 39, 2391 (1998)
B.M. Trost, Comprehensive organic synthesis (Pergamon, Oxford, 1991), pp. 341–394
B.C. Ranu, R. Jana, Eur. J. Org. Chem. 3767 (2006)
C. Li, Chem. Rev. 93, 2023 (1993)
A. Hoz, A. Moreno, E. Vazquez, Synlett 608 (1999)
N. Britto, V.G. Gore, R.S. Mali, A.C. Ranade, Synth. Commun. 19, 1899 (1989)
B.P. Bandgar, L.S. Uppalla, D.S. Kurule, Green Chem. 1, 243 (1999)
P. Wasserscheid, W. Keim, Angew. Chem. Int. Ed. 39, 3772 (2000)
S.B. Phadtare, K.J. Jarag, G.S. Shankarling, Dyes. Pigm. 97, 105 (2013)
I. Bandres, R. Alcalde, C. Lafuente, M. Atilhan, S. Aparicio, J. Phys. Chem. B 115, 12499 (2011)
S.L. Perkins, P. Painter, C.M. Colina, J. Phys. Chem. B. 117, 10250 (2013)
D. Saberi, J. Akbari, S. Mahdudi, A. Heydari, J. Mol. Liq. 196, 208 (2014)
Q. Zhang, K.D. Vigier, S. Royer, F. Jerome, Chem. Soc. Rev. 41, 7108 (2012)
B.S. Singh, H.R. Lobo, D.V. Pinjari, K.J. Jarag, A.B. Pandit, G.S. Shankarling, Ultrason. Sonochem. 20, 287 (2013)
I. Hawkins, S.T. Handy, Tetrahedron 69, 9200 (2013)
A.P. Abbott, D. Boothby, G. Capper, D.L. Davies, R.K. Rasheed, J. Am. Chem. Soc. 126, 9142 (2004)
N. Azizi, E. Gholibeglo, M. Babapour, H. ghafuri, S.M. Bolourtchian, Compet. Rendus Chimie 15, 768 (2012)
N. Jain, A. Kumar, S. Chauhan, Tetrahedron 61, 1015 (2005)
Y. Cai, Y. Peng, G. Song, Catal. Lett. 109, 61 (2006)
T. Welton, Chem. Rev. 99, 2071 (1999)
N. Azizi, S. Dezfooli, M.M. Hashemi, Compet. Rendus Chimie 16, 1098 (2013)
H. Tavakol, S. Hatami, S. Ghammamy, G. Rezaei-Behbahani, F. Shabani, Heteroat. Chem. 20, 398 (2009)
F. Keshavarzipour, H. Tavakol, Catal. Lett. 145, 1062 (2015)
Z. Mirjafary, H. Saeidian, F.M. Moghaddam, Trans. C Chem. Chem. Eng. 16, 12 (2009)
H. Valizadeh, M. Mahmoodian, H. Gholipour, J. Heterocycl. Chem. 48, 799 (2011)
A. Shockravi, H. Shargi, H. Valizadeh, M.M. Heravi, Sulfur Silicon Relat. Elem. 177, 2555 (2002)
J.R. Harjani, S.J. Nara, M.M. Salunkhe, Tetrahedron Lett. 43, 1127 (2002)
H. Valizadeh, S. Vaghefi, Syn. Comm. 39, 1666 (2009)
N.B. Darvatkar, A.R. Deorukhkar, S.V. Bhilare, D.G. Raut, M.M. Salunkhe, Syn. Commun. 38, 3508 (2008)
A.P. Abbott, G. Capper, D.L. Davies, H. Munro, R.K. Rasheed, V. Tambyrajah, Chem. Commun. 201 (2001)
Acknowledgments
This work was supported by the research affairs of Isfahan University of technology.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Keshavarzipour, F., Tavakol, H. The synthesis of coumarin derivatives using choline chloride/zinc chloride as a deep eutectic solvent. J IRAN CHEM SOC 13, 149–153 (2016). https://doi.org/10.1007/s13738-015-0722-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-015-0722-9